Abstract
The human face provides a wealth of information pertaining to the internal state and life-stage history of an individual. Facial width-to-height ratio is a size-independent sexually dimorphic trait, and estimates of aggression made by untrained adults judging own-race faces were positively associated with both facial width-to-height ratio and actual aggressive behavior. Given the significant adaptive value of accurately detecting aggressiveness based on facial appearance, we hypothesized that aggression estimates made by adults and 8-year-olds would be highly correlated with male facial width-to-height ratio even for a face category with which they had minimal experience—other-race faces. For each of the four race and age groups, estimates of aggression were positively correlated with facial width-to-height ratio irrespective of rating own-or other-race faces. Overall, the correlations between facial width-to-height ratio and ratings of aggression were stronger for adults than for children. Sensitivity to facial width-to-height ratio appears to be part of an evolved mechanism designed to detect threats in the external environment. This mechanism is likely broadly tuned and functions independently of experience.
1. Introduction
The human face is an inherently social stimulus that provides a wealth of information, such as the emotional state and age of an individual. Whereas facial cues such as emotional expressions provide only a temporary glimpse into an individual’s internal state, stable facial cues may also serve as reliable signals of an individual’s underlying traits and behavioral tendencies (e.g., Mueller & Mazur, 1997; Penton-Voak et al., 2006; Sell et al., 2009; Shoup & Gallup, 2008). Although there is debate over the accuracy of trait impressions based on facial appearance (e.g., Olivola & Todorov, 2010), a number of studies demonstrate that individuals are surprisingly accurate in making trait inferences from human faces, which suggests that person perception may be an adaptively tuned process reflective of an evolved approach/avoidance mechanism (Bar et al., 2006; Rule & Ambady, 2008; Todorov et al., 2005).
Both dynamic and static facial characteristics signal propensity for aggression; an angry facial expression signals immediate potential threat but stable characteristics of an emotionally neutral face may also convey accurate information pertaining to an individual’s aggressive potential. Sensitivity to these cues has significant adaptive value in that it allows an individual to successfully identify and respond to potentially threatening situations (Haselton & Funder, 2006). Adults are able to predict violence and aggressiveness based on facial structure; their estimates of violent potential when judging the faces of sex offenders are positively correlated with the offenders’ actual violent history. Furthermore, cues that reflect high levels of facial masculinity are positively associated with both actual and perceived violence (Stillman et al., 2010). It has been hypothesized that trait inferences may be partly driven by an overgeneralization of the emotion recognition system, in that certain facial attributes may resemble features that are characteristic of given emotional expressions, which in turn lead an observer to infer that an individual is actually expressing that emotion or possesses qualities that are consistent with that emotion (Said et al., 2009; Zebrowitz et al., 2003). For example, large eyes look brighter and may be associated with happiness, which might lead an observer to label the individual as warm and friendly. While several structural qualities of human faces have been found to contribute to trait impressions (e.g., symmetry and large eyes are positively correlated with perceived honesty (Zebrowitz et al., 1996), the exact facial cues that influence perceptions of aggression remain largely unspecified.
The facial width-to-height ratio in male faces may be a cue used for trait judgments. This ratio reflects a sexual dimorphism in the human face that is independent of the size of the body (Weston et al., 2007). Sex differences in this face characteristic emerge around puberty, concurrent with the onset of general sex differences in facial structure relative to body size that are reflective of testosterone increases in males (Verdonck et al., 1999). Individual differences in reactive aggression were correlated with male facial width-to-height ratio (Carré & McCormick, 2008), and estimates of aggression based on facial appearance were positively associated with both facial width-to-height ratio and actual aggressive behavior (Carré et al., 2009). The accuracy of participants’ estimates of aggression remained high even when faces were manipulated such that featural cues are eliminated while the facial width-to-height ratio is maintained, suggesting that the ratio guides judgments of aggression (Carré et al., 2010).
Given that estimates of aggressive potential (Carré et al., 2009; Carré et al., 2010) and threat (Oosterhof & Todorov, 2008) from neutral faces are highly consistent across viewers, it is possible that the human cognitive and perceptual system may be preferentially tuned to rapidly and accurately detect a propensity for aggression in other individuals (Cosmides & Tooby, 1992; Duntley & Buss, 2008) akin to “honest signals” that guide behavior in humans (Sundie et al., 2010) and in non-human species (e.g., Tibbetts & Dale, 2004; Tibbetts & Lindsay, 2008). Expertise in face recognition is thought to be a product of experience (MacLin & Malpass, 2001; Nelson, 2003; Rhodes et al., 2009); however, if the cognitive system is preferentially tuned to detect facial cues that convey the behavioral tendencies of others, sensitivity to such cues may be broadly tuned (i.e., independent of experience with a given face category) and may develop early in life.
The current study was designed to investigate whether humans are sensitive to differences in facial width-to-height ratio when they are asked to judge the aggressiveness of a category of faces with which they have little or no experience, such as other-race faces. (We use the terms own- and other-race to be consistent with previous literature but recognize that these are cognitive/perceptual and not biological categories.) Sensitivity to facial width-to-height ratio may be broadly tuned such that abundant experience with a particular face category is not necessary to use this ratio as a cue for aggressive potential. Furthermore, if such sensitivity is broadly tuned and independent of experience, then young children may also rely on this cue when making judgments of aggression. We tested these hypotheses by asking two groups of participants to judge aggressive potential in two sets of male faces—Caucasian and Chinese. First, Caucasian and Chinese adults judged the aggressive potential in own- versus other-race male faces. When judging facial identity, adults are less sensitive to configural cues (i.e., spacing between the major face features and the face contour) in other-race faces (Mondloch et al., 2010; Rhodes et al., 2006), a pattern that likely reflects their relative lack of experience with other-race faces (Rhodes et al., 2009). If sensitivity to facial width-to-height ratio is broadly tuned and functions independently of experience, then adults will likely be sensitive to variations in facial width-to-height ratio in other-race faces, despite their being generally less sensitive to configural cues in other-race faces. Second, 8-year-old Caucasian and Chinese children judged the same set of male faces. A positive correlation between facial width-to-height ratio and estimates of aggression by children would lend further support to the hypothesis that sensitivity to facial width-to-height ratio may be part of an evolved mechanism designed to alert individuals to threats in the environment (i.e., an “honest signal”).
2. Experiment 1
(2.1) Method
Participants
Sixteen Caucasian (8 women; age range = 18-25) and 16 Chinese (8 women; age range = 18-25) undergraduates participated in this experiment and received research credit or a small honorarium. Caucasian participants were from Brock University in Ontario, Canada, and Chinese participants were from Zhejiang Normal University in Jinhua, China.
Materials
Stimuli consisted of 24 facial photographs of Caucasian undergraduate men from XXX University and 24 facial photographs of Chinese undergraduate men from XXX University. The Caucasian faces used in this study were the same as those used in previous studies investigating sensitivity to facial width-to-height ratio (Carré et al., 2009; Carré et al., 2010). All faces had a neutral expression, did not have facial hair, and were photographed in a forward-facing position. Photographs were converted to grayscale and a black occluder was placed around each face to conceal background information. Faces were standardized using a hairline-chin distance of 400 pixels. ImageJ (NIH open-source software) was used to measure the width-to-height ratio of each face, defined as the distance between the left and right zygion divided by the distance between the upper lip and brow (Weston et al., 2007). Overall, the facial width-to-height ratio for the Caucasian face set ranged from 1.54 to 2.09, and the facial width-to-height ratio for the Chinese face set ranged from 1.68 to 2.17.
Procedure
In the current experiment and in Experiment 2, the procedures were approved by each university’s ethics board. Participants were seated in front of a 14-inch LCD monitor and told that they would be shown a series of male faces and that their task was to estimate how aggressive each person would be if he were provoked. Faces were presented using E-Prime software, and the experiment was blocked by race and counterbalanced such that half of the participants saw the 24 Caucasian faces first followed by the 24 Chinese faces, whereas the other half viewed the faces in the reverse order. Within each block, faces were fully randomized. Each trial consisted of a 500-ms fixation cross followed by a face that appeared for 2000 ms. After the presentation of the face, the question, “How aggressive would this person be if provoked?” appeared on the screen alongside a 7-point Likert scale (1 = not at all aggressive, 7 = very aggressive) and participants were given an unlimited amount of time to respond. Participants made their response by pressing the corresponding numerical button on a keyboard.
(2.2) Results
Caucasian and Chinese participants demonstrated a strong positive correlation between estimates of aggressive potential and facial width-to-height ratio in adult male faces. There was no evidence for an own-race advantage; both groups exhibited comparable correlations for own- and other-race faces. Overall, the mean correlation between estimated aggression and stimulus facial width-to-height ratio was greater for Caucasian participants than for Chinese participants, which suggests that regardless of face race, Caucasian participants are more sensitive to facial width-to-height ratio as a cue for aggressive potential than are Chinese participants.
Analysis by participant
All t-tests and correlational analyses were two-tailed. As shown in Fig. 1, estimates of aggression were highly consistent across the 16 Caucasian participants tested for Caucasian faces (Cronbach’s alpha = .89) and Chinese faces (Cronbach’s alpha = .87) and across the 16 Chinese participants tested for Caucasian faces (Cronbach’s alpha = .73) and Chinese faces (Cronbach’s alpha = .79). Single-sample t-tests comparing individual correlations to a null value of zero revealed that estimated aggression was positively correlated with faces’ width-to-height ratio for Caucasian participants rating Caucasian faces (r = .41), t15 = 8.65, p < . 001, and for Caucasian participants rating Chinese faces (r = .37), t15 = 8.75, p < .001. Likewise, estimated aggression was positively correlated with faces’ width-to-height ratio for Chinese participants rating Caucasian faces (r = .25), t15 = 5.53, p < .001, and for Chinese participants rating Chinese faces (r = .24), t15 = 6.16, p < .001. A 2 (face race: Caucasian, Chinese) × 2 (participant race: Caucasian, Chinese) repeated-measures ANOVA with participants’ individual correlations between estimated aggression and stimulus faces’ width-to-height ratio as the dependent variable revealed a main effect of participant race, F1, 30 = 9.57, p < .01. As shown in Table 1, the mean correlation between estimated aggression and stimulus facial width-to-height ratio was larger for Caucasian participants (Mean correlation ± SD = .39 ± .18) than for Chinese participants (Mean correlation ± SD = .25, ± .18). There was neither a main effect of face race nor a significant interaction, ps > .50.
Figure 1.
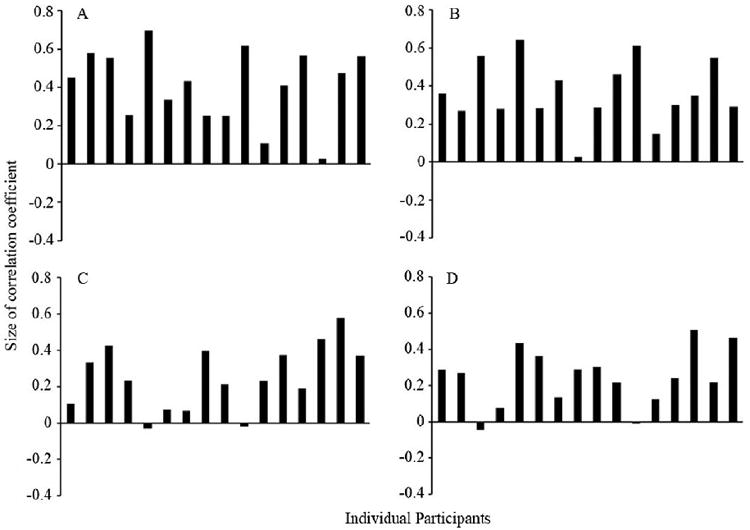
Relationships between individual participants’ estimates of the pictured individuals’ aggression and facial width-to-height ratio for (A) Caucasian adults observing Caucasian faces, (B) Caucasian adults observing Chinese faces, (C) Chinese adults observing Caucasian faces, and (D) Chinese adults observing Chinese faces.
Table 1.
Mean Correlations between Estimated Aggression and Width-to-Height Ratio Across Participants
| Participant Group | Caucasian Faces
|
Chinese Faces
|
||
|---|---|---|---|---|
| n | M (SE) | n | M (SE) | |
| Caucasian Adults | 16 | .41 (.05) | 16 | .37 (.04) |
| Chinese Adults | 16 | .25 (.05) | 16 | .24 (.04) |
| Caucasian Children | 16 | .25 (.05) | 16 | .16 (.02) |
| Chinese Children | 16 | .14 (.02) | 16 | .12 (.07) |
Analysis by stimulus face
Among Caucasian participants, the mean estimated aggressiveness of each Caucasian stimulus face across participants was positively correlated with facial width-to-height ratio, r = .64, p < .01, and the mean estimated aggressiveness of each Chinese stimulus face was positively correlated with facial width-to-height ratio, r = .61, p < .01 (See Fig. 2). To determine whether the overall correlation for Caucasian faces was greater than the overall correlation for Chinese faces, we calculated the 95% confidence interval for each correlation coefficient. Because of the great deal of overlap between the 95% confidence intervals for Caucasian faces (.21 to .86) and for Chinese faces (.16 to .85), we concluded that among Caucasian participants, the relationship between facial width-to-height ratio and estimates of aggressive potential was comparable across Caucasian and Chinese male faces.
Figure 2.
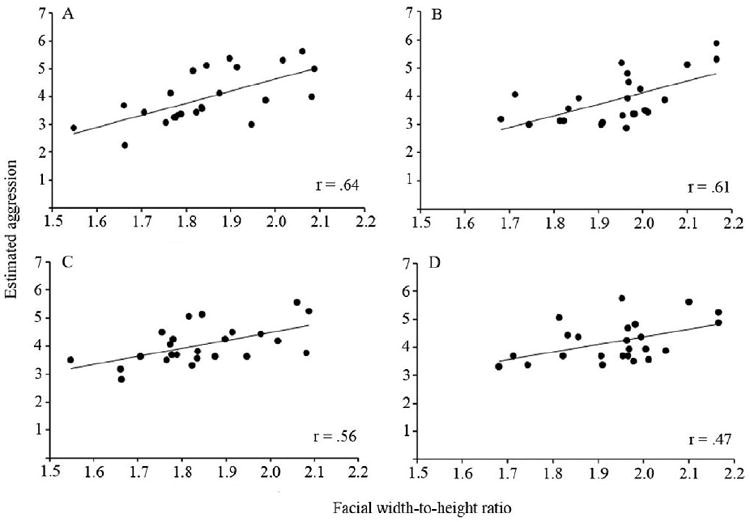
Correlation between mean estimated aggressiveness of each stimulus face across participants and facial width-to-height ratio for (A) Caucasian adults observing Caucasian faces, (B) Caucasian adults observing Chinese faces, (C) Chinese adults observing Caucasian faces, and (D) Chinese adults observing Chinese faces.
Among Chinese participants, the mean estimated aggressiveness of each Chinese stimulus face across participants was positively correlated with facial width-to-height ratio, r = . 47, p < .05, and the mean estimated aggressiveness of each Caucasian stimulus face was positively correlated with facial width-to-height ratio, r = .56, p < .01. To determine whether the overall correlation for Caucasian faces was greater than the overall correlation for Chinese faces, we calculated the 95% confidence interval for each correlation coefficient. Because of the great deal of overlap between the 95% confidence intervals for Caucasian faces (.08 to .83) and for Chinese faces (-.04 to .79), we concluded that among Chinese participants, the relationship between facial width-to-height ratio and estimates of aggressive potential was comparable across Caucasian and Chinese male faces.
3. Experiment 2
(3.1) Method
Participants
Sixteen Caucasian 8-year-olds (±6 months; 8 female) and 16 Chinese 8-year-olds (±6 months; 8 female) participated in this experiment. Caucasian children were recruited from local schools in the XXX region of XXX, Canada, and XXX children were recruited from an elementary school in XXX, China. Four additional children were tested but excluded from all analyses because they failed to pass the validation trials at the end of the task (n = 2) or did not follow task instructions (n = 2).
Materials
Test stimuli were identical to those used in Experiment 1. To assess whether children remained attentive at the end of the task, an additional three validation trials were created. Validation stimuli consisted of three Caucasian male face identities from the NimStim Face Stimulus Set (Tottenham et al., 2009). One identity had a happy expression, one had an angry expression, and one had a neutral expression. Faces were converted to grayscale and resized such that the distance from the hairline to chin was 400 pixels. A black occluder was placed around each face to conceal all background information.
Procedure
To ensure that children had an accurate understanding of aggressiveness, children were first provided with a verbal definition of aggression (e.g., “when someone yanks a toy out of your hands”) and were then told a story about three men who played a game in which they could steal points from other players. One man stole 3 points, one man stole 20 points, and one man stole 60 points. Children were then shown five cups of increasing height. The smallest cup was labeled 1, and the largest cup was labeled 5. Children were told that the size of the cup indicated how aggressive a person is and that their job was to rate how aggressive each person was in the game by pointing to the cup that best represents their level of aggression. To be included in the final data analysis, children had to rank order the three men correctly. No children were excluded on the basis of failing to meet this criterion.
After criterion trials, children were told that they were going to be shown pictures of more men who played the point collecting game and that their job was to use the 5-point cup rating scale to estimate each man’s aggressiveness. Children were seated in front of a 15-inch LCD screen and stimulus faces were presented using Cedrus Superlab Version 4.0. All components of the task were identical to Experiment 1 except that after completing the 48 test trials, children were shown three validation trials that consisted of a happy male face, a neutral male face, and an angry male face. The order in which children viewed the three faces was randomized. Children were asked to estimate how aggressive each man was likely to be when playing the point collecting game. To be included in the final data analysis, children had to rank order the three faces correctly, such that the happy face received the lowest aggression rating and the angry face received the highest aggression rating.
(3.2) Results
We converted children’s aggression ratings on a 5-point scale to ratings on a 7-point scale by multiplying each child’s original rating for each face by seven and then dividing the product by five. This conversion allowed us to make a direct comparison between Experiments 1 and 2. Similar to the adult participants in Experiment 1, both Caucasian and Chinese children demonstrated a positive correlation between estimates of aggressive potential and facial width-to-height ratio in adult male faces. There was no evidence for an own-race advantage; both Caucasian and Chinese children exhibited comparable correlations between estimates of aggression and facial width-to-height ratio for own- and other-race faces.
Analysis by participant
As shown in Fig. 3, estimates of aggression were highly consistent across the 16 Caucasian children tested for both Caucasian faces (Cronbach’s alpha = . 84) and Chinese faces (Cronbach’s alpha = .66). Estimates of aggression, however, were less consistent across the 16 Chinese children tested for both Caucasian faces (Cronbach’s alpha = . 28) and Chinese faces (Cronbach’s alpha = .48). Single-sample t-tests comparing individual correlations to a null value of zero revealed that estimated aggression was positively correlated with faces’ width-to-height ratio for Caucasian children rating Caucasian faces (r = .25), t15 = 5.27, p < .001, and for Caucasian children rating Chinese faces (r = .16), t15 = 2.64, p < .05. Likewise, estimated aggression was positively correlated with faces’ width-to-height ratio for Chinese children rating Caucasian faces (r = .14), t15 = 2.69, p < .05. The positive association between estimated aggression and stimulus faces’ width-to-height ratio was marginally significant for Chinese children rating Chinese faces (r = .12), t15 = 1.81, p = .09. As shown in Table 1, a 2 (face race: Caucasian, Chinese) × 2 (participant race: Caucasian, Chinese) repeated-measures ANOVA with participants’ individual correlations between estimated aggression and faces’ width-to-height ratio as the dependent variable revealed no main effects or interaction, ps > .20.
Figure 3.
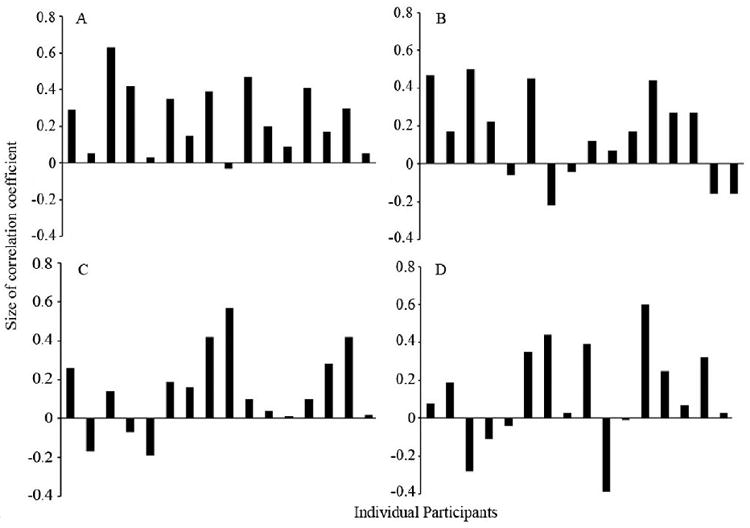
Relationships between individual participants’ estimates of the pictured individuals’ aggression and facial width-to-height ratio for (A) Caucasian children observing Caucasian faces, (B) Caucasian children observing Chinese faces, (C) Chinese children observing Caucasian faces, and (D) Chinese children observing Chinese faces.
Analysis by stimulus face
Among Caucasian children, the mean estimated aggressiveness of each Caucasian face across participants was positively correlated with facial width-to-height ratio, r = .45, p < .05, and the mean estimated aggressiveness of each Chinese face was positively correlated with facial width-to-height ratio, r = .50, p < .05 (See Fig. 4). To determine whether the overall correlation for Caucasian faces was different from the overall correlation for Chinese faces, we calculated the 95% confidence interval for each correlation coefficient. Because of the great deal of overlap between the 95% confidence intervals for Caucasian faces (-.06 to .78) and for Chinese faces (0 to .80), we concluded that among Caucasian children, the relationship between facial width-to-height ratio and estimates of aggressive potential was comparable across Caucasian and Chinese male faces.
Figure 4.
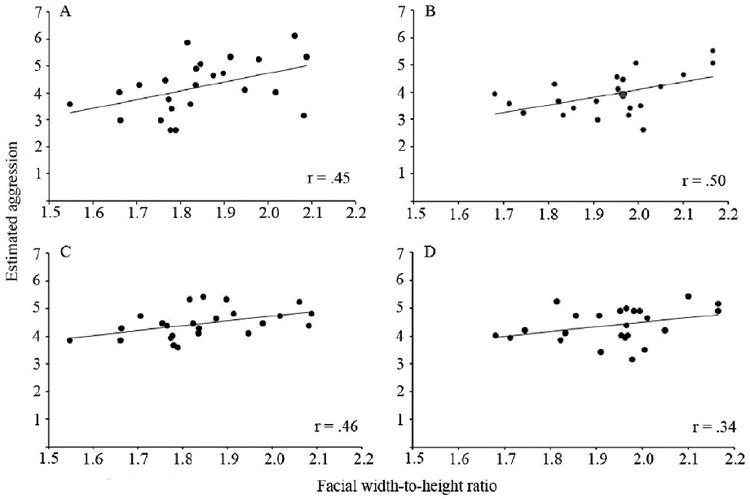
Correlation between mean estimated aggressiveness of each face across participants and facial width-to-height ratio for (A) Caucasian children observing Caucasian faces, (B) Caucasian children observing Chinese faces, (C) Chinese children observing Caucasian faces, and (D) Chinese children observing Chinese faces.
Among Chinese children, the mean estimated aggressiveness of each Caucasian face across participants was positively correlated with facial width-to-height ratio, r = .46, p < .05. Although there was a positive correlation between the mean estimated aggressiveness of each Chinese face and facial width-to-height ratio across Chinese children (r = .34), this correlation was not significant, p > .10. To determine whether the overall correlation for Caucasian faces was greater than the overall correlation for Chinese faces, we calculated the 95% confidence interval for each correlation coefficient. Because of the great deal of overlap between the 95% confidence intervals for Caucasian faces (-.05 to .78) and for Chinese faces (-.20 to .72), we concluded that among Chinese children, the relationship between facial width-to-height ratio and estimates of aggressive potential was comparable across Caucasian and Chinese male faces.
Combined analysis of Experiments 1 and 2
A repeated-measures ANOVA including both age groups of participants’ individual correlations between estimates of aggression and faces’ width-to-height ratio revealed higher correlations in Caucasian participants than in Chinese participants, F1, 60 = 7.20, p < .01, and higher correlations in adults than in children, F1, 60 = 14.49, p < .001. There were no other significant main effects or interactions, ps > .20.
4. Discussion
While facial width-to-height ratio is likely not the sole facial cue used to determine an individual’s level of aggression (e.g., Zebrowitz et al., 1996), it appears to be used by both adults and children irrespective of face race. Both age groups tested exhibited no evidence for an own-race advantage in sensitivity to facial width-to-height ratio as a cue for aggression. In contrast to adults’ reduced sensitivity to configural cues to identity in other-race faces (Mondloch et al., 2010; Rhodes et al., 2006), adult and child Caucasian and Chinese participants demonstrated comparable correlations between estimates of aggression and facial width-to-height ratio for own- and other-race faces. These results indicate that the ability to gauge a conspecific’s propensity for aggression based on facial width-to-height ratio may be part of an evolved mechanism designed to alert individuals to threats in the environment, just as aggressive behavior itself has adaptive value (Griskevicius et al., 2009). Such a mechanism is independent of experience with a given face category and may be comparable to the evolved cognitive mechanism used to assess physical formidability proposed by Sell and colleagues (2009). The lack of any own-race advantage when judging propensity for aggression is consistent with evidence that the own-race recognition advantage is eliminated when own- and other-race faces display an angry facial expression (Ackerman et al., 2006); Caucasian observers recognize Caucasian faces more accurately than African American faces when all faces display a neutral expression, but that pattern is reversed when all faces display anger. The underlying mechanism likely involves brain structures in the limbic system and the amygdala in particular, which has been implicated in the implicit assessment of various stimuli (Sander et al., 2003) and judgments of social valence such as trustworthiness (Todorov & Engell, 2008; Winston et al., 2002).
Like adults, 8-year-old children demonstrated sensitivity to facial width-to-height ratio as a cue for aggressive behavior. Our finding that children are less sensitive to variations in facial width-to-height ratio than adults may reflect age differences specific to face perception (Freire & Lee, 2001; Mondloch et al., 2002) or to age differences in visual development (Skoczenski & Norcia, 2002). Nonetheless, our finding that children’s sensitivity to facial width-to-height ratio as a cue for aggressive potential is independent of face race provides further evidence that experience with a face category is not necessary to be sensitive to variations in facial width-to-height ratio and supports our hypothesis that sensitivity to facial width-to-height ratio may be part of an evolved mechanism that functions independently of experience with a particular face category and that alerts individuals to threats in the environment.
Although sensitivity to the facial width-to-height ratio is independent of our experience with a given face category, two findings suggest that experience may serve to refine this sensitivity. The age difference suggests that sensitivity to facial width-to-height ratio may become further tuned as children gain experience in witnessing the link between high facial width-to-height ratio and overtly aggressive behaviors. Adults tend to avoid displaying overt acts of aggression in front of children and thus children may have fewer opportunities than adults to witness the link between facial width-to-height ratio and aggression. Furthermore, children’s primary social interactions are with their peers, whose faces do not show systematic variations in facial width-to-height ratio until puberty (Weston et al., 2007). Similar to children’s attractiveness preferences (Cooper et al., 2006), sensitivity to facial width-to-height ratio may be shaped by the type of faces that children predominantly encounter in their everyday environment; if children are rarely exposed to faces that systematically differ in width-to-height ratio, their sensitivity to this metric as a cue for aggression may be less well refined than that of adults. Such an explanation leads to the prediction that adult-like sensitivity to differences in the facial width-to-height ratio may develop during early adolescence. Second, Caucasian participants were overall more sensitive than Chinese participants to facial width-to-height ratio as a cue for aggressive potential. A potential explanation for this difference may be related to differences between North American and Asian societies, perhaps linked to collectivist versus individualist cultures. In a collectivist culture, there is greater emphasis on cooperation and working together for the benefit of all (e.g., Kim & Markus, 1999; Triandis, 1995), which likely discourages the use of overtly aggressive actions. Adults in collectivist cultures are thus likely to have fewer opportunities than those in individualist cultures to witness the association between high facial width-to-height ratio and aggressive behavior; as such, the ability to accurately identify aggressive potential by way of facial width-to-height ratio may have less adaptive value in a collectivist culture. Therefore, although individuals in collectivist cultures are sensitive to differences in facial width-to-height ratio, their ability to use the ratio as a cue for aggressive potential may be less well refined than those in individualist cultures and they may be less apt to use such a ratio when gauging a man’s propensity to commit an aggressive act. Such an interpretation is speculative, however, and we are currently examining the relationship between facial width-to-height ratio and actual aggressive behavior in Chinese participants.
The results of the present study are the first to demonstrate that expertise with a given face category is not necessary for individuals to be sensitive to variations in facial width-to-height ratio although experience in witnessing the association between facial width-to-height ratio and aggressive behavior may further refine the ability to accurately gauge an individual’s propensity for aggression. Sensitivity to facial width-to-height ratio itself appears to be part of an evolved mechanism designed to detect threats in the external environment. This mechanism functions independently of experience with a given face category and is likely influenced by subcortical structures implicated in the implicit assessment of various types of stimuli. Sensitivity to such systematic variations in facial width-to-height ratio may thus be akin to “honest signals” of aggressive behavior described in non-human species (e.g., Tibbetts & Lindsay, 2008).
Acknowledgments
Research support was provided by Social Sciences and Humanities Research Council grants (xxx, xxx), by a Vanier Canada Graduate Scholarship (xxx), and by NIH (xxxx).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman JM, Shapiro JR, Neuberg SL, Kenrick DT, Becker DV, Griskevicius V, Maner JK, Schaller M. They all look the same to me (unless they’re angry) Psychological Science. 2006;17:836–840. doi: 10.1111/j.1467-9280.2006.01790.x. 10.1111/j.1467-9280.2006.01790.x. [DOI] [PubMed] [Google Scholar]
- Bar M, Neta M, Linz H. Very first impressions. Emotion. 2006;6:268–278. doi: 10.1037/1528-3542.6.2.269. 10.1037/1528-3542.6.2.269. [DOI] [PubMed] [Google Scholar]
- Carré JM, McCormick CM. In your face: Facial metrics predict aggressive behavior in the laboratory and in varsity and professional hockey players. Proceedings of the Royal Society of London: Series B Biological Sciences. 2008;275:2651–2656. doi: 10.1098/rspb.2008.0873. 10.1098/rspb.2008.0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, McCormick CM, Mondloch CJ. Facial structure is a reliable cue of aggressive behavior. Psychological Science. 2009;20:1194–1198. doi: 10.1111/j.1467-9280.2009.02423.x. 10.1111/j.1467-9280.2009.02423.x. [DOI] [PubMed] [Google Scholar]
- Carré JM, Morrissey MD, Mondloch CJ, McCormick CM. Estimating aggression from emotionally neutral faces: Which facial cues are diagnostic? Perception. 2010;39:356–377. doi: 10.1068/p6543. 10.1068/p6543. [DOI] [PubMed] [Google Scholar]
- Cooper PA, Geldart SS, Mondloch CJ, Maurer D. Developmental changes in perceptions of attractiveness: A role of experience? Developmental Science. 2006;9:530–543. doi: 10.1111/j.1467-7687.2006.00520.x. 10.1111/j.1467-7687.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- Cosmides L, Tooby J. Cognitive adaptations for social exchange. In: Barkow JH, Cosmides L, Tooby J, editors. The adapted mind. New York: Oxford University Press; 1992. pp. 163–228. [Google Scholar]
- Duntley JD, Buss DM. Victim adaptations. In: Duntley J, Shackelford T, editors. Evolutionary forensic psychology. New York: Oxford University Press; 2008. pp. 201–229. [Google Scholar]
- Freire A, Lee K. Face recognition in 4- to 7-year-olds: Processing of configural, featural, and paraphernalia information. Journal of Experimental Child Psychology. 2001;80:347–371. doi: 10.1006/jecp.2001.2639. 10.1006/jecp.2001.2639. [DOI] [PubMed] [Google Scholar]
- Griskevicius V, Tybur JM, Gangestad SW, Perea EF, Shapiro JR, Kenrick DT. Aggress to impress: Hostility as an evolved context-dependent strategy. Journal of Personality and Social Psychology. 2009;96:980–94. doi: 10.1037/a0013907. 10.1037.a0013907. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Funder D. The evolution of accuracy and bias in social judgment. In: Schaller M, Kenrick DT, Simpson JA, editors. Evolution and social psychology. New York: Psychology Press; 2006. pp. 15–37. [Google Scholar]
- Kim H, Markus HR. Deviance or uniqueness, harmony and conformity? Journal of Personality and Social Psychology. 1999;77:785–800. 10.1037/0022-3514.77.4.785. [Google Scholar]
- MacLin OH, Malpass RS. Racial categorization of faces: The ambiguous-race face effect. Psychology, Public Policy, and Law. 2001;7:98–118. 10.1037/1076-8971.7.1.98. [Google Scholar]
- Mondloch CJ, Elms N, Maurer D, Rhodes G, Hayward WG, Tanaka JW, Zho G. Processing underlying the cross-race effect: An investigation of holistic, featural, and relational processing of own-race and other-race faces. Perception. 2010;39:1065–1085. doi: 10.1068/p6608. 10.1068/p6608. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Le Grand R, Maurer D. Configural face processing develops more slowly than featural face processing. Perception. 2002;31:553–566. doi: 10.1068/p3339. 10.1068/p3339. [DOI] [PubMed] [Google Scholar]
- Mueller U, Mazur A. Facial dominance in Homo sapiens as honest signaling of mate quality. Behavioral Ecology. 1997;8:569–579. 10.1093/beheco/8.5.569. [Google Scholar]
- Nelson CA. The development of face recognition reflects an experience-expectant and activity-dependent process. In: Pascalis O, Slater A, editors. The development of face processing in infancy and early childhood. New York: Nova; 2003. pp. 79–97. [Google Scholar]
- Olivola CY, Todorov A. Fooled by first impressions? Reexamining the diagnostic value of appearance-based inferences. Journal of Experimental Social Psychology. 2010;46:315–324. 10.1016/j.jesp.2009.12.002. [Google Scholar]
- Oosterhof NN, Todorov A. The functional basis of face evaluation. Proceedings of the National Academy of Sciences: USA. 2008;105:11087–11092. doi: 10.1073/pnas.0805664105. 0.1073/pnas.0805664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton-Voak IS, Pound N, Little AC, Perrett DI. Personality judgments from natural and composite facial images: More evidence for a “kernel of truth” in social perception. Social Cognition. 2006;24:490–524. 10.1521/soco.2006.24.5.607. [Google Scholar]
- Rhodes G, Ewing L, Hayward WG, Maurer D, Mondloch CJ, Tanaka JW. Contact and other-race effects in configural and component processing of faces. British Journal of Psychology. 2009;100:717–728. doi: 10.1348/000712608X396503. 10.1348/000712608X396503. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Hayward WG, Winkler C. Expert face coding: Configural and component coding of own-race and other-race faces. Psychonomic Bulletin & Review. 2006;13:499–505. doi: 10.3758/bf03193876. [DOI] [PubMed] [Google Scholar]
- Rule NO, Ambady N. The face of success: Inferences from chief executive officers’ appearance predict company profits. Psychological Science. 2008;19:109–111. doi: 10.1111/j.1467-9280.2008.02054.x. 10.1111/j.1467-9280.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- Said CP, Sebe N, Todorov A. Structural resemblance to emotional expressions predicts evaluation of emotionally neutral faces. Emotion. 2009;9:260–264. doi: 10.1037/a0014681. 10.1037/a0014681. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: An evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Sell A, Cosmides L, Tooby J, Sznycer D, von Rueden C, Gurven M. Human adaptations for the visual assessment of strength and fighting ability from the body and face. Proceedings of the Royal Society B: Biological Sciences. 2009;276:575–584. doi: 10.1098/rspb.2008.1177. 10.1098/rspb.2008.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoup ML, Gallup GG. Men’s faces convey information about their bodies and their behavior: What you see is what you get. Evolutionary Psychology. 2008;6:469–479. [Google Scholar]
- Skoczenski AM, Norcia AM. Late maturation of visual hyperacuity. Psychological Science. 2002;13:537–541. doi: 10.1111/1467-9280.00494. 10.1111/1467-9280.00494. [DOI] [PubMed] [Google Scholar]
- Stillman TF, Maner JK, Baumeister RF. A thin slice of violence: Distinguishing violent from nonviolent sex offenders at a glance. Evolution and Human Behavior. 2010;31:298–303. 10.1016/j.evolhumbehav.2009.12.001. [Google Scholar]
- Sundie JM, Kenrick DT, Griskevicius V, Tybur JM, Vohs KD, Beal DJ. Journal of Personality and Social Psychology. 2010 Nov 1; doi: 10.1037/a0021669. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Tibbetts EA, Dale J. A socially enforced signal of quality in paper wasps. Nature. 2004;432:218–222. doi: 10.1038/nature02949. 10.1038/nature02949. [DOI] [PubMed] [Google Scholar]
- Tibbetts EA, Lindsay R. Visual signals of status and rival assessment in Polistes dominulus paper wasps. Biology Letters. 2008;4:237–239. doi: 10.1098/rsbl.2008.0048. 10.1098/rsbl.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Engell AD. The role of the amygdala in the implicit evaluation of emotionally neutral faces. Social Cognitive and Affective Neuroscience. 2008;3:303–312. doi: 10.1093/scan/nsn033. 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Mandisodza AN, Goren A, Hall CC. Inferences of competence from faces predict election outcomes. Science. 2005;308:1623–1626. doi: 10.1126/science.1110589. 10.1126/science.1110589. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triandis HC. Individualism and collectivism. Boulder, CO: Westview Press; 1995. [Google Scholar]
- Verdonck A, Gaethofs M, Carels C, de Zegher F. Effect of low-dose testosterone treatment on craniofacial growth in boys with delayed puberty. European Journal of Orthodontics. 1999;21:137–143. doi: 10.1093/ejo/21.2.137. 0.1093/ejo/21.2.137. [DOI] [PubMed] [Google Scholar]
- Weston EM, Friday AE, Liò P. Biometric evidence that sexual selection has shaped the hominin face. PLoS ONE. 2007;2:1–8. doi: 10.1371/journal.pone.0000710. 10.1371/journal.pone.0000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5:277–283. doi: 10.1038/nn816. 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Fellous J, Mignault A, Andreoletti C. Trait impressions as overgeneralized responses to adaptively significant facial qualities: Evidence from connectionist modeling. Personality and Social Psychology Review. 2003;7:194–215. doi: 10.1207/S15327957PSPR0703_01. 10.1207/S15327957PSPR0703_01. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Voinescu L, Collins MA. “Wide-eyed” and “crooked-faced”: Determinates of perceived and real honesty across the life span. Personality and Social Psychology Bulletin. 1996;22:1258–1269. 10.1177/01461672962212006. [Google Scholar]


