Abstract
Background:
The use of adjuvant tamoxifen therapy in the treatment of estrogen receptor (ER) expressing breast carcinomas represents a major advance in personalized cancer treatment. Because there is no benefit (and indeed there is increased morbidity and mortality) associated with the use of tamoxifen therapy in ER-negative breast cancer, its use is restricted to women with ER expressing cancers. However, correctly classifying cancers as ER positive or negative has been challenging given the high reported false negative test rates for ER expression in surgical specimens. In this paper I model practice recommendations using published information from clinical trials to address the question of whether there is a false negative test rate above which it is more efficacious to forgo ER testing and instead treat all patients with tamoxifen regardless of ER test results.
Methods:
I used data from randomized clinical trials to model two different hypothetical treatment strategies: (1) the current strategy of treating only ER positive women with tamoxifen and (2) an alternative strategy where all women are treated with tamoxifen regardless of ER test results. The variables used in the model are literature-derived survival rates of the different combinations of ER positivity and treatment with tamoxifen, varying true ER positivity rates and varying false negative ER testing rates. The outcome variable was hypothetical 10-year survival.
Results:
The model predicted that there will be a range of true ER rates and false negative test rates above which it would be more efficacious to treat all women with breast cancer with tamoxifen and forgo ER testing. This situation occurred with high true positive ER rates and false negative ER test rates in the range of 20-30%.
Conclusions:
It is hoped that this model will provide an example of the potential importance of diagnostic error on clinical outcomes and furthermore will give an example of how the effect of that error could be modeled using real-world data from clinical trials.
Keywords: Breast cancer, cancer biomarker, medical error
INTRODUCTION
Breast cancer remains the most common malignancy affecting women in developed countries.[1] A major advance in the treatment of breast cancer was the discovery that tamoxifen, a selective estrogen receptor (ER) modulator reduces the risk of recurrence and death as well as providing effective palliation for patients with metastatic disease.[1] Tamoxifen also has a role in the primary prevention of breast cancer in high risk populations.[2–4]
While it is clear that there is a survival benefit from administering tamoxifen to women with breast cancers that express the estrogen receptor (ER positive breast cancers), not all breast cancers express these receptors. Swain[5] summarized available data on the utility of tamoxifen in ER negative breast cancers and concluded that there was no survival benefit to the administration of tamoxifen to women with ER-negative breast cancer. In fact, administration of tamoxifen to women with ER-negative breast cancers appears to increase overall mortality as the use of tamoxifen has been associated with an increased risk of venous thromboembolism (including stroke and pulmonary embolus), gastrointestinal cancers, endometrial cancer, and fatal myocardial infarction.[6–8] The current recommendation, therefore, is that women with ER-positive breast cancers of any size should receive tamoxifen but women with ER-negative tumors should not.[9]
A major practical problem, however, with the use of tamoxifen has been the accurate determination of ER status given the reported high false negative rates of ER receptor testing. For example, over the past decade, a number of laboratories in the United States, Europe, and Canada have reported false negative rates of 15-40%.[5,10] Compounding this false negative rate is the fact that some laboratories have traditionally counted cases with 10-20% of ER-positive cells as negative,[1,5] despite the fact that cases with 1-10% positive cells can still show benefit from tamoxifen.[11]
Intuitively, we might expect that if tamoxifen reduces the overall mortality rate in ER-positive tumors, but increases the mortality rate in ER-negative tumors (because of an increased risk of other adverse events), then in the presence of a high false negative rate in ER testing (meaning that many women were improperly denied tamoxifen treatment), there may be a false negative ER testing rate above which the treatment emergent adverse events cause fewer deaths than the reduction in recurrent or new cancers afforded by treating women with ER-negative cancers (some of which will be false negatives). In other words, there may be a false negative ER test rate above which it is more efficacious to treat everyone regardless of ER test status.
I approached this problem by modeling different practice recommendations using published information. Specifically, I used literature-derived data from clinical trials to model the overall mean expected survival of different hypothetical cohorts of women with early breast cancer. These hypothetical populations varied in their true ER-positive rate, in their false negative ER test rate, and finally in their treatment regime (treat only ER test positive cases with tamoxifen, or treat all individuals with tamoxifen regardless of ER test status). Another variable (node positive rate) was held constant in the model. Ten-year survival rates for each of the groups within the hypothetical cohorts were obtained from literature values, and the overall mean 10-year survival rate for each hypothetical cohort was calculated.
METHODS
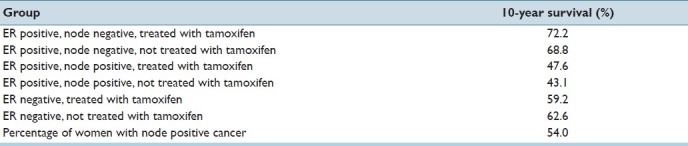
I constructed a dynamic mathematical model to estimate the overall 10-year mortality in a hypothetical cohort of women with early breast cancer. The inputs to the model were population breast cancer true ER positive rate, false negative ER rest rate, breast cancer lymph node positive rate, and the associated 10-year survival rates for each of these groups treated and not treated with tamoxifen for at least 2 years. Parameterization for the model was obtained from the Early Breast Cancer Trialists′ Collaborative Group's 1998 review of 55 tamoxifen randomized trials [Table 1].[12] For ER-positive rates, I considered the clinically feasible range of 0.60-1.00. For false negative ER test rates, I considered the clinically feasible range of 0.00-0.30. I modeled two different scenarios: the first was if only breast cancer patients with an ER-positive test result were treated with tamoxifen. The second scenario was if all patients were treated with tamoxifen regardless of ER test results.
Table 1.
Parameters used in the model. All estimates of 10-year survival were taken from the early breast cancer trialists’ collaborative group (12)
The calculations were performed by constructing an excel spreadsheet to input the mortality rates associated with each of the cohort groups. A visual basic program then used these values to calculate estimated 10-year survival rates for each of the combinations of true ER rates and false negative test rates for each of the two treatment scenarios [Table 2]. The output of these simulations was then graphically represented using SPSS Version 15.0 for Windows.
Table 2.
Equations used in the model

RESULTS
The model predicted that in the scenario where only individuals with ER-positive test results were treated, the greatest overall survival was seen in populations with a high overall ER-positive rate and a low false negative rate (survival rates shown in green in Figure 1). For the scenario where all individuals were treated regardless of ER test result, the false negative test rate was irrelevant and there was much less variation in the overall survival rate in different hypothetical populations. As Figure 1 shows, there was a range of true ER positive rates and false negative test rates where the greatest overall survival benefit was obtained by treating everyone with tamoxifen instead of only treating patients with ER-positive test results. In general, an overall survival advantage was seen in scenarios with lower false negative error rates and higher true ER-positive rates. For true ER positivity rates of 80-90% the tipping point where treating all patients and forgoing testing resulted in the longest mean survival occurred at a false negative rate of 20-30% [Figure 1].
Figure 1.
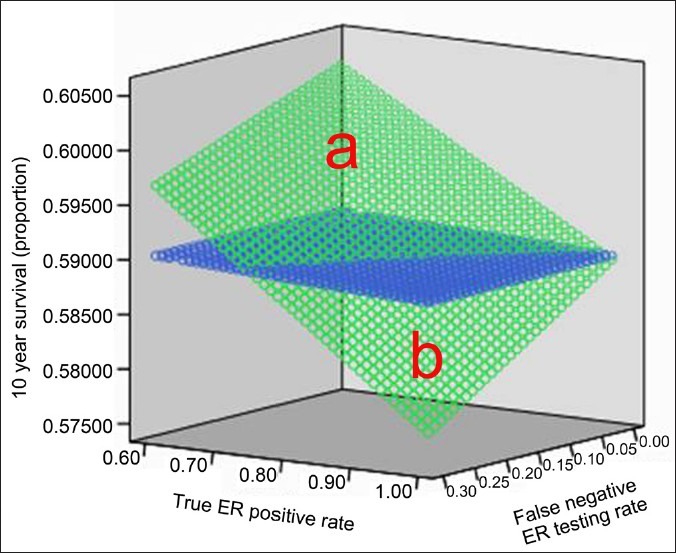
Model of 10-year survival given varying estrogen receptor true positive rates (the clinically plausible range of 60-100%) and varying estrogen receptor false negative testing rates (the clinically plausible range of 0-30%). The green area indicates the predicted 10-year survival for the scenario where only patients with a positive estrogen receptor result are treated with tamoxifen. The blue area indicates the predicted 10-year survival for the scenario all patients are treated with tamoxifen regardless of their estrogen receptor status. Populations in region “a” had the highest overall survival rate if only ER patients were treated with tamoxifen; region “b” populations had a higher overall survival rate if every patient was treated with tamoxifen regardless of ER status
CONCLUSIONS
In this paper I presented a model which demonstrates the potential effect of diagnostic error in ER testing of breast cancer cases. In cohorts with high false negative test rates, the effect of this error could potentially be such that greater overall survival may have been achieved by not testing breast cancers for ER status at all and instead treating all patients with tamoxifen regardless of the ER test results of their tumor.
The first and most important criticism of this model is that the predictions are only as good as the survival data used as inputs. Although the survival data were obtained from a meta-analysis of many randomized controlled trials, these trials are now as much as several decades old. In particular, the data used to estimate survival of ER-negative patients treated with tamoxifen were based on a relatively small number of patients which met this criterion from the randomized controlled trials. Even when considering the treatment groups with more robust numbers of participants such as ER-positive patients treated with tamoxifen, the true utility of tamoxifen was likely underestimated because up to 20% of women prescribed tamoxifen in the early trials either did not take it or stopped it prematurely.[12] The value of this analysis may also be limited in the future by the fact that a newer class of drugs, the Aromatase inhibitors, are replacing tamoxifen as first line treatment agents for early breast cancer.[13,14]
It could also be argued that the high false negative error rates for ER testing that I considered for this model were the product of a bygone era and that error rates this high would not be tolerated in modern laboratories. I suspect that for the most part this is true; however it should be noted that the 40% false negative ER testing rate reported from Newfoundland is less than 6 years old. Nevertheless it should be noted that in regions of the world where rigorous quality assurance may not yet be the norm, there may exist a range of true ER positive and false negative test rates where the greatest 10-year survival of breast cancer patients would be obtained by treating all patients with early breast cancer with tamoxifen and forgoing ER testing until an acceptably low false negative testing rate can be assured.
It is hoped that the model presented here will provide an example of the potential importance of diagnostic error in the pathology laboratory as well as providing an example of how the effect of that error could be modeled using real-world data from clinical trials.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2012/3/1/19/95452
REFERENCES
- 1.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–18. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 3.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 4.Fabian CJ, Kimler BF. Selective estrogen-receptor modulators for primary prevention of breast cancer. J Clin Oncol. 2005;23:1644–55. doi: 10.1200/JCO.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Swain SM. Tamoxifen for patients with estrogen receptor-negative breast cancer. J Clin Oncol. 2001;19(18 Suppl):93S–97S. [PubMed] [Google Scholar]
- 6.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18:937–47. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier CR, Jick M. Tamoxifen and risk of idiopathic venous thromboembolism. Br J Clin Pharmacol. 1998;45:608–12. doi: 10.1046/j.1365-2125.1998.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMichele A, Troxel AB, Berlin JA, Weber AL, Bunin GR, Turzo E, et al. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol. 2008;26:4151–9. doi: 10.1200/JCO.2007.14.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adjuvant therapy for breast cancer. National Institutes of Health Consensus Development Statement. 2000. Nov 1-3, [Last accessed on 2012 Jan 16]. Available from: http://odp.od.nih.gov/consensus/cons/114/114_intro.htm .
- 10.Allred DC. Commentary: hormone receptor testing in breast cancer: A distress signal from Canada. Oncologist. 2008;13:1134–6. doi: 10.1634/theoncologist.2008-0184. [DOI] [PubMed] [Google Scholar]
- 11.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 12.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451–67. [PubMed] [Google Scholar]
- 13.Sehdev S, Martin G, Sideris L, Lam W, Brisson S. Safety of adjuvant endocrine therapies in hormone receptor – positive early breast cancer. Curr Oncol. 2009;16(Suppl 2):S14–23. doi: 10.3747/co.v16i0.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–29. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]


