Abstract
A 60-year old woman presented with dyspnoea and fatigue. She was frail and cachectic (BMI 17.5) with a pancytopenia. Previously she had received chemotherapy for chronic lymphatic leukaemia. She relapsed one year ago necessitating a reduced intensity conditioning allogeneic haematopoietic cell transplantation. Subsequently, graft versus host disease required high-dose immunosuppressants. Computerized tomography on admission showed bilateral lung nodules and a suspicious cardiac mass. Bronchial biopsies demonstrated abundant hypae consistent with Aspergillus fumigatus infection. Echocardiography demonstrated a large fungus ball attached to the right coronary cusp of the aortic valve with near complete obliteration of the left ventricular outflow tract. Due to the high risk of embolization this was resected under cardiopulmonary bypass. The mass was attached subvalvularly to the ventricular septal free wall and eroding through it. It peeled off leaving intact aortic leaflets. Unresectable fungal deposits were discovered on the interventricular septum, the left ventricle free wall and posterior aortic wall. High-dose systemic antifungal therapy (Voriconazole and Amphoteracin B) was given for 4 months. After discharge she remained well till a 4-month follow-up, after which she eventually succumbed to her disease. We discuss the clinical difficulties in managing patients with fungal infective endocarditis and present a brief review of cardiac aspergillosis management.
Keywords: Aspergillosis, Infective endocarditis, Aortic valve
INTRODUCTION
Invasive cardiac aspergillosis is a severe and extremely life-threatening disease with few reported cases [1]. It is commonly described in transplant patients who are immunosuppressed [2]. It may present as a single organ disease or with ‘malignant’ disseminated form to locations such as the lung, kidneys, brain and retroperitoneium. The burden of diagnosis still lies with clinicians: they need a high index of suspicion the entity by understanding the organisms and the patients who are likely to be infected. We present an exceptional case with near complete obliteration of the left ventricular outflow tract (LVOT), followed by complete resection and survival.
CASE
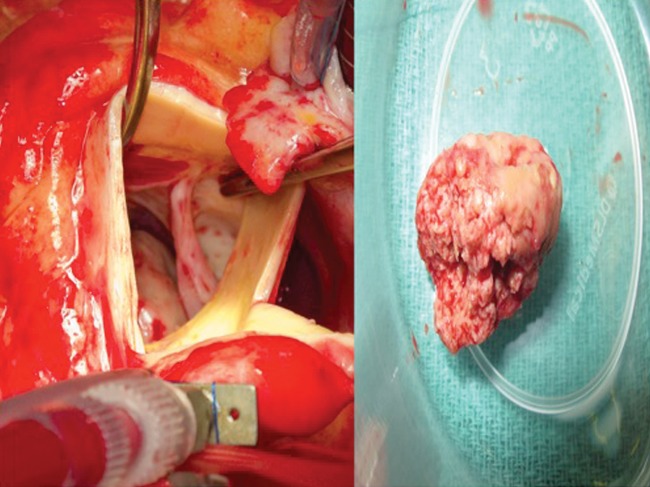
A 60-year old woman presented with dyspnoea and fatigue. Having been diagnosed with chronic lymphatic leukaemia 5 years previously, she was treated with Fludarabine, Cyclophosphamide and Alemtuzumab. She relapsed one year ago necessitating a reduced intensity conditioning allogeneic haematopoietic cell transplantation using matched sibling and unrelated donors. Unfortunately, she developed graft versus host disease requiring high-dose steroids and Mycophenolate mofetil. The patient was frail and cachectic on examination (BMI 17.5). She was pancytopenic on admission. CT as part of a septic screen demonstrated bilateral lung nodules (Fig. 1) with biopsies at bronchoscopy demonstrating abundant hypae consistent with Aspergillus fumigatus infection. Echocardiography demonstrated a large filling defect (2.5 cm × 1.7 cm) with near obliteration of the LVOT. This was attached to the right coronary cusp of the aortic valve (Fig. 2, Supplementary Videos 1 and 2). The patient subsequently developed endopthalmitis and a brain computer tomogram demonstrated cerebral microemboli. Due to the high risk of further embolization she was offered surgical excision of the mass and consented to aortic valve replacement. Her logistic EuroSCORE was 42.97% with an additive score of 14. She underwent excision of the left ventricular mass under cardiopulmonary bypass. The mass was attached subvalvularly to ventricular septal free wall and eroding through it (Fig. 2). The fungal mass peeled off the aortic leaflets easily leaving a morphologically normal native aortic valve, hence not necessitating an aortic valve replacement. The cardiopulmonary bypass time was 58 min with an aortic cross-clamp time of 33 min. She had evidence of disseminated invading fungal deposits on the interventricular septum, epicardial surface of the left ventricle and eroding fungus into the posterior aortic wall. The fungal spread was too extensive for complete debridement. She was returned to intensive care where she required a resteronotomy on day one post-operatively to evacuate haematoma. The microbiology from the excised specimen confirmed a diagnosis of Aspergillus fumigatus. Despite a prolonged stay of two months in intensive care the patient made a full recovery on high dose systemic antifungal therapy (Voriconazole and Amphoteracin B). She remained well till a 4-month follow-up, after which she eventually succumbed to her disease.
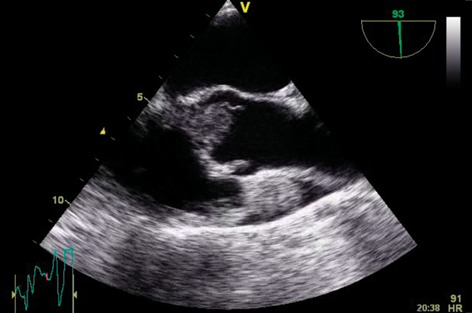
Supplementary Video 1: Parasternal long-axis view demonstrating the near complete LVOT obstruction with the fungal mass.
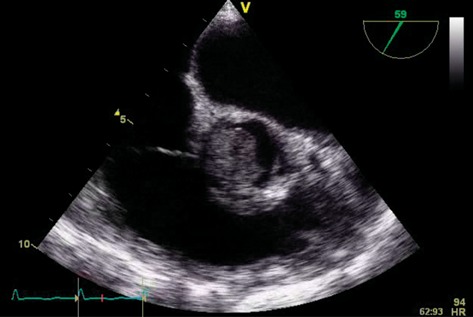
Supplementary Video 2: Parasternal short-axis view demonstrating the fungus mass association with aortic valve leaflets.
Figure 1:
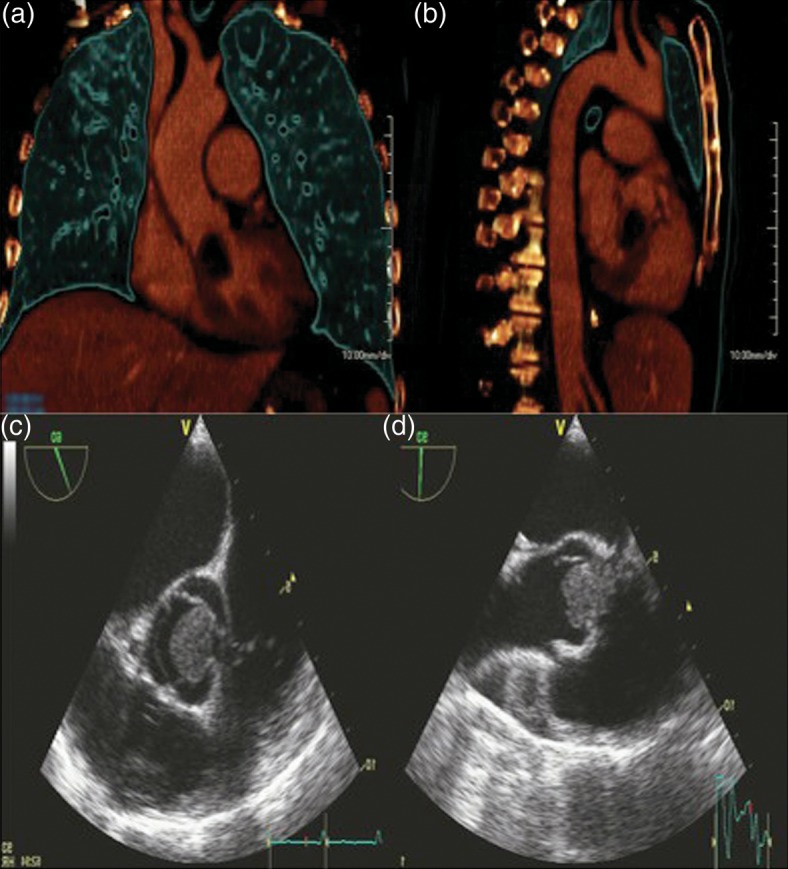
3D reconstruction of coronal and sagittal views of the chest demonstrating the large cardiac Aspergilloma. Parasternal long- and short-axis views on transthoracic echocardiography showing LVOT mass.
Figure 2:
Intra-operative transaortic view of the aortic valve after Aspergillus mass resection. The fungus mass that was removed can be seen.
DISCUSSION
Fungal endocarditis is a rare but increasingly prevalent entity. It is usually seen in patients receiving broad-spectrum antibiotics or invasive interventions including long-term central venous catheters or prosthetic heart valves [1]. More than 180 different species of Aspergillus have been described to date. The conidia are readily aerosolized and cause pulmonary infection as in our case. Physical barriers such as the mucociliary clearance are less effective against Aspergillus fumigatus, which has small, 2–3 µm, hypae. It is therefore a most invasive form of disease, most commonly involving the lung (91%), followed by the central nervous system and rarely the heart [3]. The incidence of cardiac Aspergillosis varies in the literature from 0.7 to 6% amongst the immunosuppressed [4, 5]. The usual mode of spread of cardiac aspergillosis is haematogenous from the pulmonary vascular bed or directly. Skin lesions can spread to the right heart in intravenous drug users [6].
The clinical presentation as in our case is usually with septic symptoms including fevers, night sweats and lethargy. Cardiac dysfunction with symptoms of cardiac failure and embolic phenomena ensue. Most reported cases of cardiac aspergillosis are diagnosed as an autopsy finding and Aspergillus is not normally cultured from post-mortem blood samples. As in our case, direct tissue biopsy (for instance, pulmonary nodules) is needed for confirmation of the disease. It accounts for morbidity and mortality in transplant patients and in this group the cumulative one-year incidence of invasive aspergillosis is 0.007% [7].
Successful treatment of Aspergillus endocarditis requires combination of antifungal therapy and surgical debridement. Voriconazole with or without liposomal Amphoteracin formulation is regarded as the medical treatment of choice in most cases with invasive Aspergillus infections [8]. Voriconazole has been related to improved survival, less nephrotoxicity, electrolyte imbalance and fewer infusion related events. Surgical debridement is essential for the survival in almost all cases of Aspergillus endocarditis. In the literature only approximately 4% of patients were successfully treated with medical therapy alone [9].
The overall prognosis of aspergillus endocarditis remains very poor with only around 32% of cases surviving the acute episode with cardiac involvement. This patient is in a minority to have recovered enough to be discharged from hospital, be it only for a brief period of time. The poor outlook is due to the immunosuppressed state, diagnostic delay and embolic complications.
In conclusion, aspergillus endocaditis is a very rare clinical entity related usually to a device or prosthesis in immunocompromised host, or as a consequence of cardiac transplantation. Clinicians should maintain a high index of suspicion in patients with a history of haematological malignancies, recent cardiothoracic surgery, intravenous drug use and immunosuppressed state where there is culture negative endocarditis. Treatment is early aggressive surgical debridement with concomitant antifungal treatment that is usually continued indefinitely.
Conflict of interest: none declared.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
REFERENCES
- 1.Pierrotti LC, Baddour LM. Fungal endocarditis, 1995–2000. Chest. 2002;122:302–10. doi: 10.1378/chest.122.1.302. [DOI] [PubMed] [Google Scholar]
- 2.McCormack J, Pollard J. Aspergillus endocarditis 2003–2009. Med Mycol. 2011;49(Suppl 1):S30–4. doi: 10.3109/13693786.2010.498449. [DOI] [PubMed] [Google Scholar]
- 3.Ellis ME, Al-Abdely H, Sandridge A, Greer W, Ventura W. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Inf Dis. 2001;32:50–62. doi: 10.1086/317550. [DOI] [PubMed] [Google Scholar]
- 4.Lorthholary O, Meyohas MC, Dupont B, Cardranel J, Salmon-Ceron D, Peyramond D, et al. Invasive aspergillosis in patients with acquired immunodeficiency syndrome: report of 33 cases. French Cooperative Study Group on Aspergillosis in AIDS. Am J Med. 1993;95:177–87. doi: 10.1016/0002-9343(93)90258-q. [DOI] [PubMed] [Google Scholar]
- 5.Lang W, Miklossy J, Deruaz JP, Pizzolato GP, Probst A, Schaffner T, et al. Neuropathology of the acquired immune deficiency syndrome (AIDS): a report of 135 consecutive autopsy cases from Switzerland. Acta Neuropathol. 1989;77:379–90. doi: 10.1007/BF00687372. [DOI] [PubMed] [Google Scholar]
- 6.Petrosillo N, Peppicelli AM, Cicalini S, Conte A, Goletti D, Palmieri F. Endocarditis caused by aspergillus species in drug users. Clin Infect Dis. 2001;;33::e97–9. doi: 10.1086/323564. [DOI] [PubMed] [Google Scholar]
- 7.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, et al. Invasive fungal infections among organ transplant recipients: results of the transplant-associated infection aurveillance network (TRANSNET) Clin Infect Dis. 2010;50:1101–11. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 8.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 9.Kalokhe AS, Rouphael N, El Chami MF, Workowski KA, Ganesh G, Jacob JT. Aspergillus endocarditis: a review of the literature. Int J Infect Dis. 2010;14e:1040–7. doi: 10.1016/j.ijid.2010.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.