Abstract
We describe monozygotic twin sisters, born to consanguineous Moroccan parents, who are highly discordant for the manifestations of Gaucher disease. Both carry Gaucher genotype N188S/N188S. One has severe visceral involvement, epilepsy, and a cerebellar syndrome. Her twin does not manifest any symptoms or signs of Gaucher disease but suffers from type 1 diabetes mellitus. The concurrence of a mild Gaucher mutation with a severe phenotype, as well as the occurrence of highly discordant phenotypes in a pair of monozygotic twins, is discussed.
Keywords: Gaucher disease, Monozygotic twins, Glucocerebrosidase, Seizures
Introduction
Gaucher disease is one of the most common lysosomal storage diseases resulting from mutations in the glucocerebrosidase gene located on chromosome 1q21. Intralysosomal accumulation of glucocerebroside in macrophages throughout the body leads to this multi-systemic disease. Based upon the presence or absence and rate of progression of neurological manifestations, Gaucher disease is divided into three types. Type 1 Gaucher disease can onset at any age and is characterized by visceral and/or skeletal involvement. Type 2 is known as ‘acute neuronopathic’ Gaucher disease, has the onset of severe central nervous system involvement in infancy, and usually leads to death by the age of 2 years. Type 3 Gaucher disease is known as ‘chronic neuronopathic’ Gaucher disease, with an onset of central nervous system involvement in childhood, adolescence, or early adulthood and a more indolent course.
More than 200 different mutations have been identified in patients with Gaucher disease, some of which are predictive of a particular phenotype. For example, the N370S mutation has only been found in patients with type 1 Gaucher disease, and homozygosity for L444P is associated with type 3 disease [2]. Conversely, patients with the same DNA mutations and even the same genotypes may exhibit marked variability in disease expression and severity; the R463C mutation, for example, is associated with type 1 as well as type 3 Gaucher disease [2]. In this report, we describe the case of a pair of monozygotic twin sisters, born to consanguineous Moroccan parents, who are highly discordant for the manifestations of Gaucher disease.
Case report
Twin A
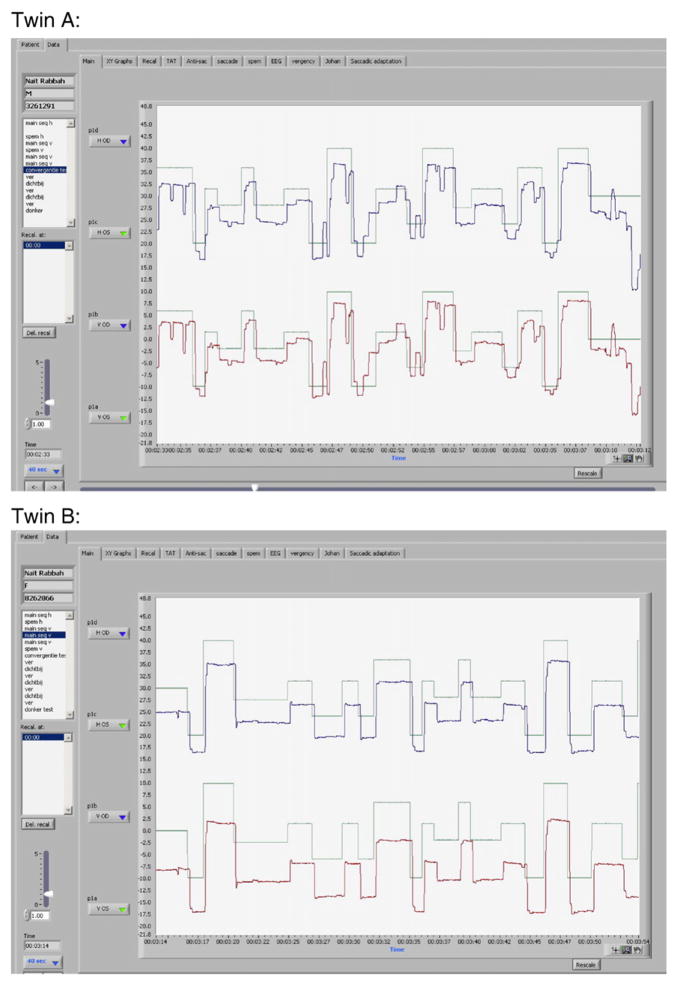
In 2003, a 22-year-old female presented at the internal medicine outpatient clinic with stomach pain and chronic fatigue. Physical examination revealed hepatosplenomegaly and subsequent laboratory investigations showed moderate cytopenia (platelets 97×109/l, Hb 11.3 g/dl). As cytopenia worsened, a bone marrow biopsy was performed, yielding typical Gaucher cells. Subsequently, the diagnosis of Gaucher disease was confirmed by establishing decreased glucocerebrosidase activity in leucocytes (1.9 nmol/mg/h (reference value 10–25 nmol/mg/h)). Mutation analysis revealed homozygosity for the N188S mutation. A chitotriosidase level of 30,000 nmol/ml/h (wt, reference value 7–187 nmol/ml/h) was found. A thorough history revealed that she had been experiencing 3 to 4 complex partial seizures per year until the age of 19 years. Enzyme replacement therapy with imiglucerase was started. After 3 years of therapy, her epilepsy recurred. Brain MRI did not show any abnormalities, but electroencephalography showed epileptic abnormalities. Valproic acid was started and after some time replaced by levetiracetam because of the persistence of seizures despite high doses. As our patient had Gaucher disease with severe visceral involvement together with seizures, reclassification to type 3 Gaucher disease was considered. Further neurological examination revealed signs of central nervous system involvement; she was unable to make rapid alternating movements with hands and feet and could not hop. Moreover, she had symmetric increased deep tendon reflexes with plantar down responses. Analysis of her eye movements showed dysmetric horizontal and vertical saccades, manifested by gaze-evoked horizontal and vertical square wave jerks (see Fig. 1).These changes are seen with cerebellar pathology. She had no difficulty with saccade initiation and slowed horizontal saccades, characteristic of type 3 Gaucher disease, were absent. Thirty months later, repeated neurological examination and eye movement analysis did not indicate further deterioration. Repeated brain MRIs, however, showed slight cerebellar atrophy. An extensive search for an underlying cerebellar disease was performed, including vitamin levels, thyroid function and mutation analysis of spinocerebellar ataxia and Friedreich’s ataxia genes. These were unremarkable except for an alteration in the SCA 13 gene (c.123G>T (p.Gln41His)). This mutation is not known to cause disease.
Fig. 1.
Horizontal eye movements. The patient is asked to follow a horizontally moving red dot. Green line= movement of red dot, blue line = movement of right eye, red line = movement of left eye.
Twin B
As her monozygous twin sister (monozygosity confirmed by DNA fingerprinting) was diagnosed with Gaucher disease, twin B was examined as well. The diagnosis of Gaucher disease was confirmed: glucocerebrosidase activity showed an identical decreased activity (1.4 nmol/mg/h) and homozygosity for the N188S mutation was established. A chitotriosidase level of 3000 nmol/ml/h was found being 10-fold less than her twin sister. To date, she has not developed any signs of Gaucher disease. Liver and spleen volumes are within the normal range, there are no signs of bone disease, and she has normal blood counts. Neurological examination and eye movement analysis revealed no abnormalities. Mutation analysis of spinocerebellar ataxia was not performed. At the age of 14, she was found to have type 1 diabetes mellitus and treatment with insulin was needed.
DNA analysis
Cells and DNA from the twins were evaluated at the National Institutes of Health, Bethesda. Total sequencing of all exons and flanking intronic regions of GBA confirmed the N188S mutation in both patients, and no other mutations, deletions, or rearrangements were found. To exclude the possibility of a GBA recombination event or GBA gene deletion, Southern blots were performed using the restriction enzymes Ssp1 and HincII [9], which showed normal results. The possibility of whole deletion or recombination outside of the two enzyme sites, however, has not been excluded. The sequence of the 5′ and the 3′ regions of GBA gene were normal. Furthermore, the DNA sequence from each sister was evaluated to detect potential alterations (i.e., acquired mutations) that could account for the difference in phenotype. Several intronic polymorphic sites were examined, but no differences were detected. LIMP-2 has recently been identified as a protein involved in the transport of glucocerebrosidase to the lysosome [5]. Sequence of the 12 exons of this gene was normal in both sisters, as well as the levels of LIMP-2 expression.
Discussion
A pair of female monozygotic twins is described where one has symptomatic type 1 Gaucher disease, while the other lacks any Gaucher symptoms at all. The glucocerebrosidase genotype identified in these twin sisters and the highly discordant phenotypes despite monozygosity are both of interest.
In 1996, the N188S mutation was first identified in a series of Korean and Chinese patients with Gaucher disease, and the three patients described had a rather mild phenotype [1]. In the following years, at least fifteen other heterozygous cases have been described [2,8]. Most of them had Gaucher type 3 disease with or without myoclonic epilepsy, and in these patients, the second mutant allele was a null mutation. Since homozygosity for null mutations results in early lethality [7], the second allele encountered in individuals heterozygous for a null allele is presumably less severe. In contrast to the abovementioned cases, twin A has a severe phenotype despite homozygosity for this presumably mild N188S mutation. It has been known for many years that the same DNA mutations can cause different types or clinical presentations of Gaucher disease [6]. Even twins with varying degrees of disease severity have been described [3], suggesting that additional factors contribute to the Gaucher phenotype. It is increasingly clear that modifiers and environmental factors are critical in defining the phenotype [2,6].
The notable different degree of disease severity between the twin sisters is puzzling. Monozygotic twins are considered to be genetically identical. However, variable contribution of intrauterine environmental exposures to the epigenome of different tissues may affect the phenotypic variability of a disease [4]. Besides, genetic alterations increase during life as a result of non-shared environmental influences and such an acquired mutation might account for the different penetrance of the mutant alleles. DNA from the twins was evaluated for acquired mutations that could account for the discordant phenotypes, but none were found. Furthermore, few differences in environmental factors can be established in these two sisters; they have co-habited all their lives and serological studies showed that both had IgG antibodies to Epstein-Barr virus (EBV) and cytomegalovirus (CMV). The only discordant medical history is that twin B has suffered from diabetes since the age of 14. It is difficult to understand how diabetes could have influenced Gaucher disease expression.
This report on identical twins emphasizes the limitations of the predictive value of genotyping, confirming the role of modifiers and/ or environmental factors on the initiation and progression of Gaucher disease. Further evaluation of potential factors contributing to the phenotypical differences in these twin sisters may provide insight into the role of modifiers and environmental factors in the development of clinical Gaucher disease.
References
- 1.Kim JW, Liou BB, Lai MY, et al. Gaucher disease: identification of three new mutations in the Korean and Chinese (Taiwanese) populations. Hum Mutat. 1996;7:214–218. doi: 10.1002/(SICI)1098-1004(1996)7:3<214::AID-HUMU5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 2.Koprivica V, Stone DL, Park JK, et al. Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet. 2000;66:1777–1786. doi: 10.1086/302925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lachmann RH, Grant IR, Halsall D, Cox T. Twin pairs showing discordance of phenotype in adult Gaucher’s disease, Q JM : Mon. J Assoc Physicians. 2004;97:199–204. doi: 10.1093/qjmed/hch036. [DOI] [PubMed] [Google Scholar]
- 4.Ollikainen M, Smith KR, Joo EJ, et al. DNA methylation analysis of multiple tissues from newborn twins reveals both genetic and intrauterine components to variation in the human neonatal epigenome. Hum Mol Genet. 2010;19:4176–4188. doi: 10.1093/hmg/ddq336. [DOI] [PubMed] [Google Scholar]
- 5.Reczek D, Schwake M, Schröder J, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Stone DL, Tayebi N, Orvisky E, et al. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15:181–188. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Tajima A, Ohashi T, Hamano S, et al. Gaucher disease patient with myoclonus epilepsy and a novel mutation. Pediatr Neurol. 2010;42:65–68. doi: 10.1016/j.pediatrneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Tayebi N, Stubblefield BK, Park JK, et al. Reciprocal and nonreciprocal recombination at the glucocerebrosidase gene region: implications for complexity in Gaucher disease. Am J Hum Genet. 2003;72:519–534. doi: 10.1086/367850. [DOI] [PMC free article] [PubMed] [Google Scholar]