Abstract
We reviewed our institutional experience with congenital mediastinal masses and compared the postnatal management and outcome of patients with or without prenatal diagnosis. Between January 1997 and August 2011, 24 patients underwent surgical procedures for congenital mediastinal mass. For eight patients, the mass was detected by prenatal ultrasonography at 27 weeks of gestation (range 22–35). Postnatal management consisted in open surgery for seven patients at a mean age of 9 months (range 1 day–20 months) and sclerotherapy for one lymphangioma at 5 months of life. Sixteen patients had postnatal diagnosis at 137 months (±194) of median age. Eight bronchogenic cysts, seven bronchopulmonary foregut malformations, five teratomas, three lymphangiomas and one haemangioma were operated on. The median age at resection was 28 months (1 day–15 years). There were four emergency procedures and no surgical mortality. The median follow-up was 45 months (3–144). The duration of mechanical ventilation and hospital stay was, respectively, 4.6 h and 7.5 days for antenatal patients and 24.3 h and 14.3 days for postnatal diagnosed patients. Prenatal diagnosis allows early management of congenital mediastinal malformations. Early resection can be performed prior to the occurrence of symptoms ∼1 year of life and is associated with an excellent outcome and less morbidity.
Keywords: Mediastinal malformations, Prenatal diagnosis, Postnatal management
INTRODUCTION
Congenital mediastinal malformation (CMM) is a rare entity, estimated at between 1 in 20 000–30 000 live births [1]. It is mainly described with isolated case reports or included with CMM in review articles [2]. This study represents the 13-year experience of a single institution with the diagnosis and management of CMM. Exclusion criteria consisted in haemopathies, neurogenic tumours and cardiac rhabdomyoma. According to this definition, it is one of the most important series comparing postnatal management of children operated for CMM with and without prenatal diagnosis.
MATERIALS AND METHODS
Between January 1997 and August 2011, 24 patients (8 females; 16 males) underwent surgical procedures by three surgeons for CMM. There were 4 neonates, 8 infants and 12 children. Data were reviewed retrospectively and statistical analysis was performed by Student's t-test using GraphPad Prism° software.
Surgical approach
After selective intubation, we performed 15 postero-lateral thoracotomies, 2 thoracoscopies, 2 laparotomies, 2 laparoscopies and 2 sternotomies. Open surgery was preferred for lesions with mass effect, neonates and emergency cases. Sternotomy was the elective surgical approach for anterior mediastinal masses such as teratomas. In the other cases, thoracoscopic or laparoscopic approach was intented but for technical reasons, it had to be converted for four patients. When immediate postoperative extubation was not possible, postoperative ventilation was performed in intensive care unit.
Antenatal diagnosis
Eight of the 24 patients who underwent resection had mediastinal lesions diagnosed on antenatal ultrasound. Foetuses with an antenatal diagnosis were followed up with sonograms at regular intervals until delivery to monitor location, size and associated anomalies and mass effects. A magnetic resonance imaging (MRI) was performed around 30 weeks gestation to evaluate the lesion and the lungs.
For these eight patients, the in utero course of the malformation remained stable and there was no associated mass effect. There was no evidence of progression of hydrops, mediastinal shift or cyst enlargement in any of the foetuses on sequential scanning. Normal delivery was preferred whenever possible.
After birth, a more precise evaluation with MRI was performed during the neonatal period. In the case of size enlargement or development of clinical signs, an early surgical procedure was decided. If not, a close clinical follow-up with a new MRI or computed tomography scan on the 6th month of life led to surgery between the 9th and 12th month of life.
In the same study period, antenatal ultrasound screening detected a total of 97 thoracic lesions. Eighteen of them (18.5%) had a mediastinal location. Among them, nine did not require surgery and two were lost to follow-up. Three regressed by the end of pregnancy and are still followed-up one probable lymphangioma and two probable bronchopulmonary foregut malformations (BPFMs). There were four medical terminations of pregnancy for one teratoma with associated anomalies and three proteus syndrome. There was one foetal and one neonatal deaths for two teratomas.
RESULTS
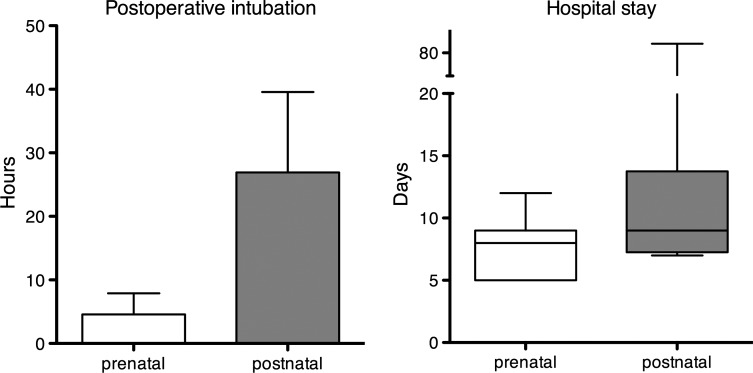
The median age at surgery was 28 months (range 1 day to 15 years). Postoperative intubation was 19 h (4–216) and hospital stay was 12.5 days (5–82). There was no operative or late mortality. The mean follow-up was 45 months (range 3–144).
Seven patients had BPFM among which four had pulmonary sequestrations (PSs) associated with enteric cysts and three had oesophagus duplications. There were eight bronchogenic cysts. The other malformations were five patients with teratomas, three patients with lymphangiomas and one with haemangioma. One patient suffered from a complex malformation syndrome. We did not find neurenteric cysts and congenital neuroblastomas. Differences in the management and outcome of patients between antenatal and postnatal diagnosis are summarized in Table 1.
Table 1:
Comparison of surgical management between prenatal and postnatal diagnosis
| Diagnosis modality | Number of patients | Mean age at diagnosis | Mean weight at surgery (kg) | Mean age at resection (months) | Emergent procedures | Morbidity (%) | Mortality (%) | Mean hospital stay (days) | Duration of ventilation (h) |
|---|---|---|---|---|---|---|---|---|---|
| Antenatal | 8 | 27 (22–35) weeks of gestation | 6 (2–13) | 7 (1–20) | 1 (12.5%) | 0 | 0 | 7.5 (6–12) | 4.6 (4–24) |
| Postnatal | 16 | 39 (3–181) months of life | 16 (4–71) | 39 (3–81) | 4 (25%) | 12 | 0 | 14.3 (7–82) | 19 (4–216) |
Antenatal diagnosis
Eight cystic lesions were diagnosed by routine sonography at a median gestation age of 27 (22–35) weeks. No therapeutic amniocentesis was performed and no mass effect was observed. Before birth, establishing the nature of the malformation was difficult. First diagnostic hypothesis was invalidated for four patients (50%) despite foetal MRI. This imaging was performed to rule out lung mass effects and associated anomalies. For these patients, amniotic fluid centesis was proposed for chromosomal analysis.
Normal delivery occurred after 38 weeks (35–41) and was possible for all patients with antenatal diagnosis. One lymphangioma suffered from a neonatal respiratory distress (Case 6), which pressed to emergency resection after intubation. One respiratory distress occurred after oral feeding because of a foregut communication (Case 8). This patient had a singular form of BPFM with coexistence of PS containing pancreatic tissue and a retro gastric cyst with gastric and pancreatic differentiation (Figs 1 and 2). We had to perform semi-urgent thoracotomy and laparotomy on the 27th day of life.
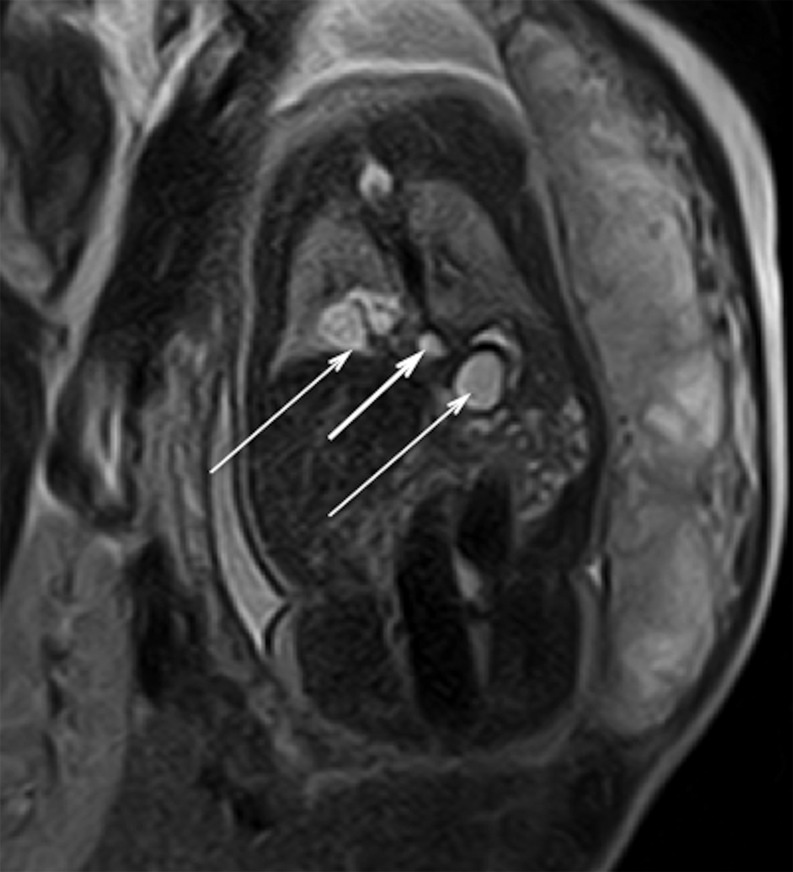
Figure 1:
Foetal MRI. Coronal HASTE T2 MR image obtained through the posterior mediastinum at a gestational age of 34 weeks. It shows the BPFM with a retrocrural cyst (short arrow) between right lower lobe and retrogastric cysts (long arrows).
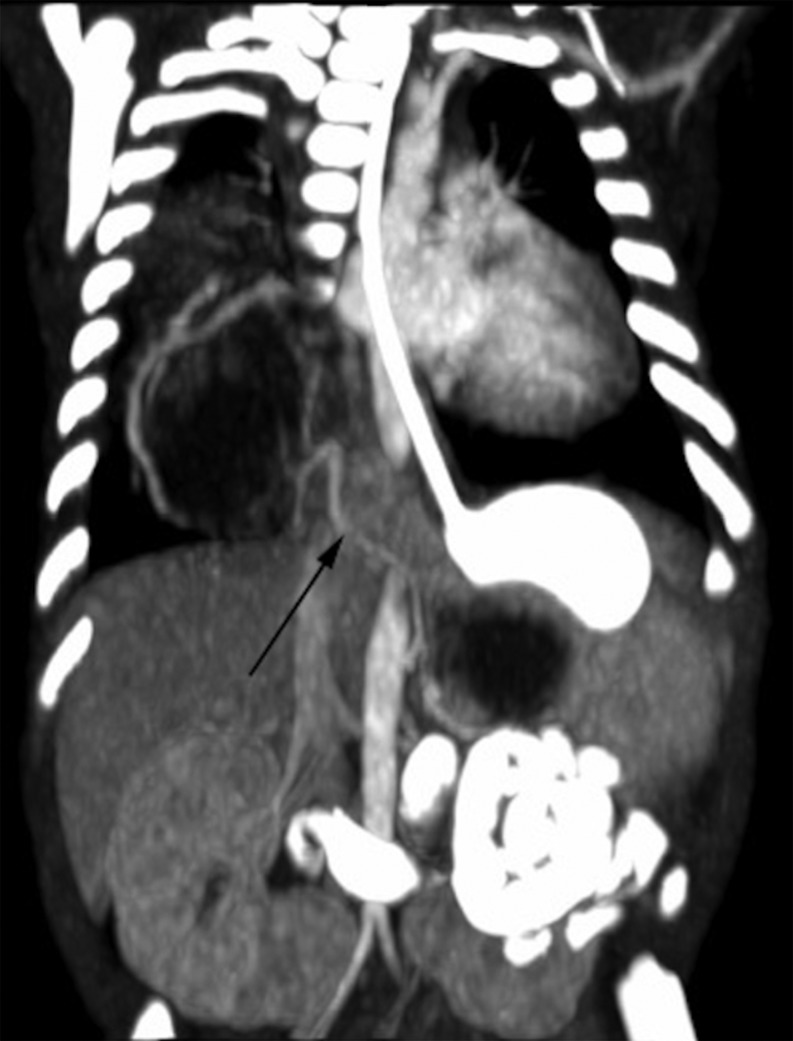
Figure 2:
Neonatal computed tomography. Coronal body contrast-enhanced CT scan after bowel opacification. It outlines cystic images with vascular aberrant supply (arrow) coming from celiac artery. Foregut communication is excluded.
The six others cases included two bronchogenic cysts, two BPFM, one lymphangioma (Fig. 3) and one haemangioma. They were managed with elective surgery between 5 and 20 months of life. For the latter lymphangioma (Case 7), a sclerotherapy was performed on the 5th month of life. Mean weight at surgery was 6.4 kg (2.8–13). Surgical procedures were performed on the 212th day of life (1–615): six thoracotomies and two laparotomies. Location, diagnosis and outcome are summarized in Table 2. Two lobectomies concerned intralobar PS associated with enteric cysts. Postoperative intubation was 4.6 h (4–24) and hospital stay was 7.5 days (6–12). After 48 (3–112)-month follow-up, there was no mortality and no surgical morbidity.
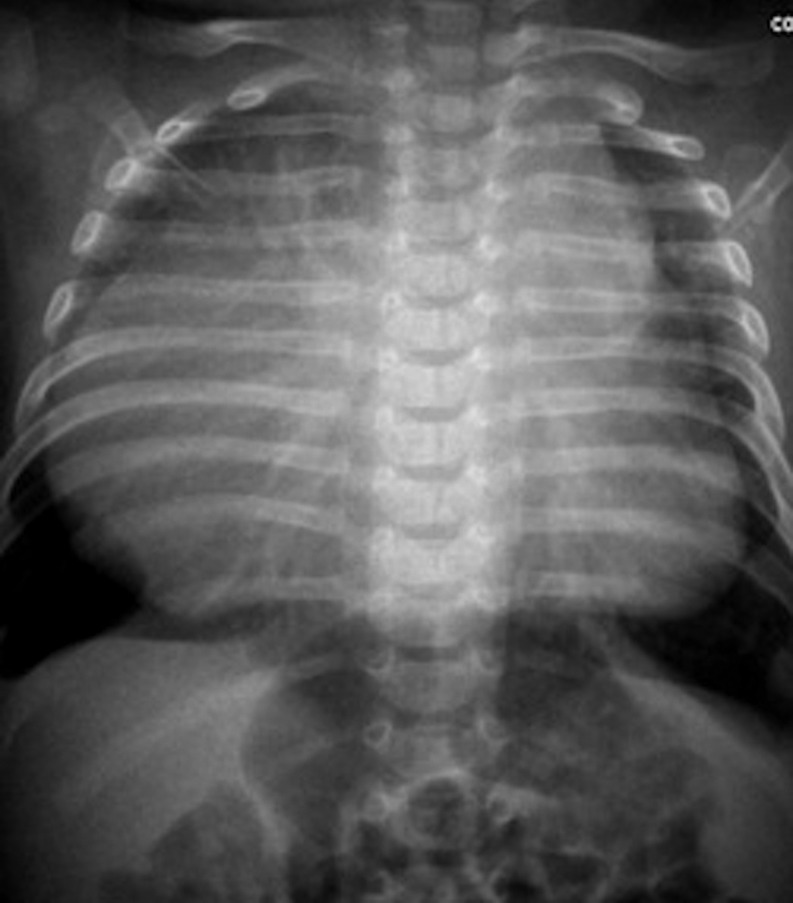
Figure 3:
Neonatal chest X-ray. Neonatal chest roentgenogram demonstrates mediastinal enlargement in an asymptomatic 1-day old boy with prenatal diagnosis of lymphangioma.
Table 2:
Main characteristics of the antenatal diagnosed patients
| Cases | Gender | GA at diagnosis (weeks) | Location | Diagnosis | Symptoms at birth | Associated findings | Treatment | Size of cyst (cm) | Age at resection (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 22 | Retrocrural | BPFM | n | n | Laparotomy at 20 days | 3 × 2 | 20 |
| 2 | M | 22 | Posterior mediastinum | Bronchogenic cyst | n | n | Right thoracotomy at 12 months | 4.2 × 3.5 | 365 |
| 3 | F | 22 | Anterior mediastinum | Hemangioma | n | n | Right thoracotomy at 6 months | 2.1 × 1.8 | 182 |
| 4 | F | 24 | Retrocrural | BPFM | n | n | Right thoracotomy at 20 months | 4 × 2 | 600 |
| 5 | F | 25 | Posterior mediastinum | Bronchogenic cyst | n | Anomaly of pulmonary venous drainage | Right thoracotomy at 12 months | 2.7 × 2 | 365 |
| 6 | M | 34 | Left hemithorax | Lymphangioma | Respiratory distress | n | Left thoracotomy at 1 day | 6.2 × 5.3 | 1 |
| 7 | M | 35 | Left hemithorax | Lymphangioma | n | n | Percutaneous sclerotherapy at 5 months | 3.3 × 2.2 | 140 |
| 8 | M | 34 | Right hemithorax | BPFM | n | Retrogastric cyst, hypospadia, urethral valva, anorectal malformation, atrial septal defect | Right thoracotomy and laparotomy at 27 days | 7 × 6.5 | 27 |
GA: gestational age; F: female; M: male; N: no.
Postnatal diagnosis
Sixteen of the 24 patients were postnatally diagnosed, at a mean age of 3.2 years (3 months, 15 years) detected mainly as respiratory distress (n = 7). Mean weight at surgery was 16 kg (4–71). They were operated 137 days after diagnosis (0–749). Initial diagnostic hypothesis was invalidated for three patients (19%). Surgical procedure was performed in emergency for four of them and a mass effect was present in six of them. For example, a 3-month old boy was operated on in emergency because of respiratory failure secondary to a huge teratoma (Figs 4 and 5). For three bronchogenic cysts, a respiratory distress occurred in the neonatal period but did not lead to diagnosis. These three patients were operated on after 1 year of life; all of them required ventilation preoperatively. One was operated on in emergency. For another one, total resection was not possible because of infectious changes. For the same reasons, two surgical procedures were necessary because of incomplete resection and hospital stay lasted 82 days for a 16-month old boy with BPFM.
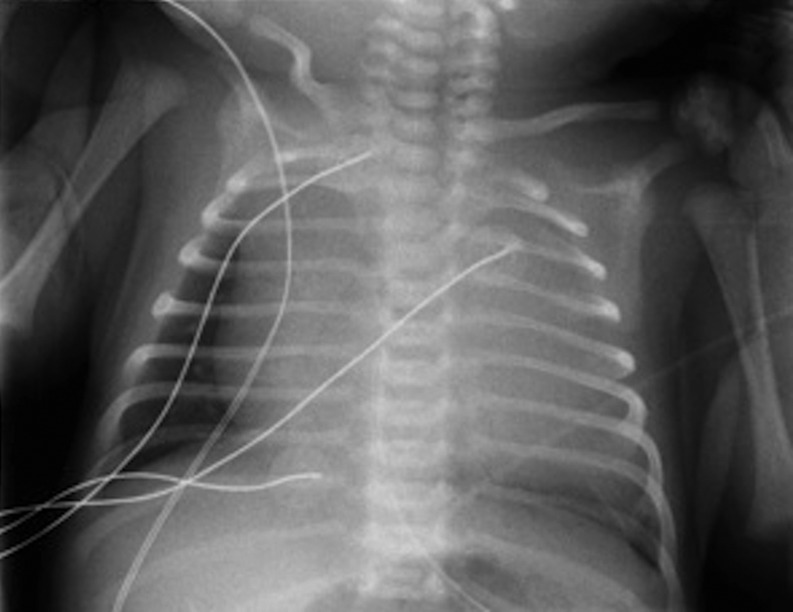
Figure 4:
Roentgen imaging of the thorax. A 3-month old boy without antenatal diagnostic was referred for respiratory distress. This radiograph shows severe enlargement of the mediastinum.
Figure 5:
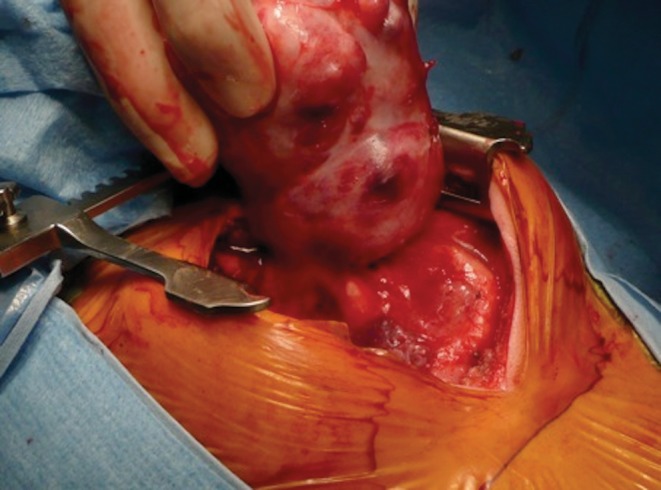
Peroperative view. Post-intubation emergent sternotomy was performed to resect a large-size immature teratoma.
Thirteen patients were extubated on the table. The mean duration of ventilation was 19 h (4–216). The mean hospital stay was 14 days (7–82). The results are shown in Fig. 6. Persistent mild symptoms were present in four patients after a 42 (6–144)-month follow-up. Surgical morbidity was 12% (one incomplete bronchogenic cyst resection with fistula, one prolonged air leak, one incomplete BPFM resection with a second-stage resection, one Claude Bernard Horner syndrome).
Figure 6:
Postoperative intubation and hospital stay. The hospital stay was longer (14.3 versus 7.5 days) and postoperative intubation was longer too (24 versus 4 h) for postnatal-detected lesions, P was not significant. The minimal hospital stay after postnatal diagnosis is 7 days when the mean hospital stay after antenatal diagnosis is <8 days.
DISCUSSION
CMM includes BPFM, bronchogenic cysts, teratomas, lymphangiomas, hemangiomas and neurenteric cysts. They are a small part of the spectrum of congenital thoracic malformations like in the present series (18.5%). Most CMM are diagnosed on the second trimester. Pregnancies can carry on until full term and lead to normal delivery after a simple foetal sonography follow-up.
In case of large lesions or associated anomalies, foetal MRI is interesting to assess the state of foetal lungs and mediastinal compression and to define the associated anomalies. It provides more information concerning the nature of many of these lesions and establishes their location, thus allowing to differentiating them from extrathoracic abnormalities such as congenital diaphragmatic hernia [3].
Isolated and small lesions have a globally good outcome. Overall prognosis then depends on the size of the mass and the secondary physiological derangement. Hydrops is the harbinger of foetal or neonatal demise [4]. In such cases, treatment in utero has been proposed. Cysts can be managed with thoracoamniotic shunting with a risk of early delivery [5].
Solid foetal mediastinal tumours have a worse prognosis than those with other locations [6]. For such lesions, antenatal intervention should be considered but is reserved for centres with appropriate expertise and possibilities of procedures like ex utero intrapartum therapy and extracorporeal membrane circulation [5, 7].
Mediastinal teratomas represent only 10% of congenital teratomas in children [8]. Usually located in the anterior and superior mediastinum, they can also be posterior. Antenatal ultrasound presentation shows multilobulated masses with cystic and solid components. Calcifications are typical for the diagnosis. Immature teratomas tend to grow faster and to a larger size, particularly in the second trimester. Teratoma represents the main cause of foetal mortality for mediastinal lesions. For large mediastinal teratomas [9], prenatal therapy is required before occurrence of foetal hydrops. In spite of close monitoring and timely intervention, a poor outcome is expected. We operated on five patients (one immature) and noted a high mortality rate related to foetal hydrops or neonatal distress.
Because of new considerations regarding the nomenclature of congenital lung disease [10], we decided to include in this group different overlapping abnormalities of the mediastinum like BPFM, PS and bronchogenic cysts. Pathological concepts are unclear, but defective budding of primitive foregut remains the most probable pathogenic theory [11]. We found seven BPFM: four had foregut cysts associated with PS and two had hybrid entities (one association with bronchogenic cyst and one association with enteric cyst) and one had oesophageal duplication. Associated congenital abnormalities are common with BPFM [11], but complex malformation syndrome (Case 8) is rare. Systematic diagnosis management should include bronchoscopy and oesophagram to exclude foregut communication.
Lymphangioma is a rare benign tumour of the lymphatic vessels. The most common location is the neck. Pure isolated mediastinal forms occur in <1% of cases and are located in the posterior mediastinum. To the best of our knowledge, there have only been five cases of prenatally diagnosed isolated mediastinal lymphangiomas [12]. Size evolution is variable. Despite its benign nature and many descriptions of spontaneous resolution, lymphangioma can infiltrate other tissues. Sclerotherapy is the first line treatment [13] and complete one-stage excision is the treatment of choice when severe mediastinal compression is present or in case of sclerotherapy failure.
The management of asymptomatic patients remains controversial. Both antenatally and postnatally, counselling of the family on a case-by-case basis is needed [1]. Some authors advocate close observation. Others favour elective resection in every case because of the risks of recurrent infection, arguing that infection and mass effect are not predictable and press to emergency procedures.
Antenatal diagnosis allowed earlier management in our series. It did not increase the rate of ceasarean section. It allowed early surgical treatment before the occurrence of complications with a low morbidity rate. Postoperative course was better. Surgical procedure was performed on older children in worse conditions. Particularly for those patients, the hospital stay and the duration of intubation were increased. The patient with 82-day hospital stay could be an isolated case, but represents a specific risk to avoid. We believe that a surgical exploration is indicated before the occurrence of respiratory distress. It reduces complication-related morbidity and parental anxiety. Most of all, it confers definitive diagnosis and treatment. We deplore three foetal and perinatal demises before surgical opportunity. It occurred in large-size teratomas for which poor outcome was expected in spite of optimum management.
Laparoscopic procedures are difficult in this period, but video-assisted thoracoscopic procedure is possible for experienced teams and it reduces morbidity [1]. In the case of retrocrural formations, video-assisted laparoscopy is the procedure of choice [14]. Robotic surgery enabling precise dissection could be a procedure of interest [15]. Surgery is mostly conservative, except for BPFM where pulmonary tissue can be involved.
CONCLUSIONS
Except for large-size teratomas, CMMs have a good global outcome, which is improved by prenatal diagnosis. If they are isolated and non-compressive, they require no antenatal intervention. Early neonatal surgical excision is required for symptomatic babies. In addition to a close follow-up and in the absence of an increase in size or respiratory symptoms, we believe that surgical resection around 12 months of age is the treatment of choice. In these conditions, an excellent outcome following surgery can be expected.
Conflict of interest: none declared.
REFERENCES
- 1.Bush A. Prenatal presentation and postnatal management of congenital thoracic malformations. Early Hum Dev. 2009;85:679–84. doi: 10.1016/j.earlhumdev.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 2.Shanmugam G, MacArthur K, Pollock JC. Congenital lung malformations—antenatal and postnatal evaluation and management. Eur J Cardiothorac Surg. 2005;27:45–52. doi: 10.1016/j.ejcts.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Williams HJ, Johnson KJ. Imaging of congenital cystic lung lesions. Paediatr Respir Rev. 2002;3:120–7. [PubMed] [Google Scholar]
- 4.Adzick NS. Management of fetal lung lesions. Clin Perinatol. 2009;36:363–76. doi: 10.1016/j.clp.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Stanton M, Davenport M. Management of congenital lung lesions. Early Hum Dev. 2006;82:289–95. doi: 10.1016/j.earlhumdev.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Sbragia L, Paek BW, Felstein VA, Farrel JA, Harrisson MR, Albanese CT, et al. Outcome of prenatally diagnosed solid fetal tumors. J Pediatr Surg. 2001;36:1244–7. doi: 10.1053/jpsu.2001.25785. [DOI] [PubMed] [Google Scholar]
- 7.Liechty KW. Ex-utero intrapartum therapy. Semin Fetal Neonatal. 2010;15:34–9. doi: 10.1016/j.siny.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs H. Perinatal (fetal and neonatal) germ cell tumors. J Pediatr Surg. 2004;39:1003–13. doi: 10.1016/j.jpedsurg.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 9.Merchant AM, Hedrick HL, Johnson MP, Wilson RD, Crombleholme TM, Howell LJ, et al. Management of fetal mediastinal teratoma. J Pediatr Surg. 2005;40:228–31. doi: 10.1016/j.jpedsurg.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Bush A. Congenital lung disease: a plea for clear thinking and clear nomenclature. Pediatr Pulmonol. 2001;32:328–37. doi: 10.1002/ppul.1126. [DOI] [PubMed] [Google Scholar]
- 11.Newman B. Congenital bronchopulmonary foregut malformations: concepts and controversies. Pediatr Radiol. 2006;36:773–91. doi: 10.1007/s00247-006-0115-4. [DOI] [PubMed] [Google Scholar]
- 12.Ruano R, Takashi E, Schultz R, Zugaib M. Prenatal diagnosis of posterior mediastinal lymphangioma by two- and three-dimensional ultrasonography. Ultrasound Obstet Gynecol. 2008;31:697–700. doi: 10.1002/uog.5327. [DOI] [PubMed] [Google Scholar]
- 13.Impellizzeri P, Romeo C, Borruto FA, Granata F, Scalfari G, De Ponte FS, et al. Sclerotherapy for cervical cystic lymphatic malformations in children. Our experience with computed tomography-guided 98% sterile ethanol insertion and a review of the literature. J Pediatr Surg. 2010;45:2473–8. doi: 10.1016/j.jpedsurg.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Joyeux L, Mejean N, Rousseau T, Couaillier JF, Piard F, Sapin E. Ectopic extralobar pulmonary sequestrations in children: interest of the laparoscopic approach. J Pediatr Surg. 2010;45:2269–73. doi: 10.1016/j.jpedsurg.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Obasi PC, Hebra A, Varela JC. Excision of esophageal duplication cysts with robotic-assisted thoracoscopic surgery. JSLS. 2011;15:244–7. doi: 10.4293/108680811X13071180406961. [DOI] [PMC free article] [PubMed] [Google Scholar]