Abstract
Aim:
To determine serum lipid, lipoproteins and oxidized low density lipoprotein (oxLDL) levels in Behçet's disease (BD) and to evaluate the relationship of these parameters with the clinical activity of the disease.
Materials and Methods:
Sixty-two patients (25 active, 37 inactive) and —26 healthy controls were included in the study. We measured serum oxLDL levels using the enzyme-linked immunosorbent assay method, and serum total cholesterol (TC), triglyceride (TG) and high density lipoprotein-cholesterol (HDL-C) levels by spectrophotometric method.
Results:
Serum TG (108±70 mg/dL and 79±40 mg/dL, respectively; P<0.05), LDL-C (124±35 mg/dL and 108±26 mg/dL, respectively; P<0.05) and oxLDL (65±19 U/L and 53±10 U/L, respectively; P<0.01) levels were significantly higher in patients than in controls, but HDL-C levels were significantly lower in patients than in controls (39±11 mg/dL and 50±13 mg/dL, respectively; P<0.05). The levels of oxLDL in patients were found to correlate with those of TC and LDL-C. Neither the lipid parameters nor the oxLDL levels in the patients with active disease (n=25) were different than those in the patients who were in inactive stage (n=37). Serum levels of oxLDL in the patients with active and inactive disease were significantly higher than those in controls (66±19 U/L, 65±19 U/L, and 53±10 U/L, respectively; P<0.05).
Conclusions:
We conclude that the increase of TG, LDL-C and oxLDL levels and the decrease of HDL-levels may indicate that there is a tendency to atherothrombotic process in patients with BD. Inflammation and immunologic reactions in BD may be caused by a response to elevated oxLDL. TG, LDL-C and oxLDL are not useful markers for the severity of the disease activity.
Keywords: Behçet's disease, serum lipid level, oxidatively modified low density lipoprotein
Introduction
Behçet's disease (BD) is a chronic disease that was first defined by a Turkish dermatologist named Hulusi Behçet in 1937 as a triad of symptoms, oral and genital ulcerations and recurrent iridocyclitis.[1] BD manifests a chronic inflammation form characterized by acute attacks and remissions. The first thing that attracts attention in histopathology is the vasculitis in which infiltration of plasma cells, monocytes, lymphocytes and neutrophils is observed in the vessel wall and peripheral vessel tissue. It was reported that chemotaxis, phagocytosis, superoxide radical anion products and lysosomal enzyme activity are increased in peripheral blood polymorphonuclear (PMN) cells in BD.[2] In the tissue damage observed in this process, there may be an important role of decrease in enzymatic activity and increase in free radical level as in many diseases.[3–5] In addition to these factors, changes in serum lipid, lipoprotein and lipid peroxidation levels may accompany the process.[6–8] Normally, there is a balance between oxidant and antioxidant systems. In cases where the antioxidant defense system is inefficient, harmful effects of free radicals arise. Lipid peroxidation occurs on cellular membrane and on cholesterols and polyunsaturated fatty acids having lipoprotein structure as well.[9] Among the oxidized lipoproteins, oxidized low density lipoprotein (oxLDL) manifests great importance in terms of atherogenesis. The oxLDL cannot be recognized by the receptors on liver and extrahepatic tissues. Instead, it is taken up by the scavenger receptors located on the macrophages.[10] As a result of uncontrolled accumulation of lipid and binding of oxLDL by scavenger receptors on vessel walls, monoctes and smooth muscle cells, foam cells are formed and atheroma plaques come into existence.[11,12] It has been reported that modified lipoproteins, especially oxLDL, give rise to humoral immune responses and antibody formation in experimental animal and human studies.[13] In the light of these information, in our study, we aimed to evaluate the importance of oxLDL in terms of diagnosis or as a marker of activity of BD by measuring this parameter in 25 active and 37 inactive (totally 62) patients (both in active and inactive phases in 13 BD patients) and 26 healthy controls.
Materials and Methods
Sixty-two BD patients, 37 inactive and 25 active, who presented to Okmeydani Educational and Research Hospital Ophthalmology Outpatient Clinic participated in the study. Informed consent was obtained from all the subjects. The diagnosis of the patients was made according to the International BD diagnosis criteria.[14] Active (n=25) and inactive (n=37) BD patients were determined by clinical parameters. In clinical evaluation, patients having at least three of the five major symptoms at the time of study (oral ulcers, genital ulcers, skin lesions, uveitis, positive pathergy test) were considered to be in the active period of the disease. Patients in remission, lacking these activity symptoms, were evaluated as inactive patients. Biopsy was not done for discrimination of active and inactive phase. Control group was composed of 26 volunteers who were found to be healthy by physical examination and laboratory findings. In BD groups, the individuals having other systemic illnesses, those under topical or systemic drug treatment and those using antioxidants and smokers were not included in the study. Venous blood samples of patient and control group for determination of oxLDL and lipid profiles were obtained after 12 hours fasting into 10 ml tubes which did not contain any anticoagulant or preservatives. In 13 BD patients, blood samples were obtained in both active and inactive phases. The samples were centrifuged at 3000 rpm for 10 minutes and then stored at –80°C till the analyzing period. Serum lipid parameters were measured photometrically on Olympus AU-5200 autoanalyser. The oxLDL measurement was performed using oxidized LDL enzyme-linked immunosorbent assay (ELISA) (Mercodia, Uppsala, Sweden) reagent on ELX50, ELX800 Micro ELISA system (Bio-Tek Instruments Inc., Vermont, United States).
The study was done in accordance with the Helsinki Declaration and informed consent was obtained from the subjects.
Statistical analysis
“SPSS for Windows 10.0” package program was used. Student's t-test was used to compare the means of two groups in dependent and independent patterns. For more than two groups, using analysis of variance (ANOVA), Tukey honestly significant difference (HSD) test that is one of the multiple comparison tests, was performed in significant differences. Correlation test was used to measure the direction and degree of relationship between the variables, and for determining the frequency distribution, Chi-square was used.
Results
The patient group in our study was composed of 62 BD patients (37 inactive and 25 active phase). Table 1 shows the distribution of clinical findings in the total BD group. The control group was composed of 26 healthy volunteers. Mean duration of disease in the inactive group was 6.63 years and in the active group was 6.87 years.
Table 1.
Distribution of clinical findings in patients with BD
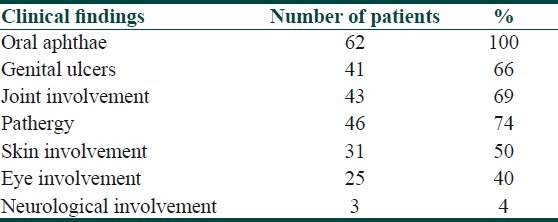
Inactive group of BD consisted of 27 male and 10 female patients; active group consisted of 18 males and 7 females and the control group had 18 males and 8 females. There was no statistically significant difference in terms of gender between the groups.
The mean±SD age of patients in the active group was 32.3±8.3 years and that in the inactive group was 33.6±7.8 years. It was 33.6±8.5 years in the control group. There was no statistically significant difference between the groups in terms of age distribution.
Serum lipid profile and oxLDL levels in total, active, inactive BD and control group are presented in Table 2. When active and inactive groups were compared, there was no statistically significant difference between the groups in terms of oxLDL (P>0.05). However, oxLDL levels of control group were significantly lower than that of active and inactive BD groups (P<0.05) [Tables 3 and 4]. When the oxLDL levels of total BD group (n=62) and control group were compared, oxLDL levels of total BD group were significantly higher than those of the control group (P<0.05) [Table 2]. In total BD group, between oxLDL levels and low density lipoprotein-cholesterol (LDL-C), very low density lipoprotein-cholesterol (VLDL-C), and total cholesterol (TC), statistically significant correlation was detected (r=0.550, r=0.378, r=0.550, respectively) (P<0.05). There was no correlation detected between oxLDL and high density lipoprotein-cholesterol (HDL-C). When the lipid profiles of BD were analyzed, it was found that LDL-C, VLDL-C and TG levels were increased (P<0.05) and HDL-C levels were decreased, which was statistically significant. However, there was no statistically significant difference in terms of TC [Table 2]. Of the 13 BD patients followed in the study, 9 were males and 4 were females and their mean±SD age was 30.5±8.5 years. The comparison of 13 BD patients’ lipid and lipoprotein parameters in active and inactive phases is presented in Table 5. Mean oxLDL level in the inactive phase was 60±15 U/L and that in the active phase was 68±17 U/L and there was no statistically significant difference betwen the groups [Table 6]. However, there was a statistically significant correlation between inactive phase oxLDL and active phase oxLDL levels (r=0.686, P<0.05) [Table 7].
Table 2.
Serum lipid profile and oxLDL levels in total, active, inactive BD patients and control group
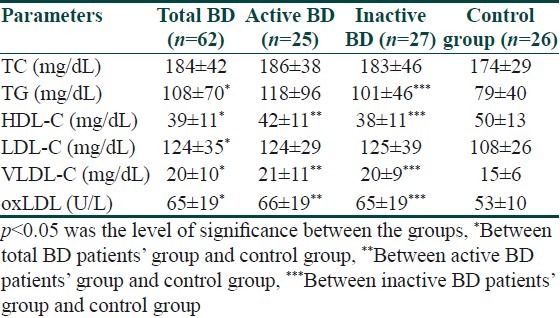
Table 3.
The comparison of oxLDL levels between active, inactive and control groups
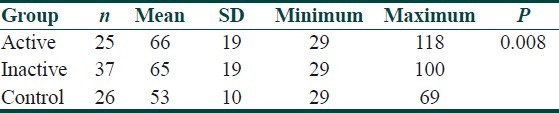
Table 4.
Multiple comparison of oxLDL levels between active, inactive and control groups
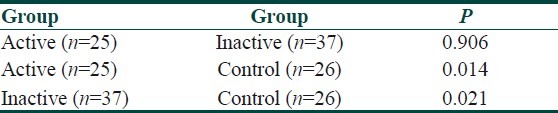
Table 5.
The comparison of lipid and lipoprotein parameters in active and inactive phases of 13 patients
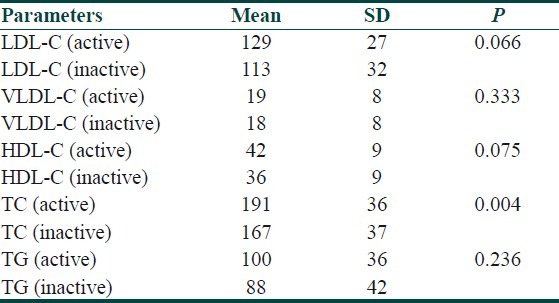
Table 6.
The comparison of oxLDL levels of 13 patients passing from inactive phase to active phase
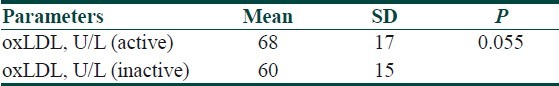
Table 7.
The correlation of oxLDL levels of 13 patients passing from inactive phase to active phase

Discussion
BD is a multisystem disorder characterized by opthalmic and dermatologic findings, mucocutenous lesions, neurologic findings, arthritis and cardiovascular system involvement. BD is a chronic recurrent inflammatory disease, and its predominant histopathology is vasculitis in which perivascular tissue is infiltrated by predominantly lymphocytes and monocytes, plasma cells, and neutrophils as well.[15] Yet, a characterized structure could not be defined for the vasculitis in various histological analyses performed. In physiologic conditions, there is a balance between oxidant factors and antioxidant mechanisms. When this balance tilts in favor of oxidants, advanced tissue damage occurs which is called oxidative stress. Oxidative damage has been thought to be the main cause of the diseases characterized by dysfunction of tissue such as aging, cardiovascular disorders, immune system diseases, degenerative diseases and cancer. Orem et al.[15] Pronai et al.,[16] Kose et al.,[17] and Sandikci et al.[18] reported that in BD, enzymatic and non-enzymatic antioxidants were decreased, and so BD patients were susceptible to oxidative stress. In BD, it has been pointed out that chemotaxis, phagocytosis, superoxide radical anion products and lysosomal enzyme activity increased in PMN cells. It has been reported that decrease in the antioxidant enzyme activity and increase in free radical levels may have an important role in the tissue damage observed in this process.[15] In addition to these factors, changes in serum lipid profile, lipoprotein and lipid peroxidation accompany the situation. Mitamura et al. performed a study in 12 BD patients and 12 healthy controls.[6] They reported that HDL-C levels were decreased in a statistically significant manner in comparison to that of control group; VLDL and LDL-C levels were increased; however, TC levels did not change. Orem et al. performed a study in 30 healthy individuals and 33 thrombogenic BD patients.[7] They reported that HDL-C and triglyceride (TG) levels were statistically significantly lower in BD patient groups in comparison to those of control group; there were no statistically significant differences in terms of LDL-C and TC levels. In another study conducted on 18 BD patients and 20 healthy individuals by Orem et al.,[8] it was reported that TC and LDL-C levels were higher and TG and HDL-C levels were lower. Again, Orem et al. performed another study on 25 healthy and 25 BD patients.[15] They reported that TC, LDL-C, TG levels were statistically significantly higher in BD patient group in comparison to the control group; HDL-C levels were statistically significantly lower in BD patient group in comparison to the control group. Orem et al., in another study,[19] analyzed 37 BD patients and 30 healthy controls and they reported that LDL-C, TC and TG levels were statistically significantly higher in BD patient group in comparison to the control group and HDL-C levels were statistically significantly lower in BD patient group in comparison to the control group. One of the lipid peroxidation processes occurs in LDL. This frequently results in oxidatively modified LDL formation which is known as atherogenic liporotein. The oxLDL plays an important role in endothelial dysfunction as well as atherosclerotic process.[15,20–22] The oxLDL cannot be recognized by the receptors on the liver and extrahepatic tissues; instead it is taken up by the scavanger receptors located on macrophages. The scavanger receptors located on monocytes, smooth muscle cells and macrophages take up oxLDL and this process results in uncontrolled lipid accumulation and foam cell formation and atheroma plaques on the vessel wall.[19,22–25] It has been reported that modified lipoproteins, especially oxLDL, trigger humoral immune response and autoantibody formation in experimental animal and human studies.[19]
The number of studies investigating the relationship between BD and oxLDL is limited. In the previously performed studies, antibodies formed against oxLDL in BD were determined,[15] but in the literature we did not encounter any study in which direct measurement of oxLDL was performed. Orem et al. performed a study on 25 healthy people and 25 BD patients.[5] They detected significantly higher oxLDL autoantibody levels in BD group compared to control group (441±173 mU/mL and 223±116 mU/mL, respectively; P<0.001). Again, Orem et al. performed another study,[19] in which they analyzed oxLDL antibody levels in 37 BD patients and 30 healthy individuals and they detected significantly higher oxLDL autoantibody levels in BD patients in comparison to control group (425±365 mU/mL and 187±132 mU/mL, respectively; P<0.05). In our study, when we analyzed the lipid profiles of total BD patients (n=62), we observed that LDL-C, VLDL-C and TG levels were statistically significantly higher in comparison to the control group (n=26) and HDL-C levels were statistically lower. However, there was no difference between the groups in terms of TC. Our findings about HDL-C and LDL-C correlated with those of Mitamura et al.[6] and Orem et al.[7,8,15,19] The finding about TC correlated with the findings of Mitamura et al.[6] and Orem et al.[7] but it was not in agreement with the findings of other studies of Orem et al.[8,15,19] The finding about TG correlated with that of Orem et al.,[17,19] but it did not correlate with that of other studies of Orem et al.[7,8] We could attribute the differences in the findings between the groups to the factors such as nutrition habits and patient group properties. When we compared the oxLDL levels of total BD group and control group, we observed a statistically significant difference between the groups. Both our study and the previously performed studies indicate that in BD, there is a prominant deterioration in serum lipid profile, giving rise to atherosclerosis. A common finding of all the studies is significant elevation of oxLDL in BD in comparison to the control group. Elevated LDL-C level is one of the factors causing damage that triggers atherosclerosis. Elevated LDL-C undergoes oxidative modification and gives rise to formation of various products having different biologic effects. One of these products is oxLDL and it was detected in high levels in parallel with LDL-C in the serum samples of our patients. Elevated oxLDL causes the secretion and transfer of adhesion molecules such as monocyte chemotactic protein-1 (MCP-1) via monocyte colony stimulating factor (MCSF) to endothelium. These events result in binding of monocytes to endothelium and migration to subendothelial surfaces. Minimally modified low-density lipoprotein (MM-LDL) causes monocytes to differentiate to macrophages and minimally modified LDL becomes more oxidized. This form of oxLDL causes accumulation of cholesterol ester via scavenger receptors. The oxLDL is a strong inhibitor for the macrophage action; it always causes the taking up of macrophages on the arterial wall. Oxidation products of LDL are cytotoxic and cytotoxicity is dangerous for the endothelial cells. It has been reported that oxLDL is especially effective for the progression of atherosclerosis.[26] The oxLDL not only causes foam cell formation, but also gives rise to chronic inflammatory reactions, cellular hyperplesia and coagulation defects and it may play an important role in the pathogenesis of endothelial dysfunction encountered in BD. Probably, the elevation in LDL subfraction, which is smaller, intense and more sensitive to oxidation, may accompany increased TG and decreased HDL-C levels that we observed in our study. Therefore, because of the elevation in this fraction, in BD, serum oxLDL levels may elevate by increased LDL-C oxidation. It also has been reported that HDL-C concentration may affect lipid peroxidation.[12] One of the reasons of high oxLDL levels that we detected in the patient group may be low levels of HDL-C observed in patients. Since we could not encounter any study concerning determination of serum oxLDL levels in BD, we could not have any opportunity to compare our findings directly. However, in two different studies performed by Orem et al.,[15,19] oxLDL antibodies in serum samples of BD patients were found to be higher in comparison to the control group. These authors reported that oxLDL could be non-specifically responsible for the endothelial dysfunction observed in BD. Our findings indirectly correlate with those of Orem et al.[15,19] When we compared oxLDL levels of active and inactive BD patients, we could not detect any statistically significant difference between them. We could not detect any statistically significant difference in either 13 patients who pass from inactive to active phase. The oxLDL levels of control group, active group, inactive group and 13 patients passing from inactive to active phase were within reference interval of our method.
Consequently, we think that oxLDL can be used as a diagnostic marker concerning oxidative stress that has a role in the etiology of BD. However, it cannot be used as a marker of activity; lipid levels were also an important factor in this process and more advanced and detailed studies should be performed to understand dynamic processes more clearly and to enlighten their roles in the pathogenesis of BD.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Behcet H. Uber rezidivierende aphtöse, durch ein virüs verursachte geschwüre am mund, am auge und an den genitalien. [Recurrent aphthous ulcers caused by a virus in the mouth, the eye and the genitals.] Dermatol Wochenschr. 1937;105:1152–7. [Google Scholar]
- 2.Deger O, Orem A, Akyol N, Bahadir S, Yildirmis S. PMN elastase levels in patients with Behçet's disease. Clin Chim Acta. 1995;236:129–34. doi: 10.1016/0009-8981(95)06033-a. [DOI] [PubMed] [Google Scholar]
- 3.Niwa Y, Miyake S, Sakane T, Shingu M, Yokoma M. Auto-oxidative damage in Behçet's disease- endothelial cell damage following the elevated oxygen radicals generated by stimulated neutrophils. Clin Exp Immunol. 1982;49:247–55. [PMC free article] [PubMed] [Google Scholar]
- 4.Dogan P, Soyuer U, Tanrıkulu G, Utas S. Changes in serum and polymorphonuclear antioxidant defense systems in Behçet's disease. Invest Dermatol. 1989;93:297–8. [Google Scholar]
- 5.Dogan P, Tanrıkulu G, Soyuer U, Kose K. Oxidative Enzymes of Polymorphonuclear Leukocytes and Plasma Fibrinogen, Ceruloplazmin and Cupper Levels in Behçet's Disease. Clin Biochem. 1995;27:413–8. doi: 10.1016/0009-9120(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 6.Mitamura T, Ohno S, Ariga H, Ohsaka T, Iwasaki N, Matsuda H, et al. Lipoprotein cholesterol concentrations in patients with Behçet's Disease. Clin Chim Acta. 1988;175:277–83. doi: 10.1016/0009-8981(88)90103-9. [DOI] [PubMed] [Google Scholar]
- 7.Orem A, Deger O, Memis O, Bahadir S, Ovali E, Cimsit G. Lp(a) lipoprotein levels as a predictor of risc for thrombogenic events in patients with Behçet's Disease. Ann Rheum Dis. 1995;54:726–9. doi: 10.1136/ard.54.9.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orem A, Efe H, Deger O, Cimsit G, Uydu HA, Vanizor B. Relationship between lipid peroxidation and disease activity in patients with Behçet's Disease. J Dermatol Sci. 1997;16:11–6. doi: 10.1016/s0923-1811(97)00613-0. [DOI] [PubMed] [Google Scholar]
- 9.Reaven PD. Mechanisms of atherosclerosis role of LDL oxidation. Adv Exp Med Biol. 1994;366:113–28. doi: 10.1007/978-1-4615-1833-4_9. [DOI] [PubMed] [Google Scholar]
- 10.Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–90. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 11.Yla-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and Human aterosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 12.Witztum JL, Steinberg D. Role of oxidized Low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–91. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes-Virella MF, Virella G. Atherosclerosis and autoimmunity. Clin Immunol Immunopathol. 1994;73:155–67. doi: 10.1006/clin.1994.1184. [DOI] [PubMed] [Google Scholar]
- 14.Criteria for diagnosis of Behcet's Disease. International Study Group for Behçet's Disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 15.Orem A, Yandi Y, Vanizor B, Cimsit G, Uydu H, Malkoc M. The evaluation of autoantibodies against oxidatively modified LDL,susceptibility of LDL to oxidation, serum lipids and lipid hydroperoxide levels, total antioxidant status, antioxidant enzyme activities, and endothelial dysfunction in patients with Behçet's disease. Clinl-Biochem. 2002;35:217–24. doi: 10.1016/s0009-9120(02)00290-4. [DOI] [PubMed] [Google Scholar]
- 16.Pronai L, Ichikawa Y, Nakazawa H, Arimori S. Enhanced superoxide generation and the decreased superoxide scavenging activity of peripheral blood leukocytes in Behcet's disease effects of colchicine. Clin Exp Rheumatol. 1991;9:227–33. [PubMed] [Google Scholar]
- 17.Kose K, Yazici C, Cambay N, Ascioglu O, Dogan P. Lipid peroxidation and erythrocyte antioxidant enzymes in patients with Behçet's disease. Tokohu J Exp Med. 2002;197:9–16. doi: 10.1620/tjem.197.9. [DOI] [PubMed] [Google Scholar]
- 18.Sandıkci R, Turkmen S, Guvenen G, Ayabakan H, Gulcan P, Koldas M, et al. Lipid peroxidation and antioxidant defence system in patients with active and inactive Behcet's disease. Acta Derm Venereol. 2003;83:342–6. doi: 10.1080/00015550310003782. [DOI] [PubMed] [Google Scholar]
- 19.Orem A, Cimsit G, Deger O, Vanizor B, Karahan S. Autoantibodies against oxidatively modified low-density lipoprotein in patients with Behçet's disease. Dermatology. 1999;198:243–6. doi: 10.1159/000018122. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg D. Low density lipoprotein Oxidation and Its Pathobiological Significance. J Biol Chem. 1997;272:20963–6. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 21.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, et al. Atherosclerosis: Basic Mechanisms.Oxidation, Inflammation, and Genetics. Circulation. 1995;91:2488–96. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg D, Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–71. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 23.Lehtimaki T, Lehtinen S, Solakivi T, Nikkila M, Jaakkola O, Jokela H, et al. Autoantibodies Against Oxidized Low Density Lipoprotein in Patients with Angiographically Verified Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 1999;19:23–7. doi: 10.1161/01.atv.19.1.23. [DOI] [PubMed] [Google Scholar]
- 24.Princen HM, Duyvenvoorde W, Buyhentek R, van der Laarse A, van Poppel G, Gevers Leuven JA, et al. Supplementation with low doses of vitamin E protects LDL from lipid peroxidation in men and women. Arterioscler Thromb Vasc Biol. 1995;15:325–33. doi: 10.1161/01.atv.15.3.325. [DOI] [PubMed] [Google Scholar]
- 25.Chisolm GM, 3rd, Hazen SL, Fox PL, Cathcart MK. The oxidation of lipoproteins by monocytes-macrophages. J Biol Chem. 1999;274:25959–62. doi: 10.1074/jbc.274.37.25959. [DOI] [PubMed] [Google Scholar]
- 26.Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, et al. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990;85:1260–6. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]


