Abstract
The inoculation of live Leishmania (L.) major to produce a single lesion is called leishmanization (LZ). LZ lesion upon cure prevents further natural infection which might be multiple lesions on unwanted sites such as face. Cutaneous leishmaniasis (CL) usually leads to a self healing lesion; though rarely the lesion persists and becomes refractory to all types of remedies. Here, we present a 41-year-old patient with a 20-year history of cutaneous lesion caused by leishmanization. The causative agent is identified as L. major. The patient did not respond to treatment with meglumine antimoniate, 20 mg/kg/day Sb+5 for three weeks and allopurinol 10 mg/kg for four weeks. After two months, the same treatment was repeated. In addition, a topical liposomal preparation containing 10% paromomycin sulfate was administered twice a day for four weeks. The lesion showed marked improvement during the treatment and was eventually completely healed.
Keywords: Leishmanization, L. major lesion, non-healing, paromomycin, treatment of cutaneous leishmaniasis
Introduction
The inoculation of live virulent Leishmania major to produce a lesion is known as leishmanization (LZ).[1] The purpose of LZ is to prevent natural infection which usually results in multiple lesions on exposed parts of the body. It was known for centuries that following recovery from a Leishmania lesion, individuals would become immune to future infection.[1,2] Originally, exudates from an active cutaneous leishmaniasis (CL) lesion were used as a source of parasites for inoculation. During the last fifty years, L. major promastigotes grown in cell-free culture media (Novy-McNeal-Nicolle; NNN) have been used for LZ in Israel[2] and Iran[3] as well as in a high-risk population in Uzbekistan.[2] Massive LZ was performed on more than two million people in Iran during the Iran-Iraq war in the 1980s.[3] Historically, LZ has shown to be the most effective preventive measure against L. major infection.[2] Lesion induced by LZ is usually a self healing lesion, but in rare cases the lesion does not heal in expected time and becomes chronic. Chronic CL lesion is usually extremely difficult to treat.[4,5]
Case Report
A 41-year-old male patient presented to us with a CL lesion that had lasted for 20 years. The patient had been inoculated about 20 years earlier in his left deltoid along with other soldiers with 0.5 × 106 L. major promastigotes harvested directly from NNN media.[3] A lesion had developed as expected in a few months after inoculation, but persisted for 20 years [Figure 1]. Although, histopathological findings were compatible, with CL, the lesion was not originally diagnosed as CL. Direct smear showed an abundance of amastigotes and inoculation subcutaneously into BALB/c mice induced leishmaniasis. Using PCR technique, the isolated Leishmania identified as L. major, the same strain that had been used for LZ.
Figure 1.
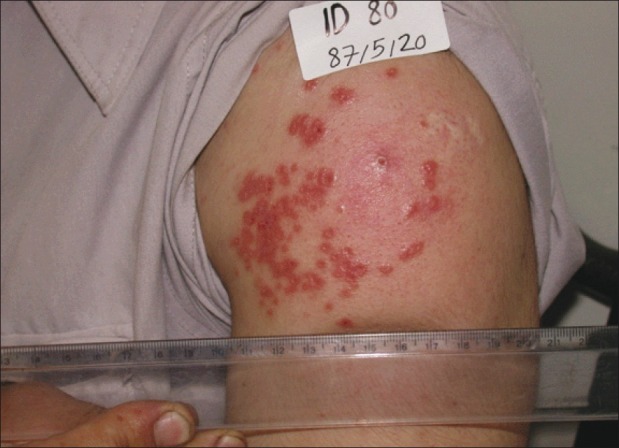
Cutaneous leishmaniasis lesion of twenty-years duration induced by leishmanization (before treatment)
The patient was treated with systemic meglumine antimoniate (Glucantime™), 20 mg/kg/day Sb+5 for three weeks and allopurinol 10 mg/kg for four weeks. The lesion did not show any sign of healing. Two months after completion of the treatment, the same treatment was repeated in combination with a topical liposomal preparation containing 10% paromomycin sulfate (TLPM)[6] twice a day for four weeks. During this treatment, the lesion showed a marked improvement [Figure 2] and was eventually completely healed, as shown in Figure 3. Regular follow up for more than 40 months after the treatment showed no sign of relapse.
Figure 2.
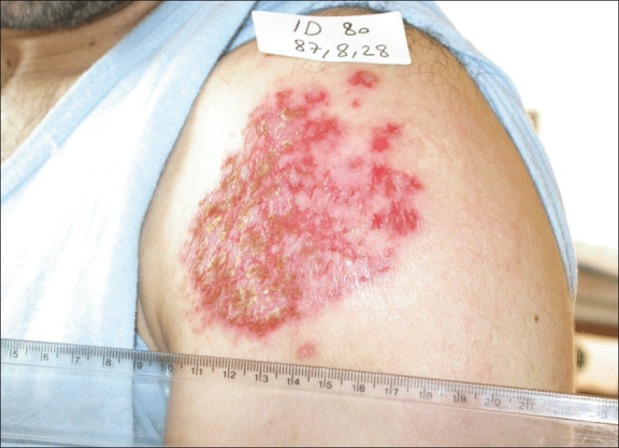
CL during treatment with systemic meglumine antimoniate 20 mg/ kg/day Sb+5 for three weeks and allopurinol 10 mg/kg and topical liposomes containing 10% paromomycin sulfate (TLPM) twice a day
Figure 3.
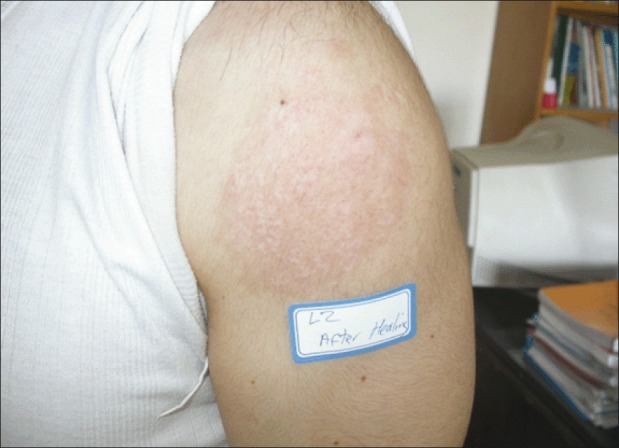
The lesion site 10 months after completion of the treatment
Discussion
CL lesions induced either by natural infection or LZ are self-healing lesions which rarely develop into the non-healing form refractory to all types of remedies and extremely difficult to treat. Lesions induced by mass LZ in Iran rarely developed into a non-healing form.[3] Here a case of non-healing CL which persisted for 20 years after LZ is presented. The lesion was not originally diagnosed as CL. Later, isolated Leishmania was identified as L. major using PCR. Also inoculation into BALB/c mice identified the parasite to be L. major, the strain which was used for LZ.
Treatment of the patient with systemic meglumine antimoniate for three weeks and allopurinol for four weeks was not successful. The patient did not receive any treatment for two months, and then the same treatment was repeated and the patient also received TLPM, twice a day for 28 days. The lesion showed signs of healing during the treatment period and was completely cured. Regular follow up for more than 3 years after the treatment showed no sign of relapse.
Paromomycin (PM) was reported to show anti-Leishmania activities and promising results against both CL and visceral leishmaniasis (VL) in clinical trials.[4,5] PM formulated in conventional ointment and cream bases were tested in clinical trials against CL and showed to be effective against CL caused by L. major.[4,5,7] The formidable barrier nature of stratum corneum (SC) of the skin does not allow the penetration of drugs with high hydrophilicity and molecular weights, like PM.[8] However, the topically applied drugs for the treatment of CL are able to target the Leishmania parasites within the phagolysosome of the infected macrophages in deep dermal layer of skin.[9]
Previously, it was shown that TLPM alone cured L. major infection in BALB/c mice.[6] It is speculated that after topical application of TLPM, some of the flexible vesicles, especially those with sizes around 100 nm (the average size of around 98% of TLPM is around 100 nm), pass through SC of even intact skin and reach the epidermis and deep dermis. In the dermis, the infected macrophages phagocyte PM-containing liposomes, and then PM is released by acidic lysosomal enzymes in the phagolysosome, where Leishmania parasites live and multiply.[9,10]
The present case report also shows that TLPM is effective in the treatment of chronic CL. It seems that the effectiveness of TLPM is due to the delivery of a high concentration of PM into the phagolysosome of Leishmania infected-macrophages in the dermis which prevents Leishmania multiplication.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Khamesipour A, Dowlati Y, Asilian A, Hashemi-Fesharki R, Javadi A, Noazin S, et al. Leishmanization: Use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine. 2005;28:3642–8. doi: 10.1016/j.vaccine.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F. Leishmaniasis vaccine candidates for development: Global overview. Indian J Med Res. 2006;123:423–8. [PubMed] [Google Scholar]
- 3.Nadim A, Javadian E, Tahvildar-Bidruni G, Ghorbani M. Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bull Soc Pathol Exot Filiales. 1983;76:377–83. [PubMed] [Google Scholar]
- 4.Modabber F, Buffet PA, Torreele E. Review open access, consultative meeting to develop a strategy for treatment of cutaneous leishmaniasis. Institute Pasteur, Paris. 13-15 June, 2006 Kinetoplastid Biology and Disease. 2007;6:1–24. doi: 10.1186/1475-9292-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatami AR, Firooz AR, Gorouhi F, Dowlati Y. Treatment of acute old world cutaneous leishmaniasis: A systematic review of the randomized controlled trials. J Am Acad Dermatol. 2007;57:335.e1–29. doi: 10.1016/j.jaad.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Jaafari MR, Bavarsad N, Fazly Bazzaz BS, Samiei A, Soroush D, Ghorbani S, et al. The Effect of topical liposomes containing Paromomycin Sulfate (PM) in the course of Leishmania major infection in susceptible BALB/c mice. Antimicrob Agents Chemother. 2009;53:2259–65. doi: 10.1128/AAC.01319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shazad B, Abbaszadeh B, Khamesipour A. Comparison of topical paromomycin sulfate (twice/day) with intralesional meglumine antimoniate for the treatment of cutaneous leishmaniasis caused by L. major. Eur J Dermatol. 2005;15:85–7. [PubMed] [Google Scholar]
- 8.Ferreira LS, Ramaldes GA, Nunan EA, Ferreira LA. In vitro skin permeation and retention of paromomycin from liposomes for topical treatment of the cutaneous leishmaniasis. Drug Dev Ind Pharm. 2004;30:289–96. doi: 10.1081/ddc-120030423. [DOI] [PubMed] [Google Scholar]
- 9.Mauel J. Macrophage parasite interactions in Leishmania infections. J Leukoc Biol. 1990;47:187–93. doi: 10.1002/jlb.47.2.187. [DOI] [PubMed] [Google Scholar]
- 10.Ashan F, Rivas IP, Khan MA, Suarez AI. Targeting to macrophages: Role of physicochemical properties of particulate carriers- liposomes and microspheres- on the phagocytosis by macrophages. J Control Release. 2002;79:29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]


