Abstract
Stability is taken as the most important parameter before opting for any transplantation technique to treat vitiligo. But, simultaneous donor site repigmentation and depigmentation of grafts at the recipient site has been noted. Similarly donor site depigmentation with complete repigmentation of the recipient area with pigment growing out from each graft has been observed. Successful repigmentation after regrafting in previous punch failure cases has also been reported. Koebner's phenomenon from history (Kp-h) and test grafting were the only available indicators to assess stability. It is quite ironic to note that even after four decades of experience in vitiligo surgery, there seems to be little consensus among workers regarding the optimal required period of stability. Moreover, the exact concept of stability in vitiligo is itself still not transparent and defined beyond doubt Overdependence on KpH or TG may be sometimes misleading in vitiligo. These two reveal the apparent clinical stability only and that may not be the true reflection of stability status of the disease at the molecular level. Antimelanocyte cytotoxic reactivity was observed among CD8+ TCC isolated from perilesional biopsies of patients with vitiligo. An attempt should be made to clearly fathom and define stability, not merely only on clinical ground but along with electron microscopy and histoenzymological analysis of the perilesional and nonlesional skin of vitiligo patients. Probably some growth factors which are responsible for both mitogenic and melanogenic stimulation of melanocytes should also be taken into account. Some serological test(s) could guide us to measure these growth factors.
Keywords: Vitiligo, vitiligo stability, vitiligo grafting
Introduction
Vitiligo persists to evade the researchers and patients in spite of the considerable advancement in basic understanding of the disease process and the treatment protocol over the years. Conservative medical therapy continues to be besieged with capricious and derisory outcome. When the disease becomes refractory to conventional therapy, transplantation techniques are the only options left to replenish the lost melanocytes.
Various surgical modalities and transplantation techniques have evolved during last few decades.[1–10]
But, till date none of the medical or surgical therapeutic choices could assure guaranteed success in all the cases. This is primarily because of the obscure etiopathogenesis and elusive activity profile of the disease itself. Not only with medical therapy but also with any of the surgical modus operandi deployed to achieve accomplishment, proper selection of cases is of paramount importance. The specific criteria for selection have been well defined.[6]
Any endeavor of defining norms or principles for selection is based on one single criterion, i.e. stability of the disease. It is taken as the most important parameter before opting for any transplantation technique to treat vitiligo.[9]
Stability is the decisive factor, the cornerstone of vitiligo therapy.
Period of stability
Even after recognizing the significance of stability and after three decades of experience in vitiligo surgery, it is quite surprising to note the little consensus regarding the optimal required period of stability. The complete lack of unanimity can be glaring in some instances [Table 1]. In one study, the minimal period of stability as a prerequisite for grafting was mentioned to be as little a period as 4 months.[11] While on the other side of the spectrum in another study, it was taken as 3 years.[7]
Table 1.
Minimum period of stability in different studies
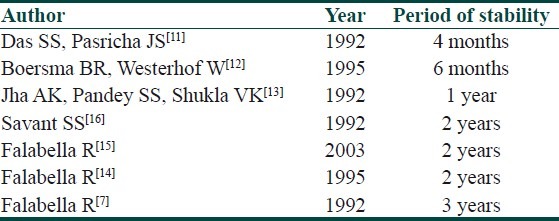
Other variable figures like 6 months, 1 year and 2 years can easily be obtained from some other studies as well.[12–16]
Even same author has considered different period of stability in different articles.[7,14] Unfortunately, none of these observations were based on any concrete logic/authentic proof. They were merely conceived on subjective basis and documented arbitrarily. A few years back, some unanswered questions were raised about this concept.[17] Even the 2 year time frame used to confirm whether lesions are progressive or stable has been a matter of divergence of opinion.[17] The IADVL taskforce recommendation to embark on vitiligo surgery after 1 year of inactivity is based on a similar supposition.[18] While discussing the severity of vitiligo, a recent article has also mentioned this ambiguity and cumbersome compromise.[19] Due to a dearth of a better alternative, this capricious criteria of minimal period of stability still holds good before opting for surgical procedure.
Area based variable status of stability
The first and foremost goal for vitiligo surgery is to induce repigmentation in depigmented part of skin. But due to some inexplicable reason, simultaneous depigmentation and repigmentation have been noticed in different areas of the same patient.[15]
Variable results were obtained over the donor site and recipient site of the same patient.[15,17,20]
Depigmentation of grafts was documented in herpes labialis-induced lip leucoderma.[21–27]
Concurrent donor site repigmentation and depigmentation of grafts at the recipient site or donor site depigmentation with complete repigmentation of the recipient area with pigment growing out from each graft have also been noticed [Figures 1 and 2].
Figure 1.
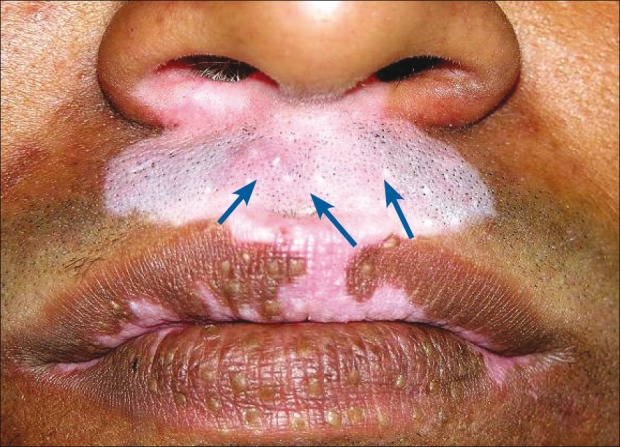
Depigmentation of grafts in the recipient area of stable vitiligo (shown with arrow)
Figure 2.
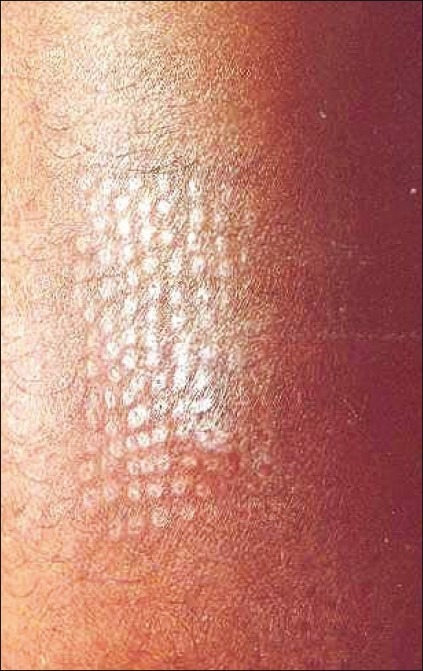
Repigmentation of the donor site in the same patient
It was suggested to observe the donor site as well, to rule out koebnerization as a side effect of surgical manipulation while commenting on the stability status of the patient by minigraft test.[20]
Simultaneous perigraft spread of pigment in one area and spread of vitiligo in another area can be found in the same patient [Figures 3 and 4].
Figure 3.
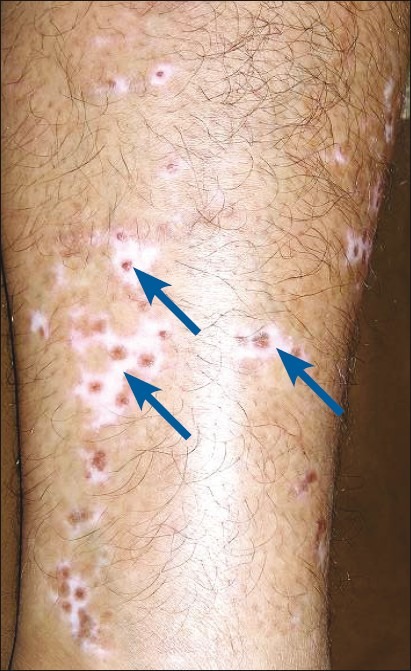
Perigraft spread of pigment (shown with arrow)
Figure 4.
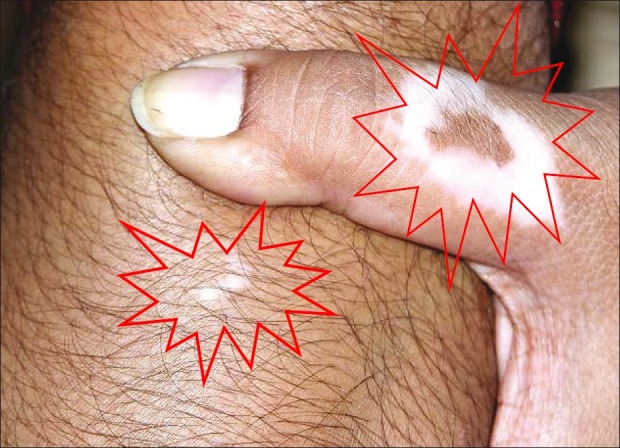
Simultaneous spread of disease over thumb and knee (shown with starburst sign)
Parallel concurrence of surgical repigmentation from the grafts and increase in size of an existing lesion in the same anatomical location was also observed [Figures 5–7].
Figure 5.
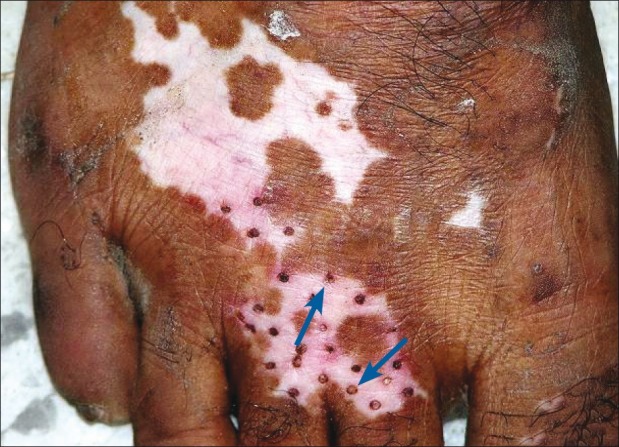
Minigrafting on dorsum of foot, first session (shown with blue arrow)
Figure 7.
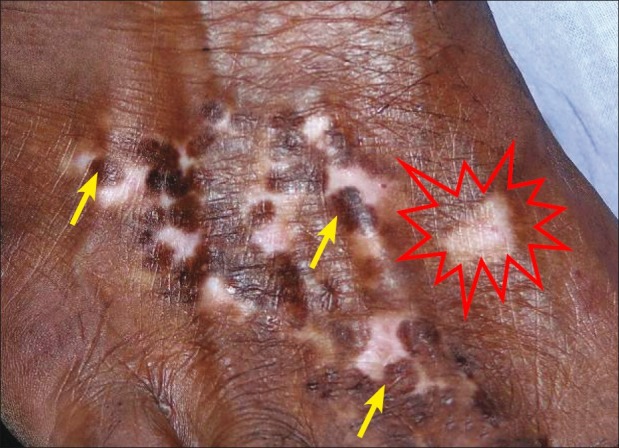
Appreciable repigmentation (yellow arrow). Reactivation of disease on the same anatomical site.(shown with red starburst)
Figure 6.
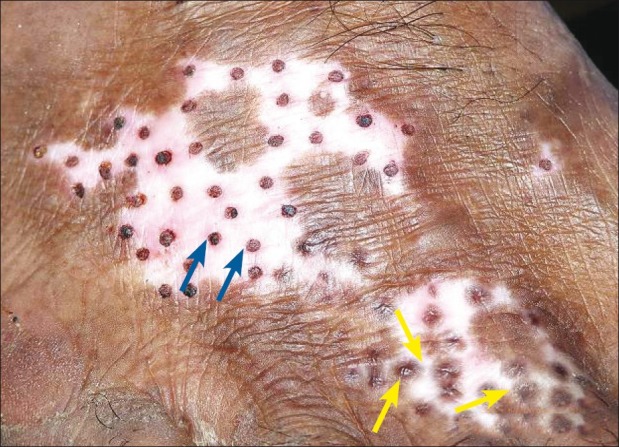
Second session, perigraft repigmentation from grafts of previous session (shown with yellow arrow)
This area based variable status of stability is obviously not related to the conventional refractory behavior of vitiligo in some typical so-called resistant anatomical sites such as palm, sole, lips, nipples, areola, glans penis, and bony prominences. Neither is this related to the type of vitiligo such as unilateral (segmental/focal) or bilateral (symmetric, vulgaris, or generalized).
Assessment of stability
Before any surgical intervention or even just to have an idea about which way the disease is progressing, evaluation of stability status of vitiligo is an essential prerequisite.
Clinically, this can be judged by three simple indicators:
History
Koebner phenomenon
Test grafting
These were elaborated and to assess the activity status of the disease some benchmark guidelines have been proposed by Falabella.[6,14] Those are:
lack of progression of old lesions within the past 2 years (in unilateral vitiligo may be shorter, and in bilateral vitiligo, stability establishes after several years);
no new lesions developing within the same period;
absence of a recent Koebner phenomenon (either from history or experimentally produced);
repigmentation of depigmented areas by medical treatment or sometimes spontaneous repigmentation;
a positive mini-grafting test; and
lack of Koebnerization at donor site.
On the backdrop of a pervasive incongruity about the minimal period of stability, an attempt was made for the first time by Falabella in 1995 to fathom stability before surgery by introducing the minigrafting test.[14]
The objective of this test vowed to serve several purposes: establishing the stability of the depigmenting process;
determine a means by which patients could be selected;
identifying patients who may respond to pigment cell transplantation;
to anticipate the response to surgical repair.
In the original suggested procedure, a few grafts (1.0-1.2 mm) were placed in the center of the depigmented lesion to be scrutinized. Dressing was done by Micropore® adhesive tape and kept for a couple of weeks. After removal of the tape, the area was exposed to sunlight for 15 min daily for a period of 3 months. No treatment was permitted during this test period.
All test sites are visualized under Wood's light. The test was considered positive if unequivocal repigmentation takes place beyond 1 mm from the border of the implanted grafts. On the other hand, if less than 1 mm or no repigmentation was observed, the test was considered as negative.[14]
In some of the biggest series, this evaluation has been termed as “test grafting” (TG) and found this to be a more reliable exercise than the unjustified dependence on period of stability alone.[25,26,28]
Over the years, this “test” has been vindicated and acknowledged as a powerful tool for detecting stable Vitiligo, which anticipates the repigmentation success in vitiligo when surgery becomes a therapeutic option.
Behl expressed some reservation while commenting on Falabella's work on mini grafting and claimed better results with thin Thiersch grafting. In a rejoinder Falabella reiterated his faith in miniature punch grafting and countered with his sets of reasons and logics in favor of punch grafting.[4,29]
But, simultaneously some valid logic of skepticism was flowing parallel to this recognition.
In the original article, no mention was there about the donor site. One of the opinions was to take the outcome of the donor site as well into consideration, concurrently along with observing the behavior of the minigrafts in the recipient site. That will give a more comprehensive idea about the status of stability.[20]
But, doubts were registered over overdependence on test grafting per se as that attaches a quite a few fallacies.[17,28,30]
TG positivity does not necessarily guarantee a favorable outcome in all cases even after perfect graft take up.
Encouraging results were obtained even after grafting of a few TG negative cases.
Simultaneous donor site repigmentation and depigmentation of grafts at the recipient site or donor site depigmentation with complete repigmentation of the recipient area are not hard to come by.
Juhlin echoed this apprehension and observed, “…neither the history nor test graft is a complete help to obtain good effect. It seems we have to wait until the method of identification and isolation of skin-homing melanocyte-specific cytotoxic T lymphocytes is available.”[31]
None other than Falabella himself expressed his doubts about the comprehensiveness of minigraft test. He observed that even when the minigrafting test is positive, “depigmenting activity” may still be present and prevent satisfactory repigmentation.[32]
More recently, Njoo et al explored the association between the experimentally induced Koebner phenomenon (KP e) and the Kobner phenomenon by history (KP-h), disease activity, and therapeutic responsiveness in vitiligo vulgaris.[33]
In the article, they proposed to measure disease activity on a 6-point scale from –1 to +4 (vitiligo disease activity [VIDA] score) and therapy-induced repigmentation grade. But the test was lacking in application potential. The authors have observed and concluded the following:
The VIDA score did not always predict a positive KP-e
The KP-e may function well as a clinical factor to assess present disease activity and may also predict the responsiveness to fluticasone propionate plus UV-A therapy but not to UV-B (311 nm) therapy.
It this context, it can be concluded that overdependence on Kp or TG may be sometimes misleading in this enigmatic disease. Because what these two reveal is the apparent clinical stability only and that may not be the true reflection of stability status of the disease at the cellular level.[28]
How stable is stability?
Another important indicator is the duration of stability status of vitiligo.
It is often hard to predict how long the disease will remain stable. Similarly difficult is to envisage when it will start to become unstable.
Repigmentation has been successfully induced in previous graft failure cases under NB-UVB (311 nm) phototherapy.[27]
It was also proposed that NB-UVB might have played the pivotal role of stabilizing the disease by promoting apoptosis of the perilesional and circulating melanocyte-specific cytotoxic CD 8 cells.[34,35]
The observation of spontaneous repigmentation of non-grafted vitiligo patches [Figures 8–10] points towards a possible release of fresh cytokines from the donor skin while stimulating the vitiliginous patches and hair follicles of the grafted sites may have played some role at the distant sites by local absorption.[36–38] Another theory was the immunogenic mechanism which was originally responsible for the development of vitiligo may have lost its antigenicity due to the autologous grafts.[35]
Figure 8.
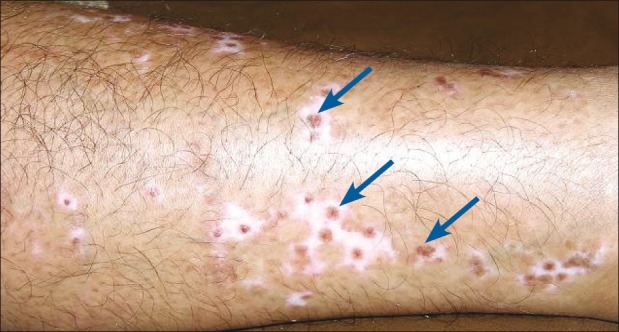
Punch grafting on medial aspect of left leg
Figure 10.
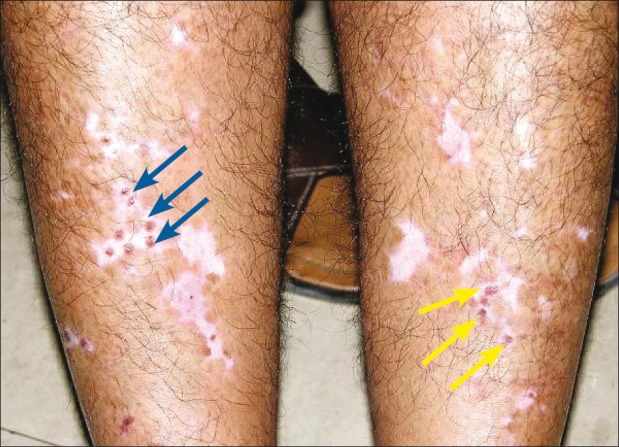
Punch grafting on right leg (shown with blue arrow). Spontaneous repigmentation over left leg (shown with yellow arrow)
Figure 9.
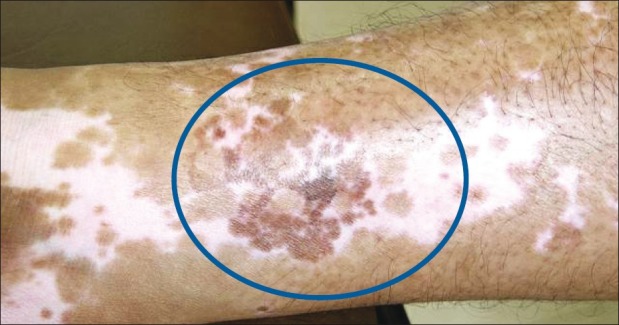
Spontaneous repigmentation of medial aspect of right leg
Successful split thickness skin graft (STSG) in PG failure cases further complicates the picture.[39]
Period of stability of repigmentation has been proposed as 1 year. That means, to qualify as stable, the repigmentated status must be maintained for a period of not less than 1 year.[40]
Understanding stability from within
The exact pathomechanism of stability is as vague as the pathogenesis of the disease itself.
The incompletely understood mechanism may involve many assortments of theories and hypothesis or may be a combination various factors. The only definite and certain fact regarding the pathogenesis of vitiligo is the absence of melanocytes in the lesion. So, it can be deducted that destruction of melanocytes is the keystone for unfolding of the disease process. Classically, there have been three hypotheses to elucidate vitiligo: the neural hypothesis, the self-destruct hypothesis, and the immune hypothesis of which the last one is gaining grounds. Aberrations of both humoral and cell-mediated immunity have been noticed. Most of the patients with generalized vitiligo were found to have circulating antibodies to cell surface antigens on normal human melanocytes; these antibodies were cytotoxic to normal melanocytes and to melanoma cells in tissue culture. Perilesional and circulating T-cells strongly suggest that melanocyte-specific cytotoxic T-cells are involved. And now it is known that UVB therapy promotes this T-cell apoptosis.[34,35] Repigmentation has been successfully induced in previous graft failure cases under NB-UVB (311 nm) phototherapy.[41]
In 1986, Grimes first reported a reduction in the number of lymphocytes and helper T cells and an increase in number of natural killer cells in vitiligo patients. She also noticed decreased helper/suppressor ratios, particularly among patients with vitiligo of less than a year's duration and among those whose serum assayed positive for autoantibodies.[42]
But D’Amelio in 1990 came up with just the reverse observation. In a study of 22 patients with vitiligo stable for a year or more demonstrated high helper T-cell levels and high helper/suppressor ratios (also in 23 first-degree relatives) compared to controls. He also reported loss of circadian rhythm of helper T cells in patients with active diseases and no loss of circadian rhythm of suppressor T cell.[43]
Cui et al. observed cytotoxic antibodies more common in patients with active than with inactive disease.[44]
An attempt was made by Hann in 1994 to compare active and inactive lesion of vitiligo by immunohistochemical methods. He documented that the percentage of B cells was higher in those with disease of recent onset (under a year) than in controls (those with later onset disease). He also noticed an increase in number of CD4 lymphocytes (especially in active lesions), but not CD8 lymphocytes, in marginal skin of active lesions. As per his observation, epidermal ICAM-1 was expressed in active but not in stable epidermal lesions.[45] In the subsequent year, Hann detected decreased helper T-cell levels with a reversed helper/suppressor ratio in 1995.[46]
In the margins of lesions, Badri found increases in CD3+, CD4+, and CD8+ T cells; many were activated and expressed the cutaneous lymphocyte-associated antigen (HECA-452+) typical of infiltrating T cells; these changes were most marked within 0.6 mm of the lesional edge. He couldn’t find any association between T-cell numbers and disease activity.[47] After that, some very interesting observations by Wankowicz-Kalinska have opened a new horizon in the basic understanding of the pathogenesis of the disease.[48]
The detection of melanocyte-reactive cytotoxic T cells in the peripheral blood of vitiligo patients and the observed correlation between perilesional T-cell infiltration and melanocyte loss in situ suggest the important role of cellular autoimmunity in the pathogenesis of this disease. T cells have been isolated from both perilesional and nonlesional skin biopsies of vitiligo patients, then cloned and their profile of cytokine production analyzed. Perilesional T-cell clones (TCC) derived from patients with vitiligo revealed a predominant Type-1-like cytokine secretion profile, but the degree of Type-1 polarization in uninvolved skin-derived TCC correlated with the process of microscopically observed melanocyte destruction in situ. Furthermore, CD8+ TCC derived from two patients also were analyzed for reactivity against autologous melanocytes. The antimelanocyte cytotoxic reactivity was observed among CD8+ TCC isolated from perilesional biopsies of patients with vitiligo.
The theory of melanocytorrhagy and apoptosis as one of the primary defects underlying melanocyte loss was discussed in another article.[45] This theory proposes that non-segmental vitiligo is a primary melanocytorrhagic disorder with altered melanocyte responses to friction and possibly other types of stress, inducing their detachment and subsequent transepidermal loss. Melanocytes detachment induces apoptosis whereas adherence to basement membrane suppresses apoptosis.[49]
Very recently, a group of worker while determining the markers of the stability of vitiligo CD 8, CD 45 RO, and FOX P3) has observed that percentage of CD8 and CD 45 RO in lesional skin biopsy may provide objective indication of lesion/area based stability. They have also observed that a higher percentage of cytotoxic CD8 cells is associated with failure of melanocyte transplantation.[50]
Conclusions
So, where do we stand?
It is obvious that before any attempt to predict the outcome of any vitiligo surgery, along with the clinical stability, assessment of “cellular” stability is of paramount importance and the cellular parameters should also be taken into consideration. When these ultarstructural investigation facilities are now available, an attempt should be made to clearly fathom stability, not merely only on clinical ground but along with electron microscopy and histoenzymological analysis of the perilesional and nonlesional skin of vitiligo patients. Probably some growth factors which are responsible for both mitogenic and melanogenic stimulation of melanocytes should also be taken into account. Some serological test(s) could guide us to measure these growth factors. Before embarking upon any surgical alternative no preconceived, biased and arbitrary formulae should guide us to assess stability of vitiligo.
The understanding of the area-based variable status and limited period of inertia or activity might necessitate test grafting at various sites at different time interval. Till those unexplored areas are charted and the cellular parameters come into existence, we should not refrain the surgical option rather we should continue surgical intervention based on logical application of our clinical knowledge.
Acknowledgement
Some figures and tables used in this article have also been used in the following chapter and articles by me and my co-authors. With the permission of the concerned editors and publisher I would like to properly acknowledge the original source:
Lahiri K, Malakar S. The concept of stability of vitiligo. In: Gupta S, Olsson MJ, Kanwar AJ, Ortonne JP, editors. Surgical Management of Vitiligo. 1st ed. Oxford: Blackwell Publishing; 2007. p.49-55.
Malakar S. Lahiri K. Minigrafting for vitiligo. In: Gupta S, Olsson MJ, Kanwar AJ, Ortonne JP, editors. Surgical Management of Vitiligo. 1st ed. Oxford: Blackwell Publishing; 2007. p. 87-95.
Lahiri K, Malakar S, Sarma N, Banerjee U. Repigmentation of vitiligo with punch grafting and narrow-band UV-B (311 nm) a prospective study. Int J Dermatol 2005;45:649-55.
Malakar S, Lahiri K, Spontaneous repigmentation in vitiligo: Why it is important. Int J Dermatol 2006;45:477-8.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Orentriech N, Selmanwitz VJ. Autograft repigmentation of leucoderma. Arch Dermatol. 1972;105:734–6. [PubMed] [Google Scholar]
- 2.Falabella R. Repigmentation of segmental vitiligo by autologous mimigrafting. J Am Acad Dermatol. 1983;9:514–21. doi: 10.1016/s0190-9622(83)70162-3. [DOI] [PubMed] [Google Scholar]
- 3.Halder RM, Breadon JY, Johnson BA. Micropigmentation for the treatment of vitiligo. J Dermatol Surg Oncol. 1989;15:1092–8. doi: 10.1111/j.1524-4725.1989.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 4.Behl PN. Treatment of vitiligo with homologous Thiersch's Skin grafts. Curr Med Pract. 1964;8:218–21. [Google Scholar]
- 5.Kiistala U, Mustakallio KK. In vivo separation of epidermis by production of suction blisters. Lancet. 1964;1:14444. doi: 10.1016/s0140-6736(64)92011-2. [DOI] [PubMed] [Google Scholar]
- 6.Falabella R. Grafting and transplantation of melanocytes for repigmenting vitiligo and other types of leucodermas. Int J Dermatol. 1989;28:363–9. doi: 10.1111/j.1365-4362.1989.tb02479.x. [DOI] [PubMed] [Google Scholar]
- 7.Falabella R, Escobar C, Borrero I. Treatment of refractory and stable vitiligo by transplantation of in vitro cultured epidermal autografts bearing melanocytes. J Am Acad Dermatol. 1992;26:230–6. doi: 10.1016/0190-9622(92)70032-b. [DOI] [PubMed] [Google Scholar]
- 8.Malakar S, Dhar S, Malakar RS. Single-hair transplant: A novel technique. Dermatology. 1999;199:370. doi: 10.1159/000018295. [DOI] [PubMed] [Google Scholar]
- 9.Olsson MJ, Juhlin L. Long-term follow-up of leucoderma patients treated with transplants of autologous cultured melanocytes, ultrathin epidermal sheets and basal cell layer suspension. Br J Dermatol. 2002;147:893–904. doi: 10.1046/j.1365-2133.2002.04837.x. [DOI] [PubMed] [Google Scholar]
- 10.Lahiri K. Evolution and evaluation of autologous mini punch grafting in vitiligo. Indian J Dermatol. 2009;54:159–67. doi: 10.4103/0019-5154.53195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das SS, Pasricha JS. Punch grafting as a treatment for residual lesions in vitiligo. Ind J Dermatol Venereol Leprol. 1992;58:315–9. [Google Scholar]
- 12.Boersma BR, Westerhof W. Repigmentation in vitiligo vulguris by autologous minigrafting: Results in 19 patients. J Am Acad Dermatol. 1995;33:990–5. doi: 10.1016/0190-9622(95)90292-9. [DOI] [PubMed] [Google Scholar]
- 13.Jha AK, Pandey SS, Shukla VK. Punch grafting in vitiligo. Ind J Dermatol Venereol Leprol. 1992;58:328–30. [Google Scholar]
- 14.Falabella R, Arrunategui A, Barona MI, Alzate A. The minigrafting test for vitiligo: Detection of stable lesions for melanocyte transplantation. J Am Acad Dermatol. 1995;32:228–32. doi: 10.1016/0190-9622(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 15.Falabella R. Surgical treatment of Vitiligo: Why, when and how. (Editorial) J Eur Acad Dermatol Venereol. 2003;17:518–20. doi: 10.1046/j.1468-3083.2003.00718.x. [DOI] [PubMed] [Google Scholar]
- 16.Savant SS. Autologous miniatures punch grafting in vitiligo. Ind J Dermatol Venereol Leprol. 1992;58:310–4. [Google Scholar]
- 17.Malakar S, Lahiri K. How unstable is the concept of stability in surgical repigmentation of vitiligo? Dermatology. 2000;201:182–3. doi: 10.1159/000018449. [DOI] [PubMed] [Google Scholar]
- 18.Parsad D, Gupta S. Standard guidelines of care for vitiligo surgery. Indian J Dermatol Venereol Leprol. 2008;74(Suppl):S37–45. [PubMed] [Google Scholar]
- 19.Ramam M, Krishna SG. Measuring the severity of vitiligo. Indian J Dermatol Venereol Leprol. 2012;78:5–7. doi: 10.4103/0378-6323.90939. [DOI] [PubMed] [Google Scholar]
- 20.Westerhof W, Boersma B. The minigrafting test for vitiligo: Detection of stable lesions for melanocyte transplantation. J Am Acad Dermatol. 1995;33:1061. doi: 10.1016/0190-9622(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 21.Malakar S, Dhar S. Rejection of punch grafts in three cases of herpes labialis induced lip leucoderma, caution and precaution. Dermatology. 1997;195:414. doi: 10.1159/000246007. [DOI] [PubMed] [Google Scholar]
- 22.Malakar S, Dhar S. Acyclovir can abort rejection of punch grafts in herpes-simplex induced lip leucoderma. Dermatology. 1999;199:75. doi: 10.1159/000018189. [DOI] [PubMed] [Google Scholar]
- 23.Malakar S, Lahri K. Successful repigmentation of six cases of Herpes labialis induced lip leucoderma by micropigmentation. Dermatology. 2001;203:194. [PubMed] [Google Scholar]
- 24.Lahiri K, Malakar S. Herpes simplex induced lip leucoderma: Revisited. Dermatology. 2004;208:182. doi: 10.1159/000077336. [DOI] [PubMed] [Google Scholar]
- 25.Malakar S, Dhar S. Treatment of stable and recalcitrant vitiligo by autologous mimiature punch grafting: A prospective study of 1,000 patients. Dermatology. 1999;198:133–9. doi: 10.1159/000018089. [DOI] [PubMed] [Google Scholar]
- 26.Malakar S, Lahiri K. Punch grafting for lip leucoderma. Dermatology. 2004;208:125–8. doi: 10.1159/000076485. [DOI] [PubMed] [Google Scholar]
- 27.Lahiri K, Malakar S, Sarma N, Banerjee U. Repigmentation of vitiligo with punch grafting and NB UVB (311 nm) - a prospective study. Int J Dermatol. 2006;45:649–55. doi: 10.1111/j.1365-4632.2005.02697.x. [DOI] [PubMed] [Google Scholar]
- 28.Lahiri K, Malakar S, Banerjee U, Sarma N. Clinico-Cellular stability of vitiligo in surgical repigmentation: An unexplored frontier. Dermatology. 2004;209:170–1. doi: 10.1159/000079612. [DOI] [PubMed] [Google Scholar]
- 29.Falabella R. Reply. J Am Acad Dermatol. 1995;33:1061. doi: 10.1016/0190-9622(95)91282-7. [DOI] [PubMed] [Google Scholar]
- 30.Lahiri K. Stability in vitiligo? What's that? J Cutan Aesthet Surg. 2009;2:38–40. doi: 10.4103/0974-2077.53100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juhlin L. How unstable is the concept of stability in surgical repigmentation of vitiligo. Dermatology. 2000;201:183. doi: 10.1159/000018450. [DOI] [PubMed] [Google Scholar]
- 32.Falabella R, Escobar C, Borrero I. Treatment of refractory and stable vitiligo by transplantation of in vitro cultured epidermal autografts bearing melanocytes. J Am Acad Dermatol. 1992;26:230–6. doi: 10.1016/0190-9622(92)70032-b. [DOI] [PubMed] [Google Scholar]
- 33.Njoo MD, Das PK, Bos JD, Westerhof W. Association of the Koebner phenomen with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135:414. doi: 10.1001/archderm.135.4.407. [DOI] [PubMed] [Google Scholar]
- 34.Ortonne JP. Vitiligo pathogenesis: What's new? J Eur Acad Dermatol Venereol Barcelona, Spain. 2003;17(Suppl 3):30. [Google Scholar]
- 35.van den Wijngaard RM, Aten J, Scheepmaker A. Expression and modulation of apoptosis regulatory molecules in human melanocytes: Significance in vitiligo. Br J Dermatol. 2000;143:573–81. doi: 10.1111/j.1365-2133.2000.03712.x. [DOI] [PubMed] [Google Scholar]
- 36.Malakar S. Spontaneous repigmentation of vitiligo patches other than the grafted site. Indian J Dermatol. 1997;472:68–70. [Google Scholar]
- 37.Malakar S, Dhar S. Spontaneous repigmentation of vitiligo patches distant from the autologous skin graft sites: A remote reverse Koebner's phenomenon? Dermatology. 1998;197:274. doi: 10.1159/000018013. [DOI] [PubMed] [Google Scholar]
- 38.Malakar S, Lahiri K. Spontaneous repigmentation in vitiligo: Why it is important. Int J Dermatol. 2006;45:478–9. doi: 10.1111/j.1365-4632.2006.02657.x. [DOI] [PubMed] [Google Scholar]
- 39.Malakar S. Successful split thickness skin graft in stable vililigo not responding to autologous miniature punch grafts. Indian J Dermatol. 1997;42:215–8. [Google Scholar]
- 40.Parsad D, Pandhi R, Dogra S, Kumar BJ. Clinical study of repigmentation patterns with different treatment modalities and their correlation with speed and stability of repigmentation in 352 vitiliginous patches. J Am Acad Dermatol. 2004;50:63–7. doi: 10.1016/s0190-9622(03)00786-2. [DOI] [PubMed] [Google Scholar]
- 41.Lahiri K, Malakar S. Inducing repigmentation by regrafting and phototherapy (311nm) in punch failure cases of lip vitiligo-a pilot study. Indian J Dermatol Venereol Leprol. 2004;70:156–8. [PubMed] [Google Scholar]
- 42.Grimes PE, Ghoneum M, Stockton T, Payne C, Kelly AP, Alfred L. T cell profiles in vitiligo. J Am Acad Dermatol. 1986;14:196–201. doi: 10.1016/s0190-9622(86)70021-2. [DOI] [PubMed] [Google Scholar]
- 43.D’Amelio R, Frati C, Fattorossi A, Aiuti F. Peripheral T-cell subset imbalance in patients with vitiligo and in their apparently healthy first degree relatives. Ann Allergy. 1990;65:143–5. [PubMed] [Google Scholar]
- 44.Cui J, Arita Y, Bystryn JC. Cytolytic antibodies to melanocytes in vitiligo. J Invest Dermatol. 1993;100:812–5. doi: 10.1111/1523-1747.ep12476636. [DOI] [PubMed] [Google Scholar]
- 45.Ahn SK, Choi EH, Lee SH, Won JH, Hann SK, Park YK. Immunohistochemical studies from vitiligo—comparison between active and inactive lesions. Yonsei Med J. 1994;35:404–10. doi: 10.3349/ymj.1994.35.4.404. [DOI] [PubMed] [Google Scholar]
- 46.Hann SK, Kim JB. Detection of antibodies to human melanoma cell in vitiligo. Yonsei Med J. 1995;36:457–61. doi: 10.3349/ymj.1995.36.5.457. [DOI] [PubMed] [Google Scholar]
- 47.Badri AM, Todd PM, Garioch JJ, Gudgeon JE, Stewart DG, Goudie RB. An immunohistological study of cutaneous lymphocytes in vitiligo. J Pathol. 1993;170:149–55. doi: 10.1002/path.1711700209. [DOI] [PubMed] [Google Scholar]
- 48.Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, et al. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest. 2003;83:683–95. doi: 10.1097/01.lab.0000069521.42488.1b. [DOI] [PubMed] [Google Scholar]
- 49.Kumar R, Parsad D. Melanocytorrhagy and apoptosis in vitiligo: Connecting jigsaw pieces. Indian J Dermatol Venereol Leprol. 2012;78:19–23. doi: 10.4103/0378-6323.90942. [DOI] [PubMed] [Google Scholar]
- 50.Rao A, Gupta S, Dinda AK, Sharma A, Sharma VK, Kumar G, et al. Study of clinical, biochemical and immunological factors determining stability of disease in patients with generalized vitiligo undergoing melanocyte transplantation. Br J Dermatol. 2012 doi: 10.1111/j.1365-2133.2012.10886.x. [In press] [DOI] [PubMed] [Google Scholar]


