Abstract
We present a case that used argon plasma coagulation (APC) for the healing of bronchopleural fistulas (BPF), which most probably developed secondary to tracheobronchial anastomotic failure (TBAF). We aimed to show this procedure as an alternative treatment for the small fistulas that could develop after pneumonectomy. In a 56-year old male patient, right upper lobe squamous cell carcinoma was detected in 2009. Sleeve pneumonectomy was done because of the carina and major fissure invasion. There was no morbidity in the early post-operative period. The patient was discharged on the seventh day without any problems. Three cycles of chemotherapy were applied. In the third month after operation, the patient complained of a cough, and odorous sputum starting 15 days earlier. Two fistula orifices (1 and 3 mm) were detected in the fibre-optic bronchoscopy (FOB). No sign of tumour recurrences was detected in either chest computed tomography (CT) or FOB. BPF had entered the mediastinal chamber, which isolated the infection from the pleural cavity. The APC procedure was applied using FOB under local anaesthesia. The processing time was 30 min. There were no complications during or after the procedure. FOB was repeated 30 days later, and none of the previously opened orifices were observed. The patient was followed up for 18 months without any symptoms. APC was generally used for the treatment of oesophageal and intestinal fistula. We could not find any cases in the literature about APC application to treat BPF. APC could be an alternative treatment for the selected cases with small, uncomplicated BPF.
Keywords: Argon plasma coagulation, Pneumonectomy, Bronchopleural fistula
INTRODUCTION
Bronchopleural fistula (BPF) is defined as a communication between the bronchus and the pleural space. It continues to represent an important clinical problem and is associated with significant morbidity and mortality. The majority of the cases occur after pneumonectomy with incidence varying from 4.5 to 20% [1]. Right pneumonectomy was associated with the significantly increased risk of the presence of BPF, along with preoperative radiation therapy, mediastinal lymph node resection and carcinoma at the bronchial stump [2].
Sleeve pneumonectomy carries special difficulties, either during surgery or in the management of post-operative complications. BPF, entering a mediastinal pouch, develops at around the third post operative month. A variety of fibre-optic bronchoscopy (FOB) techniques have been used to help the sealing of BPFs, such as bronchoscopic glue instillation and the injection of a tissue expander. Here we present a case with BPF that had most probably developed secondary to tracheobronchial anastomotic failure (TBAF), which was treated with argon plasma coagulation (APC). We aimed to show this approach as an alternative treatment, in particular for small fistulas, which develop after sleeve pneumonectomy and in patients with increased risk of surgical intervention.
CASE PRESENTATION
In a 56-year old male patient, right upper lobe squamous cell carcinoma was detected in December 2009. Sleeve pneumonectomy was done because of the tumour extension to the carina and major fissure invasion. There was no morbidity in the post-operative period. The patient was discharged on the seventh day without any problems, and classed as pT4N1M0 stage IIIA. Three-cycle chemotherapy was given in the adjuvant setting.
In the third postoperative month, the patient complained of a cough, and odorous sputum starting 15 days earlier. No fever or leukocytosis was detected. The chest computed tomography (CT) revealed post-pneumonectomic changes. No evidence of recurrence was noted on CT. The cavity was filled with fluid with no hydroaeric level (Fig. 1). FOB has been used to diagnose and localize only when any recurrence or postoperative fistulas develop. Two fistulas, (1 and 3 mm) which were located on the tracheobronchial anastomosis, were detected by FOB. There was no macroscopic evidence of recurrence. Histopathological analysis of biopsy materials obtained from the stump showed non-specific bronchitis on the mucosa.
Figure 1:
Thorax CT image revealed post-pneumonectomic changes.
Since the anastomosis had a left-sided tracheobronchial localization and had no stump like that of pneumonectomy, submucosal injection techniques with of a tissue expander or bronchoscopic instillation of n-butyl-cyanoacrylate glue were not suitable techniques for this case. Surgical repair of these fistulas via thoracotomy 3 months after surgery was also difficult and associated with high morbidity. Since it was noted that the fistulas were entering a pouch, a conservative approach was planned.
APC procedure was applied with FOB under local anaesthesia for the treatment of fistula. A 1.5-mm diameter probe, developed by ERBE Corp., was used. The APC equipment used was a combination of a high-frequency electrosurgical generator and an argon source. Argon flow and electrical power were set at 1.5 l/min and 60 W, respectively. It was applied all around the cleft as a non-contact thermal method with a depth of penetration of roughly 2–3 mm. The processing time was 30 min. There were no complications during or after the procedure. Endoscopy was done as a control on the 10th day, and a small area of fibrosis was detected around the treatment region. FOB was repeated 30 days later and none of the previously opened orifices were observed (Fig. 2). The patient was followed up for 18 months without any symptoms and recurrences.
Figure 2:
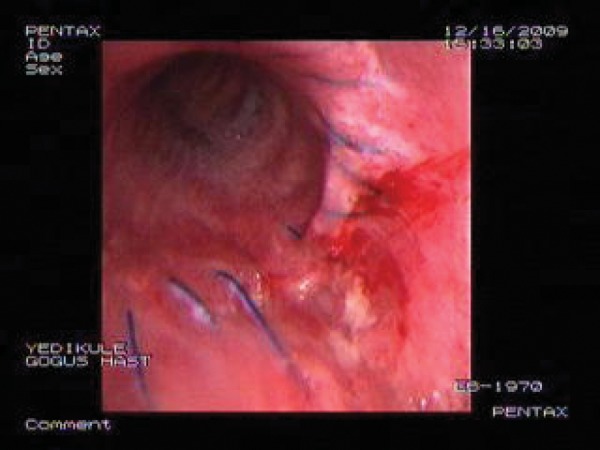
Bronchoscopy image 30 days after the procedure.
DISCUSSION
Despite the advances in the surgical techniques, BPF is still an important clinical problem with high morbidity and mortality particularly after right pneumonectomy [1]. Bronchoscopic and radiological evaluation are most popular for the localization/confirmation of the fistula [3, 4]. Bronchoscopic evaluation is critical for the early diagnosis of a small fistula when the symptoms of the fistula are not clear. Our case presented with a non-productive persistent cough and odorous sputum, and bronchoscopic evaluation revealed two orifices sized 3 and 1 mm.
We know that the post-pneumonectomy cavity fills up with fluid. At the same time, a cortex enrolling the cavity develops. This cortex also covers the bronchial stump and even causes difficulties in the repair of late BPFs. In the presented case, anastomotic failure developed, possibly at the third month postoperatively and the newly developed cortex created a chamber between the mediastinum and cortex. Two small fistulas entered this mediastinal chamber.
The management of the BPF varies according to the initial type of surgery, the size and the shape of the fistula, the patient's general condition and the physiology of the remaining lung. A number of non-surgical techniques have been developed for attempting endobronchial closure of BPFs in recent years. Two different techniques have been used successfully, either alone or as a combined treatment [5]. Although they have been used widely for the treatment of BPFs in recent years, endoscopic treatment is reserved only for small fistulas or for patients with poor general condition [6–8]. It should be noted that the efficacy of the endoscopic repair decreases proportionally with increasing fistula diameter [2, 5].
APC is a method of non-contact electrocoagulation with a penetration depth of roughly 2–3 mm. It has been popular in gastrointestinal endoscopy for superficial coagulation of large mucosal surfaces and has been used to repair gastrointestinal fistulas. The flexibility of the argon gas flow gives an opportunity to reach bends and corners in the bronchial tree. In addition, coagulated tissue has a higher resistance that automatically drives the flow to the nearby untreated tissue. Therefore, APC is advantageous for treating bronchial segments such as apical and posterior segments of the upper lobes or the apical lower lobe segments [9].
APC generates electrical energy flows to the target tissue and allows for rapid coagulation and mechanical trauma. The healing of the fistula can be attributed to mechanical trauma and wound healing, and it may enhance inflammatory reaction, re-epithelialization, and stimulate angiogenesis and fibrosis. Although no data have been found in the literature indicating APC for the treatment of BPF, successful management has been shown for the gastrocutaneous fistula and tracheoesophageal fistula using APC [10].
In the presented case, fibrine glue and submucosal injection were not available. The application of a tracheobronchial stent was also planned in case APC failed. APC is a technically applicable, easy to use, cheap and non-invasive method. Although more experience needs to be evaluated, we were able to show the feasibility and capacity of this technique for the treatment of fistulas in a single case. APC is a feasible alternative treatment for small BPFs, in selected cases where the surgical risk is high.
Conflict of interest: none declared.
REFERENCES
- 1.McManigle JE, Fletcher GL, Tenholder MF. Bronchoscopy in the management of bronchopleural fistula. Chest. 1990;97:1235–8. doi: 10.1378/chest.97.5.1235. doi:10.1378/chest.97.5.1235. [DOI] [PubMed] [Google Scholar]
- 2.Asamura H, Naruke T, Tsuchiya R, Goya T, Kondo H, Suemasu K. Bronchopleural fistulas associated with lung cancer operations: univariate and multivariate analyses of risk factors, management, and outcome. J Thorac Cardiovasc Surg. 1992;104:1456–64. [PubMed] [Google Scholar]
- 3.Kim EA, Lee KS, Shim YM, Kim J, Kim K, Kim TS, et al. Radiographic and CT findings in complications following pulmonary resection. Radiographics. 2002;22:67–86. doi: 10.1148/radiographics.22.1.g02ja0367. [DOI] [PubMed] [Google Scholar]
- 4.Misthos P, Konstantinou M, Kokotsakis J, Skottis I, Lioulias A. Early detection of occult bronchopleural fistula after routine standard pneumonectomy. Thorac Cardiovasc Surg. 2006;54:264–7. doi: 10.1055/s-2005-872975. doi:10.1055/s-2005-872975. [DOI] [PubMed] [Google Scholar]
- 5.García-Polo C, León-Jiménez A, López-Campos JL, Arnedillo A, González-Moya E, Fernández-Berni JJ, et al. Endoscopic sealing of bronchopleural fistulas with submucosal injection of a tissue expander: a novel technique. Can Respir J. 2010;17:e23–4. doi: 10.1155/2010/385036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sirbu H, Busch T, Aleksic I, Schreiner W, Oster O, Dalichau H. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg. 2001;7:330–6. [PubMed] [Google Scholar]
- 7.Sipple JM, Chesnutt MS. Bronchoscopic therapy for bronchopleural fistulas. J Bronchol. 1988;5:61–9. [Google Scholar]
- 8.Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128:3955–65. doi: 10.1378/chest.128.6.3955. doi:10.1378/chest.128.6.3955. [DOI] [PubMed] [Google Scholar]
- 9.Bolliger CT, Sutedja TG, Strausz J, Freitag L. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258–71. doi: 10.1183/09031936.06.00013906. doi:10.1183/09031936.06.00013906. [DOI] [PubMed] [Google Scholar]
- 10.Yankovic F, Castillo C, Saenz R, Navarrete C. Endoscopic argon plasma coagulation in recurrent tracheoesophageal fistula: clinical series and review of the literature. Gastroenterol Hepatol. 2009;32:600–4. doi: 10.1016/j.gastrohep.2009.06.012. doi:10.1016/j.gastrohep.2009.06.012. [DOI] [PubMed] [Google Scholar]



