Abstract
Background and aims
The cumulative incidence of and risk factors for perianal Crohn’s disease for findings other than fistulas are unknown.
Methods
The medical records of 310 incident cases of Crohn’s disease from Olmsted County, Minnesota, diagnosed between 1970 and 2004, were reviewed for evidence of perianal disease findings other than fistulas. Cumulative incidence was estimated using the Kaplan-Meier method, and associations between baseline factors and time to first event were assessed using proportional hazards regression. Four types of lesions were studied: anorectal strictures, deep anal canal ulcers, anal fissures, and perianal skin tags.
Results
The 10-year cumulative probability from time of diagnosis was 5.8% (95% confidence interval [CI], 2.6%-8.8%) for anorectal strictures, 6.6% (3.6%-9.6%) for deep anal canal ulcers, 10.5% (6.8%-14.1%) for anal fissures, and 18.7% (13.9%-23.3%) for perianal skin tags. The cumulative probability for any perianal lesion other than fistulas was 21.3% (16.5%-25.8%) at 5 years and 29.2% (23.5%-34.5%) at 10 years. Baseline factors associated with time to first perianal lesion other than fistulas were age (hazard ratio [HR] per 10 years, 0.9; 95% CI, 0.8-0.98; p=0.026), female gender (HR, 1.7; 95% CI, 1.1-2.7; p=0.013), and presence of extraintestinal manifestations (HR, 1.7; 95% CI, 1.03-2.8; p=0.038).
Conclusion
Perianal lesions other than fistulas occurred frequently during the clinical course of Crohn’s disease. Female gender and extraintestinal manifestations were associated with increased risks for perianal lesions other than fistulas, while older age at diagnosis was associated with a slightly decreased risk.
Keywords: Crohn’s disease, natural history, anorectal strictures, anal ulcers, perianal tags, anal fissures
INTRODUCTION
Perianal Crohn’s disease encompasses both fistulizing lesions (fistulas, abscesses and rectovaginal fistulas) and findings other than fistulas (anal fissures, deep anal canal ulcers, anorectal strictures, perianal skin tags, hemorrhoids and cancer) and. Primary lesions are caused by intestinal inflammation and include superficial fissures, deep cavitating anal canal ulcers and lymphedema1, 2. Secondary lesions are fistulas and abscess. Strictures are associated with primary or secondary perianal lesions or are a long-term consequence of the inflammatory process1, 2. Hemorrhoids are incidental lesions that are not directly related to Crohn’s disease.
Most attention to perianal Crohn’s disease has focused on the diagnosis, epidemiology and treatment of perianal abscess and fistula. By contrast, few data exist on perianal lesions other than fistulas. Available data suggest that perianal lesions other than fistulas may occur frequently during the course of Crohn’s disease2. However, only referral center studies have reported the epidemiology of perianal Crohn’s disease findings other than fistulas in adults. Anorectal strictures and anal canal ulcerations were reported in 7 to 9%3-5 and 10 to 50%4-11 of patients with Crohn’s disease patients, respectively. A recent pediatric inception cohort study found that among 276 children with Crohn’s disease, 13 (4.7%) had only perianal skin tags and anal fissures within 30 days of diagnosis12. Overall, the cumulative incidence of and risk factors for lesions other than fistulas are unknown.
Perianal Crohn’s disease lesions other than fistulas can be disabling and often requires medical or surgical treatment2. A better knowledge of their natural history may help physicians managing these lesions. The aim of this study was therefore to estimate the cumulative incidence of and assess factors potentially associated with perianal Crohn’s disease findings other than fistulas in a well-defined population-based cohort.
METHODS
Patient Selection
The population-based inception cohort of subjects with Crohn’s disease in Olmsted County, Minnesota, has been previously described 13, 14 and was updated through July 2009 for this study.
Study Setting
Olmsted County is situated in southeastern Minnesota and had approximately 124,000 people at the 2000 U.S. Census. In 2000, 89% of the population was non-Hispanic white. Although 25% of county residents are employed in health care services (vs 8% nationwide), and the level of education is higher (30% have completed college vs 21% nationwide), the residents of Olmsted County are otherwise socioeconomically similar to the U.S. white population15.
Rochester Epidemiology Project
The Rochester Epidemiology Project (REP) is a unique medical records linkage system developed in the 1960s and funded in part by the National Institutes of Health. It exploits the fact that virtually all of the health care for the residents of Olmsted County is provided by two organizations: Mayo Medical Center (Mayo Clinic and its hospitals, Rochester Methodist and Saint Marys); and Olmsted Medical Center (a multispecialty clinic and its hospital, Olmsted Community Hospital). Diagnoses generated from all outpatient visits, emergency room visits, hospitalizations, nursing home visits, surgical procedures, diagnostic studies, autopsy examinations, and death certificates for all county residents seen at these two institutions as well as a small number of additional health care providers serving the local population have been recorded in a central diagnostic index since 1908 15, 16. In any three-year period, over 90% of county residents are examined at one of the two major health care systems15. Thus, it is possible to identify all diagnosed cases of a given disease, and all diagnostic examinations, including radiological studies, performed in these subjects. The resources of the REP have been used to identify Olmsted County residents diagnosed with Crohn’s disease from 1940 to 200413, 14.
Case Ascertainment
With approval from the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center, the records of 310 patients who had not denied permission for research access to their medical records and who were first diagnosed with Crohn’s disease between January 1, 1970 and December 31, 2004 were reviewed. Demographic information including date of birth, date of symptom onset, date of diagnosis, disease type and extent were previously determined13, 14. Beginning with the date of diagnosis, all clinical, endoscopic and proctologic records were reviewed to identify the presence of perianal lesions other than fistulas. Four types of lesions were studied: anorectal strictures, deep anal canal ulcers, anal fissures, and perianal skin tags. Patients were followed through their medical records from the date of symptom onset to the date of last follow-up in their medical record or up to July 2009.
Statistical Analysis
The cumulative incidence (1 minus survival free) of each type of perianal lesion was estimated using the Kaplan-Meier method, and 95% confidence intervals (CI) estimated using the log transform method (with modified lower limit). The associations between baseline demographic and clinical factors and time from diagnosis to initial occurrence (including those present at diagnosis) of specific findings (anorectal strictures, deep anal canal ulcers, anal fissures, perianal skin tags, and separately, the earliest of these) were assessed using Cox proportional hazards regression models. The risks for specific findings were summarized as hazard ratios (HR), [95% CI] calculated from the estimated coefficients (and their standard errors) in these models.
RESULTS
Baseline Characteristics (Table 1)
Table 1.
Baseline characteristics of the 310 patients at the time of Crohn’s disease diagnosis.
| Characteristic | N (%) |
|---|---|
| Gender | |
| Male | 156 (50.3) |
| Female | 154 (49.7) |
| Age | |
| Less than 16 years (A1) | 33 (10.6) |
| Age between 17 and 40 years (A2) | 177 (57.1) |
| Age more than 40 years (A3) | 100 (32.3) |
| Disease Location | |
| Small Bowel (L1) | 96 (31.2) |
| Colitis (L2) | 102 (33.1) |
| Ileocolonic (L3) | 103 (33.4) |
| Gastroduodenal (L4) | 7 (2.3) |
| Not documented | 2 |
| Disease behavior | |
| Non-Stricturing/Non-Penetrating (B1) | 248 (81.3) |
| Stricturing (B2) | 14 (4.6) |
| Penetrating (B3) | 43 (14.1) |
| Not documented | 5 |
| Perianal Disease prior to or within 90 days of diagnosis | |
| Yes | 51 (16.7) |
| No | 254 (83.3) |
| Smoking history | |
| Current smokers | 98 (32.9) |
| Former smokers | 45 (15.1) |
| Non-smokers | 155 (52.0) |
| Not documented | 12 |
| Family History of IBD | |
| Yes | 43 (14.4) |
| No | 255 (85.6) |
| Not documented | 12 |
| Extra-intestinal manifestation | |
| Yes | 46 (15.1) |
| No | 259 (84.9) |
| Not documented | 5 |
Half of the 310 patients were male. Corresponding to the Montreal classification17, only 10.6% of patients were diagnosed below the age of 16 years (A1), 57.1% of patients had an age between 17 and 40 years (A2), and approximately one third of patients had an age at diagnosis above 40 years (A3). Approximately one third of patients each had ileitis, colitis, or ileocolitis at the time of diagnosis. Approximately 8 out of 10 patients had nonstricturing, nonpenetrating disease (B1), almost 5% of patients had stricturing disease (B2) and 15% had penetrating disease (B3). Perianal disease was noted in 16.7% of patients prior to or within 90 days of Crohn’s disease diagnosis. Half of patients were non-smokers, while one third of patients were current smokers. A familial history of IBD was reported by 15% of patients. Extra-intestinal manifestations at the time of diagnosis were noted in 15% of patients.
Cumulative Incidence of Perianal Lesions Other Than Fistulas
Among the 310 patients, 22 subjects had an anorectal stricture at the time of or after the diagnosis of Crohn’s disease. Only two patients developed anorectal strictures within the first year of diagnosis. In one patient, the stricture was not passable by the examining finger or endoscope. The cumulative probability of developing an anorectal stricture from the time of diagnosis was 1.0% (95% CI, 0.2%-2.2%), 5.8% (2.6%-8.8%) and 17.5% (8.2%-27.8%) at 5, 10 and 30 years, respectively (Figure 1a).
Figure 1.
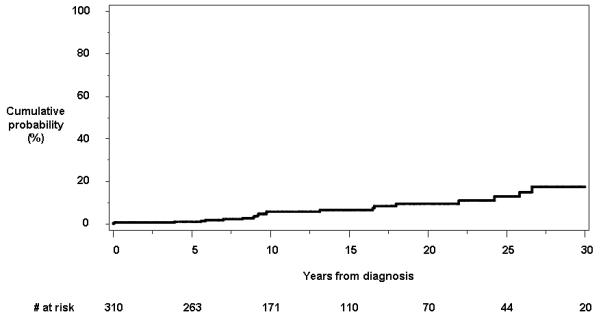
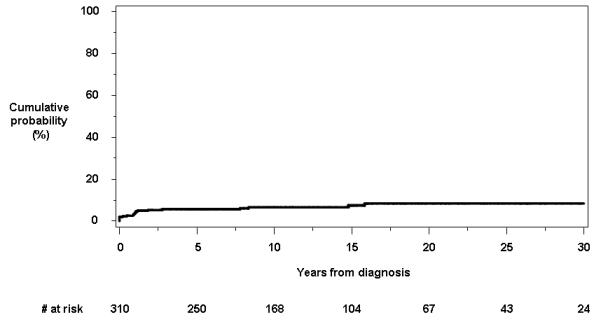
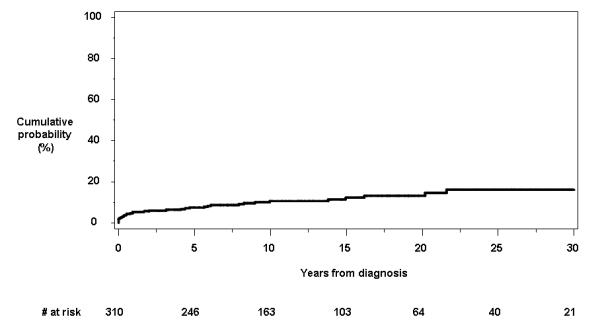
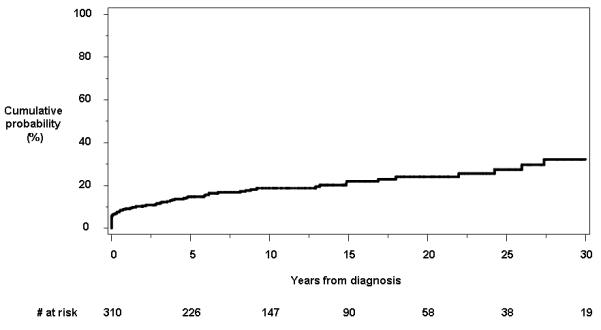
Cumulative probability of developing individual perianal findings other than fistulas from the time of diagnosis of Crohn’s disease: a) anorectal strictures; b) anal canal ulcers; c) anal fissures; and d) perianal skin tags.
Beginning with the date of diagnosis, a total of 21 patients developed at least one deep anal canal ulcer. Eleven individuals had an anal canal ulcer within the first year of diagnosis. In 15 patients (71.4%), the anal canal ulcer was symptomatic. The cumulative probability of having or developing an anal canal ulcer at or from time of diagnosis was 5.6% (95% CI, 3.0%-8.3%), 6.6% (3.6%-9.6%) and 8.4% (4.5%-15.6%) at 5, 10 and 30 years, respectively (Figure 1b).
Thirty-four patients had or developed anal fissures at the time of (n=5) or after (n=29) the diagnosis of Crohn’s disease. Sixteen individuals had anal fissures within the first year of diagnosis. In 29 out of 34 patients (85.3%), anal fissures were symptomatic. The cumulative probability of having anal fissures at or following the date of diagnosis was 7.3% (95% CI, 4.3%-10.3%), 10.5% (6.8%-14.1%), and 16.0% (9.8%-24.9%) at 5, 10 and 30 years, respectively (Figure 1c).
A total of 63 patients had or developed perianal skin tags at the time of or after Crohn’s disease diagnosis. Twenty-eight individuals had perianal skin tags within the first year of diagnosis. In 32 of these patients (50.8%), the perianal skin tags were symptomatic. The cumulative probability of having perianal skin tags at or following the date of diagnosis was 14.7% (95% CI, 10.6%-18.7%), 18.7% (13.9%-23.3%) and 32.2% (22.1%-42.3%) at 5, 10 and 30 years, respectively (Figure 1d).
For the 93 patients who developed at least one of anorectal stricture, deep anal canal ulcer, perianal fissure, and/or perianal skin tags, the cumulative probability of having or developing any perianal lesion other than fistulas (earliest of the four) was 21.3% (95% CI, 16.5%-25.8%), 29.2% (23.5%-34.5%) and 44.8% (34.0%-57.4%) at 5, 10 and 30 years, respectively (Figure 2).
Figure 2.
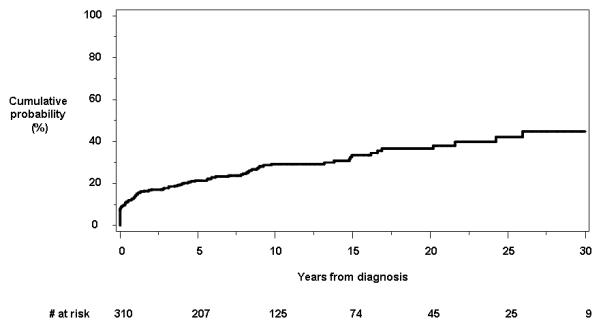
Cumulative probability of developing any type of perianal lesions other than fistulas from time of diagnosis.
Risk Factors for Perianal Lesions Other Than Fistulas
We assessed potential associations between time to perianal lesion other than fistulas and 6 baseline characteristics: age, gender, disease location, smoking status, family history of inflammatory bowel disease, and the presence of extra-intestinal manifestations. In univariate proportional hazards regression analyses, the only baseline factor associated with time to first anal canal ulcer was the presence of extraintestinal manifestations (HR, 2.9; 95% CI, 1.2-7.2; p=0.02). None of the factors studied was associated with time to first anal fissure. The baseline factors univariately associated with time to first anal canal ulcer or anal fissure were age (HR per 10 years, 0.8; 95% CI, 0.7-0.96; p=0.018) and the presence of extraintestinal manifestations (HR, 2.5; 95% CI, 1.4-4.5; p=0.003). The baseline factors univariately associated with time to first perianal skin tags were female gender (HR, 2.9; 95% CI, 1.7-5.1; p<0.001), former cigarette smoker status relative to non-smokers HR, 0.2; 95% CI, 0.05-0.87; p=0.03), and the presence of extraintestinal manifestations (HR, 2.9; 95% CI, 1.7-5; p<0.001). The baseline factors univariately associated with time to earliest of any perianal lesion other than fistulas were age (HR per 10 years, 0.8; 95% CI, 0.7-0.97; p=0.017), female gender (HR, 1.9; 95% CI, 1.2-2.8; p=0.004), and the presence of extraintestinal manifestations (HR, 2; 95% CI, 1.2-3.3; p=0.005) (Table 2).
Table 2.
Univariate associations between baseline factors and time to earliest of any perianal lesion other than fistulas in 310 patients from Olmsted County, Minnesota.
| Characteristic | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Gender | |||
| Male | 1.0w | Ref. | |
| Female | 1.9 | 1.2-2.8 | 0.004 |
| Age | 1.0 | 0.99-1.9 | 0.43 |
| Disease Location | |||
| Small Bowel (L1) | 0.7 | 0.4-1.3 | 0.27 |
| Colitis (L2) | 1.0 | Ref. | |
| Ileocolonic (L3) | 1.0 | 0.6-1.6 | 0.1 |
| Gastroduodenal (L4) | 0.9 | 0.2-4 | 0.96 |
| Disease behavior at the 90-day baseline | |||
| Non-Stricturing/Non-Penetrating (B1) | 1.0 | Ref. | |
| Stricturing (B2) | 0 | 0 | 1 |
| Penetrating (B3) | 0.7 | 0.3-1.3 | 0.2 |
| Smoking history | |||
| Current smokers | 1.3 | 0.8-2 | 0.26 |
| Former smokers | 0.5 | 0.2-1.1 | 0.08 |
| Non-smokers | 1.0 | Ref. | |
| Family History of IBD | |||
| Yes | 1.0 | 0.5-1.7 | 0.9 |
| No | 1.0 | Ref. | |
| Extra-intestinal manifestation | |||
| Yes | 2 | 1.2-3.3 | 0.005 |
| No | 1.0 | Ref. | |
In a multiple variable proportional hazards regression model, the baseline factors independently associated with time to earliest of any perianal lesion other than fistulas were age (HR per 10 years, 0.9; 95% CI, 0.8-0.98; p=0.026), female gender (HR, 1.7; 95% CI, 1.1-2.7; p=0.013), and the presence of extraintestinal manifestations (HR, 1.7; 95% CI, 1.03-2.8; p=0.038) (Table 3).
Table 3.
Multiple variable model retaining significant baseline factors for time to earliest of any perianal lesion other than fistulas in 310 patients from Olmsted County, Minnesota.
| Characteristic | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Gender | |||
| Male | 1.0 | Ref | |
| Female | 1.7 | 1.1-2.7 | 0.013 |
| Age per 10 years | 0.9 | 0.8-0.98 | 0.026 |
| Extra-intestinal manifestation | |||
| Yes | 1.7 | 1.03-2.8 | 0.038 |
| No | 1.0 | Ref. | |
DISCUSSION
This is the first study investigating the cumulative incidence of and risk factors for perianal Crohn’s disease other than fistulas in a population-based cohort. One pediatric population-based study assessed the frequency of perianal lesions at Crohn’s diagnosis12. All other available studies on the epidemiology of non-fistulizing perianal lesions were referral center-based2. In addition, most studies focused on prevalence and clinical course of these lesions or only reported their crude incidence rate2. The cumulative incidence of perianal Crohn’s disease other than fistulas remained unknown.
In this population-based cohort, the cumulative probability of developing anorectal strictures from time of diagnosis was 5.8% (95% CI, 2.6%-8.8%) at 10 years. This figure is consistent with findings among 754 patients with Crohn’s disease seen between 1960 and 1965 at Mayo Clinic, Rochester, Minnesota, US, where approximately 7% patients had anorectal stenosis5. Anorectal stricture occurred in 12 of 160 patients (7.5%) with Crohn’s disease involving the colon and seen at The Mount Sinai Hospital, New York, US3. Among 202 patients with Crohn’s disease who had been examined in Birmingham, United Kingdom, during the year 1984 to assess the frequency of perianal disease, 19 (9%) had anal stricture4.
In this population-based cohort, the cumulative probability of having a deep anal canal ulcer or an anal fissure from time of diagnosis was 17.1% (95% CI, 12.5-21.6) at 10 years. Among 754 patients with Crohn’s disease seen between 1960 and 1965 at Mayo Clinic, Rochester, Minnesota, US, approximately 10% patients had anal fissures5. Among 233 new patients with Crohn’s disease referred to Saint Mark’s Hospital, London, UK, between 1977 and 1983, 61 (26%) had anal fissures7. Among 202 patients with Crohn’s disease who had been examined during the year 1984 to assess the frequency of perianal disease, 12 (6%) had anal ulcers and 38 (19%) had anal fissures4. Among 153 patients attending a Crohn’s follow-up clinic at the Department of surgery in Birmingham, England, 53 (35%) had developed deep anal canal ulceration8. The charts of 1,098 patients seen at the Lahey Clinic between 1957 and 1978 were reviewed; anal fissures were noted in 29% of patients9. By reviewing the case notes of 151 consecutive patients with Crohn’s disease presenting to surgeons, Hobbiss and Schofield identified 23 (15%) patients who had anal canal ulceration10. In a retrospective study, among 225 patients with luminal Crohn’s disease anal canal ulcerations were present in 50% of patients6. Among 101 patients consecutively referred to a French referral center for active Crohn’s disease between 1991 and 1994 and prospectively evaluated by experienced proctologists, 42.5% had anal canal ulceration11.
While anal canal ulcerations are classically described as painless, pain has been reported up to 70% of patients in referral center-based series7, 18. We found that 71.4% and 85.3% of patients with Crohn’s disease had symptomatic anal canal ulcers and anal fissures, respectively. This is line with the results of a retrospective study from a US referral center showing that anal fissures were symptomatic in 84% of cases18 and a French prospective study in which anal canal ulcers were associated with unremitting pain in 56% of cases11.
In this population-based cohort, the cumulative probability of having perianal skin tags was 18.7% (95% CI, 13.9-23.3) at 10 years. Among 153 patients attending a Crohn’s follow-up clinic at the Department of surgery in Birmingham, England, 37 (24.2%) had perianal skin stags associated with anal fissures or fistulas8. Among 202 patients with Crohn’s disease who had been examined during the year 1984 to assess the frequency of perianal disease, 75 (37%) had perianal skin tags4, and that 86% of these lesions were asymptomatic4. Recently, perianal skin tags were photographed in 170 consecutive patients with inflammatory bowel disease and perianal skin tags in the course of 1 year19. Perianal skin tags were found in 75.4% of patients with Crohn’s disease19. In contrast to anorectal strictures and anal canal ulcerations, perianal skin tags are less often symptomatic and usually do not require specific management. In this population-based cohort, the proportion of symptomatic perianal skin tags was relatively high, occurring in half of the 63 patients who developed this type of lesions after Crohn’s diagnosis. It is therefore possible that physicians pay less attention to these lesions and do not systematically mention their presence on proctology and endoscopy reports. Therefore, even though we confirmed that the most common manifestation was of perianal skin tags, the frequency of perianal skin tags might have been underestimated in our population-based study.
To our knowledge, the risk factors for having perianal Crohn’s disease other than fistulas have never been formally assessed. Due to the relatively small number of events, we were only able to assess for risk factors for developing any type of lesions other than fistulas (anorectal strictures, anal canal ulcers, anal fissures, and/or perianal skin tags). Among baseline characteristics, we identified female gender as having the strongest association with lesions other than fistulas in multivariate analysis. Females were more likely to develop any lesion other than fistulas. Two risk factors were positively associated with the development of lesions other than fistulas, namely age and presence of extraintestinal manifestations. However, due to low statistical significance, this remains to be confirmed in independent population-based cohorts.
In conclusion, perianal lesions other than fistulas occurred frequently during the clinical course of Crohn’s disease, with a cumulative risk of almost 30% at 10 years. Female gender, extraintestinal manifestations and age were negatively or positively related to the presence of perianal lesions other than fistulas.
AUTHOR INVOLVEMENT.
Peyrin-Biroulet: Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript.
Loftus: Study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtaining funding, study supervision.
Tremaine: Critical revision of the manuscript.
Harmsen: Analysis and interpretation of data, critical revision of the manuscript.
Zinsmeister: Analysis and interpretation of data, critical revision of the manuscript.
Sandborn: Study concept and design, critical revision of the manuscript, obtaining funding.
Acknowledgments
Supported by the Mayo Foundation for Medical Education and Research, and made possible by the Rochester Epidemiology Project (Grant Number R01 AG034676 from the National Institute on Aging).
Footnotes
Presented in part at the Annual Meeting of the American Gastroenterological Association, New Orleans, LA, May 1-5, 2010 (Gastroenterology 2010;138(5 Suppl 1):S-199).
No conflicts of interest to disclose.
REFERENCES
- 1.Ingle SB, Loftus EV., Jr The natural history of perianal Crohn’s disease. Dig Liver Dis. 2007;39:963–9. doi: 10.1016/j.dld.2007.07.154. [DOI] [PubMed] [Google Scholar]
- 2.Bouguen G, Siproudhis L, Bretagne JF, Bigard MA, Peyrin-Biroulet L. Nonfistulizing perianal Crohn’s disease: Clinical features, epidemiology, and treatment. Inflamm Bowel Dis. 2010;16:1431–42. doi: 10.1002/ibd.21261. [DOI] [PubMed] [Google Scholar]
- 3.Greenstein AJ, Sachar DB, Kark AE. Stricture of the anorectum in Crohn’s disease involving the colon. Ann Surg. 1975;181:207–12. doi: 10.1097/00000658-197502000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keighley MR, Allan RN. Current status and influence of operation on perianal Crohn’s disease. Int J Colorectal Dis. 1986;1:104–7. doi: 10.1007/BF01648416. [DOI] [PubMed] [Google Scholar]
- 5.Wolff BG, Culp CE, Beart RW, Jr., Ilstrup DM, Ready RL. Anorectal Crohn’s disease. A long-term perspective. Dis Colon Rectum. 1985;28:709–11. doi: 10.1007/BF02560279. [DOI] [PubMed] [Google Scholar]
- 6.Bernard D, Morgan S, Tasse D. Selective surgical management of Crohn’s disease of the anus. Can J Surg. 1986;29:318–21. [PubMed] [Google Scholar]
- 7.Sweeney JL, Ritchie JK, Nicholls RJ. Anal fissure in Crohn’s disease. Br J Surg. 1988;75:56–7. doi: 10.1002/bjs.1800750120. [DOI] [PubMed] [Google Scholar]
- 8.Buchmann P, Keighley MR, Allan RN, Thompson H, Alexander-Williams J. Natural history of perianal Crohn’s disease. Ten year follow-up: a plea for conservatism. Am J Surg. 1980;140:642–4. doi: 10.1016/0002-9610(80)90048-3. [DOI] [PubMed] [Google Scholar]
- 9.Williams DR, Coller JA, Corman ML, Nugent FW, Veidenheimer MC. Anal complications in Crohn’s disease. Dis Colon Rectum. 1981;24:22–4. doi: 10.1007/BF02603444. [DOI] [PubMed] [Google Scholar]
- 10.Hobbiss JH, Schofield PF. Management of perianal Crohn’s disease. J R Soc Med. 1982;75:414–7. doi: 10.1177/014107688207500609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siproudhis L, Mortaji A, Mary JY, Juguet F, Bretagne JF, Gosselin M. Anal lesions: any significant prognosis in Crohn’s disease? Eur J Gastroenterol Hepatol. 1997;9:239–43. doi: 10.1097/00042737-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Keljo DJ, Markowitz J, Langton C, Lerer T, Bousvaros A, Carvalho R, Crandall W, Evans J, Griffiths A, Kay M, Kugathasan S, LeLeiko N, Mack D, Mamula P, Moyer MS, Oliva-Hemker M, Otley A, Pfefferkorn M, Rosh J, Hyams JS. Course and treatment of perianal disease in children newly diagnosed with Crohn’s disease. Inflamm Bowel Dis. 2009;15:383–7. doi: 10.1002/ibd.20767. [DOI] [PubMed] [Google Scholar]
- 13.Loftus EV, Jr., Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–8. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 14.Loftus CG, Loftus EV, Jr., Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, 3rd, Sandborn WJ. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254–61. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 16.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245:54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 17.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleshner PR, Schoetz DJ, Jr., Roberts PL, Murray JJ, Coller JA, Veidenheimer MC. Anal fissure in Crohn’s disease: a plea for aggressive management. Dis Colon Rectum. 1995;38:1137–43. doi: 10.1007/BF02048328. [DOI] [PubMed] [Google Scholar]
- 19.Bonheur JL, Braunstein J, Korelitz BI, Panagopoulos G. Anal skin tags in inflammatory bowel disease: new observations and a clinical review. Inflamm Bowel Dis. 2008;14:1236–9. doi: 10.1002/ibd.20458. [DOI] [PubMed] [Google Scholar]


