Abstract
Pectus excavatum and pectus carinatum represent the most frequent chest wall deformations. However, the pathogenesis is still poorly understood and research results remain inconsistent. To focus on the recent state of knowledge, we summarize and critically discuss the pathological concepts based on the history of these entities, beginning with the first description in the sixteenth century. Based on the early clinical descriptions, we review and discuss the different pathogenetic hypotheses. To open new perspectives for the potential pathomechanisms, the embryonic and foetal development of the ribs and the sternum is highlighted following the understanding that the origin of these deformities is given by the disruption in the maturation of the parasternal region. In the second, different therapeutical techniques are highlighted and based on the pathogenetic hypotheses and the embryological knowledge potential new biomaterial-based perspectives with interesting insights for tissue engineering-based treatment options are presented.
Keywords: Pectus excavatum, Funnel chest, History, Hypotheses, Development, Chest wall deformity
INTRODUCTION
Congenital chest wall deformities can be classified into rare entities such as the cleft sternum, pentalogy of Cantrell, asphyxiating thoracic dystrophy (Jeune syndrome), Poland syndrome and spondylothoracic dysplasia (Jarcho–Levin syndrome), which represent ∼3–4% of all cases, with pectus excavatum (PE) and pectus carinatum (PC) representing 95–97% of all chest wall deformities. PE represents a depression in the anterior chest wall as a result of dorsal deviation of the sternum and the third to seventh rib or costal cartilage, and is the most common chest wall deformity, representing 90% of all cases. Depending on the severity of PE, deviations of thoracic organs and spine deformities are known. Although PE in most instances has little or no influence on the function of the inner organs, the cosmetic appearance of the patients leads to psychological impairment which requires therapy. The most established treatment options are surgical interventions. There are a variety of operation methods which have been developed. Regarding the formal pathogenesis of PE, several hypotheses exist, although the underlying pathomechanisms are not at all clearly understood. Furthermore, questions arise about the role of developmental processes in the formation of PE.
The present report summarizes the pathophysiological hypotheses based on an historical overview of the description of PE and highlights the development of the sterno-clavicular joint. Summarizing the different therapeutical techniques, we highlight potential new perspectives.
THE HISTORY OF PECTUS EXCAVATUM DESCRIPTION
The first description of a funnel-formed chest wall came from Bauhinus [1] in the sixteenth century. Another documented description of an appearance of the thorax could be found in 1860 by Woillez [2]. In 1863, von Luschka [3] reported about a 6-cm deep depression in the thorax wall of a 24-year-old man. In 1870, Eggel [4] published the first comprehensive case report of a patient with a funnel-formed thorax depression calling it a ‘miraculum naturae’. He assumed that the reason for the deformity would be a weakness and an abnormal flexibility of the sternum caused by nutritional disturbance or by developmental failure. Individual case reports followed by Williams [5], Flesch [6] and Hagmann [7]. The latter believed that overgrowth of the ribs causes the depression of the chest. In contrast, Langer and Zuckerkandel [8] favoured the hypothesis of a developmental failure, taking place in utero, in which the lower jaw of the foetus is responsible for the deformity by pushing on the sternum as a result of too high intrauterine pressure. Regarding surgical correction, Meyer performed the first operation of PE in 1911 with the removal of the rib cartilage [9]. He also analysed the removed cartilage microscopically and identified an unspecific degeneration. However, he did not link the histological findings with the pathogenesis of PE.
EPIDEMIOLOGY AND CLINICAL PICTURE OF PECTUS EXCAVATUM
The incidence of PE has a ratio between 0.1 and 0.8 per 100 persons [10]. Interestingly, males are more often affected, with a gender distribution between 2:1 and 9:1 [11]. Even if PE occurs sporadically, a genetic predisposition seems likely, since a positive family history could be found in up to 43% of PE cases [12, 13]. However, a specific genetic defect has not yet been found. Most cases of PE could be noted clinically within the first year of life, but primary occurrence in puberty is also described. Mostly, chest wall deformities represent a single anomaly, but they could also be one manifestation of various genetic disorders. In this context, Kotzot and Schwabegger [14] gave a comprehensive overview about all syndromes associated with chest wall deformities. Colombani [15] mentioned that only <1% of patients with PE have an underlying connective tissue disorder.
Clinically, PE is associated with a typical posture [14]: thin, tall patients with a pot-belly and forward-drifted shoulders, which could lead to permanent scoliosis [15, 16]. The depression of the sternum can displace the heart and reduce the lung volume [13, 17]. As a result of the anatomical changes, chest pain [12, 13, 15], fatigue [15], dyspnoea on exertion [12, 13, 15, 18], respiratory infections [13], asthma symptoms [13], palpitations [12] or heart murmurs could occur [13]. Several cases with mitral valve prolapse [13, 15, 19], mitral valve regurgitation and ventricle compression could be found [15, 17, 19]. For the latter, Coln et al. [19] demonstrated that 95% of 123 patients had cardiac compression. Even a single case report of syncopal symptoms has been reported. The pulmonary and cardiovascular functions of patients with PE deformities were analysed in many investigations and have revealed measurable deficiencies [12]. Fonkalsrud et al. [13] reported that the symptoms of many untreated PE patients become progressively worse with age and he recommended an operational intervention for both young and adult patients.
In contrast to these descriptions of more or less severe clinical signs, symptoms affecting daily life activities are either rare [20]. Therefore, some authors described the indication for a PE correction to be primarily cosmetic.
Numerous clinical studies described an improvement of pulmonary and/or cardiovascular symptoms and improvement in the subjective well-being after surgical correction [9, 13, 16–18]. Malek et al. [18, 21] concluded that an operative intervention improves cardiovascular but not pulmonary function. Guntheroth [22] and Spiers as well as Johnson et al. [23] re-evaluated the source data of Malek's meta-analyses and stated that due to relevant methodological deficits, these data failed to demonstrate any improvement of cardiac function [23]. In this context, Aronson et al. [24] could not show an improvement in lung function parameters after Nuss procedure. Numerous authors highlight the psychological factors due to the physiognomic features of the chest wall deformity [15]. The deformities are thought to cause relevant social discrimination, especially during adolescence, leading to the socio-psychologic problems [15]. A multicentre study demonstrated that the surgical repair of PE patients improves these socio-psychologic problems [25].
HYPOTHESES AND PATHOPHYSIOLOGICAL CONCEPTS
There exist several hypotheses regarding its pathogenesis. Bauhinus [1] gave the first pathophysiological hypothesis, mentioning a hypertension of the diaphragm during embryonic development as the pathophysiologic factor. At the end of the nineteenth and beginning of the twentieth century, one leading opinion on pathogenesis was intrauterine pressure on the sternum through an abnormal position of the embryo [5, 8]. An alternative idea invoked acquired damage caused by a permanent mechanical stress through an extreme position, given by cobblers. Further hypotheses highlighted other diseases such as syphilis or rickets as the reason for PE. In contrast, Brown [26] published in 1939 his observations of a thickened ligamentum substernale, which should lead to a retraction of the sternum. A further hypothesis favoured an imbalance between the anterior and posterior musculature of the muscle fibres of the anterior part of the diaphragm with a movement of the xiphoid and sternum backwards.
Today's leading hypotheses focused on a defective metabolism in the sternocostal cartilage, resulting in a biomechanical weakness and an overgrowth of the sternocostal cartilage [27]. The latter hypothesis was proven by Fokin et al. [28], who found a variable cellularity and matrix disorganization in the cartilage of PE patients. However, Nakaoka et al. [29] demonstrated that the costal cartilage on the side of the deepest impression is no longer compared with that of the contralateral side.
A systematic analysis of the histological changes in the sternocostal cartilage of PE patients revealed a premature ageing of the cartilage [30]. Regarding the cause for an inadequate degradation, an ultrastructural and biochemical study demonstrated abnormalities in the content of trace elements in the costal cartilage from PE patients, namely decreased levels of zinc and increased levels of magnesium and calcium [31], who demonstrated that the lack of zinc in the diet results in a lower metabolic activity of chondrocytes. Feng et al. [32] were able to demonstrate the deficit of the biomechanical qualities in the cartilage from PE patients. These findings give interesting insights in the correlation of metabolic lesions and mechanical properties of the cartilage in PE.
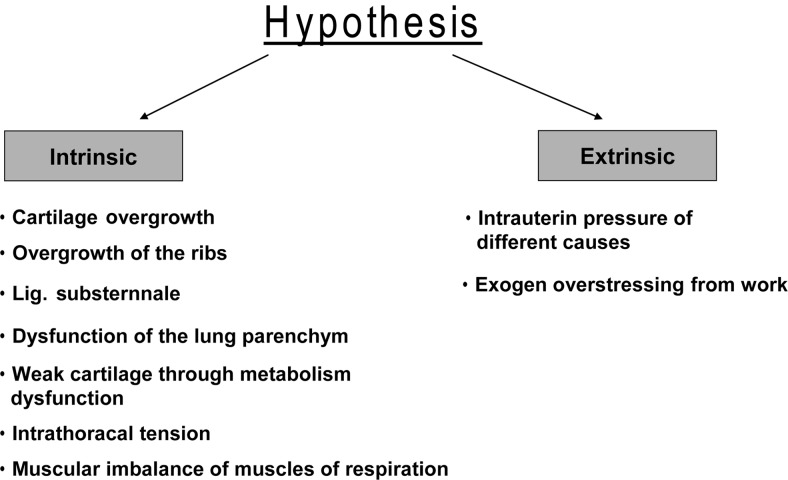
Finally, two main hypotheses for the pathogenesis of PE exist: a developmental disorder or cartilage overgrowth. In the latter, overgrowth caused by maturation disturbances is discussed. Taking these two aspects together, the potential relationship to the development of the ribs and the sternocostal joint is of interest. A summary of the pathophysiological concepts is given in Fig. 1.
Figure 1:
Schematic depiction of the different hypotheses about intrinsic and extrinsic pathogenetic factors.
EMBRYONIC DEVELOPMENT OF THE RIBS, STERNUM AND STERNOCOSTAL JUNCTIONS
The trunk develops from the mesoderm. These cells undergo an epithelial–mesenchymal transition (EMT), creating mesenchymal cells, which are arranged on each side of the chorda dorsalis and result in the paraxial mesoderm. The mesenchymal cells condense to cell clusters and hence lead to the segmental-oriented somites. During the fourth week, the somites differentiate in the ventromedial sclerotome and the dorsolateral dermomyotome. The ribs are formed through resegmentation of the sclerotomes with the beginning of the fifth embryological week. They begin growing from the thoracic vertebrae towards the ventral body wall where they fuse with the sternum 1 week later. The fusion of the ribs with the sternum occurs when the fusion of the two parallel sternal bands is completed. The chondral templates of the ribs ossify by endochondral ossification, beginning at the rib angle in the sixth week.
The sternum develops from a pair of lateral mesenchymal bands. The sternal bands move towards each other and fuse starting from the cranial end. There is evidence that the ribs develop independently from the sternum [33]. Interestingly, the sternal bands move together but not by the elongation of the ribs [33]. When the sternum bars attach together at the cranial endpoint, a single interclavicular blastema occurs which generates the manubrium and clavicle. At the end of the embryonic period, a chondral model of the manubrium sterni, the sternum itself and the processus xiphoideus are generated. The first ossification centre of the sternum appears at the sixth month and ossification is completed after the 12th year.
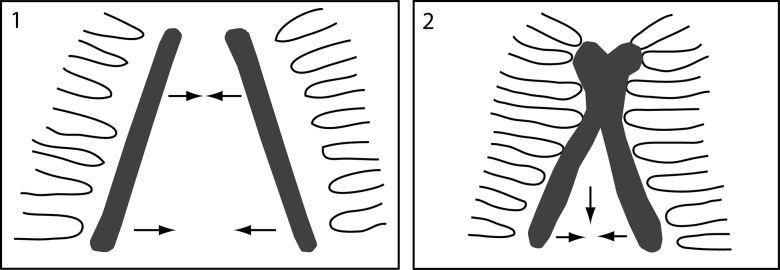
The development of the sternocostal joint is correlated with that of the sternum and the ribs [33]. The ribs come into contact wherever fusion of the sternum bands is completed. The presence of the sternal tissue band is a prerequisite for the formation of the ventral part of the rib (Fig. 2). However, until today, it is not clear whether mechanical stimulation, cell–cell interactions or messenger substances are principally responsible for the normal formation of the sternum. During development of the sternocostal articulation, scattered chondroblasts are involved [33]. The separation between the ribs and the sternum starts by the invasion of cells from the perichondrium between the ribs and the sternum. First, they produce layers of cells between the ribs and the sternum. Then, the cells in the middle layers orientate in a radial direction and form the basis for the sternocostal joints. It remains a matter of debate as to what extent the regulation of these processes is responsible for the pathophysiology of chest wall deformities. In this context, the analyses of the dynamic processes of ossification and cartilage maturation could open innovative perspectives.
Figure 2:
Schematic depiction of the embryological development of the sternum and the sterno-costal articulation via fusion of the sternal bands from cranial to caudal.
TREATMENT OF PECTUS EXCAVATUM
After the first documented surgical correction of PE by Meyer [9], Ravitch [34] performed a trend-setting open intervention technique in 1949. Six years later, Rehbein and Wernicke [35] used crossed metal blades during the operation to stabilize the chest wall.
The open approach of subperichondrial resection of all deformed costal cartilages, xiphoid resection and sternal osteotomy with anterior fixation of the sternum using mostly multiple bar implantation represented the gold standard up to the beginning of the last decade. The procedure consists of a median longitudinal incision along the sternum in males and a submammary skin incision in females, resection of the deformed costal cartilages and a complete mobilization of the sternum, usually requiring excessive retrosternal dissection and sternal transversal osteotomies. The final correction form of the ventral thorax aperture is fixed and stabilized using different metallic bar forms in a different number of implanted bars and their positioning, longitudinally and/or transversally, depending on the surgeon's preference. The operation time needed for the open correction ranges from 2 to 5 h and often a perioperative blood transfusion is necessary. Moreover, chest tubes and multiple wound drains are used in the majority of cases. Patient-controlled intravenous analgesia (PCA) or epidural catheter and/or opioid therapy for pain control are used most of the times. Patients are discharged from the hospital after 6–8 days on average. The implanted bars are removed 1 year after surgery during a short hospital stay of 2 to 3 days.
In 1998, Nuss et al. [36] introduced a minimally invasive technique as an alternative to the standard open repair, the so-called minimally invasive repair of pectus excavatum or minimal access repair of pectus excavatum. The Nuss procedure, which raises the sternum with a retrosternal metallic bar which is placed under thoracoscopic control, is based on the flexibility of the thorax in young subjects, making effective correction possible without the need for extensive costal cartilage resection or sternal osteotomy. The surgical procedure of the Nuss procedure consists of identifying the most depressed zone and the most everted costal line on the left and right side of the sternum, preparing the rectilinear metallic bar using flexible templates for the modulation of the bar length and curve shape. A slight overcorrection of the sternum is aimed for while the stem is modulated. For this procedure, two skin incisions of 3–4 cm in length are made at the right and left middle axillary lines. Next, skin flaps are raised and tunnelled up to the predetermined points at the most-everted intercostals space. A thoracoscope is inserted between the two intercostal spaces below right thoracic incision for the visual control of instrumentation in the retrosternal space and to identify the deepest point of sternal depression. A metal introducer is entered intrathoracally at the predetermined points at the most everted intercostals space on the patient's right side facing forward, thus dissecting the plane separating the sternum from the pericardium. The guide is then exteriorized via the left side at the same level and then pushed through the skin incision. A tie is attached to the end of the introducer bar and the (trial bar) introducer is then removed backwards on the right side under thoracoscopic guidance, which allows the passage of the tie from left to right. The tie is then attached to the final bar, so that it can be inserted with the concave side facing forward from right to left, guided by the tie, while it is pulled up on the left. Under thoracoscopy, the retrosternal curve bar is rotated by 180° around its axis, so that the concave side faces backwards, thus pushing the sternum in ventral direction.
The bar is connected with one stabilizer on each side and attached to the lateral muscles of the chest wall. Some surgeons use one stabilizer on the right side only, but others additionally wire the bar to a rib with two or more subperiostal cable wires. At the end of the procedure, the thoracic cavity is explored by the thoracoscope to ensure that there are no organic lesions or that the lung is trapped between the bar and the chest wall, and to evaluate possible bleeding. No systematic thoracic drainage or wound drains are left postoperatively, and the minor degree of pneumothorax often seen on postoperative plane chest X-ray has no consequences. The operative duration is 50 min on average and usually no ICU care is required. Pain control is managed in a manner identical to the description above and the length of hospital stay is usually 4–5 days. Previously, the bar was removed 2 years after surgery, although in the last years it is recommended to leave the bar in situ for 3 years. A modification for reducing severe complications such as cardiac perforation during Nuss procedure was established by Ohno et al. [37], who recommended, based on their experience, that the tip of the introducer has to be observed at all time under right and left thoracoscopic guidance.
Other operational techniques described in the literature for the correction of PE wall are the method of Leonard or Robicsek, which both represent modifications of the Ravitch operation. In the approach of the Leonard modification, a curvilinear incision is made over the sternum and after mobilization of the pectoral muscles the lower costal cartilage is removed, whereas the perichondrium is left in space. A wedge osteotomy is performed and instead of a bar there is a wire placed behind the sternum which is pulled up through separate stab incisions fixed to an external brace. The brace is worn for about 3 months [38].
During the method of Robicsek et al. [39], there is bilateral resection of the defective costal cartilages and a transverse wedge sternotomy. After mobilization of the sternum in the correct position, it is stabilized from behind through a Marlex mesh which is fixed to the cartilage remnants.
Different minimal invasive approaches are the so-called Erlangen technique and the magnetic mini-mover procedure: the operation with the ‘Erlangen technique’ is characterized by minimal resection of the cartilage and reduced anterolateral mobilization. The sternum is mobilized by retrosternal dissection via an anterior incision, and an elastic metal bar is implanted transsternally through stitch incisions. Minimal resection of a cartilage is provided by intraoperative tensiometry. This technique measures, at defined intervals, the necessary forces to elevate the chest wall and determines whether complete division is necessary. The metal bar will be removed after 1 year [40].
During the magnetic mini-mover procedure, a magnet is placed during this operational intervention upon the sternum. With a second magnet on a brace which must be worn by the patient, the magnetic force pulls the sternum out of its depressed position. The brace must be worn several months. In this operation, a metal plate is brought behind the sternum used as a counter-support for the magnet which will be placed on the sternum. Harrison et al. [41] reported that they were able to terminate this operation within 30 min. The operation needs general anaesthesia, but it is possible that patients can leave the hospital at the same day of surgical intervention. The detailed outcomes will be reported when Phase II of the clinical trial will be completed.
There is one surgical treatment of PE via sternochondroplasty introduced by Lacquet without the use of prosthetic material and with good long-term results [42]. In a recent survey, the sternochondroplasty is described to be superior to the Nuss procedure in the cases of asymmetric PE [43].
A non-surgical alternative in the treatment of PE represents the vacuum bell. Placed on the thorax, the patient produces a negative pressure with a handpump each day over several months. Schier et al. [44] demonstrated that the vacuum bell could be an alternative to the surgical treatment in less severe cases and in the preoperative preparation. Haecker [45] published 133 patients and confirmed the conclusions of Schier et al. [44]. However, long-term results are still lacking.
COMPARISON OF THE DIFFERENT SURGICAL INTERVENTIONS
Taking the different treatment options together, it becomes obvious that not all surgical methods are applicable for all manifestations of PE. In this context, Harrison et al. [41] demonstrated that an asymmetry of the sternum poses significant problems for the magnetic mini-mover. According to Coelho et al. [43], the sternochondroplasty is predominant in comparison to the Nuss procedure in the cases of asymmetric PE. A recent meta-analysis of the experience of the Ravitch and the Nuss procedure identified no differences in overall complications and length of hospital stay between these procedures [46]. In this meta-analysis, the Ravitch procedure revealed lower rates of re-operation, postoperative haemothorax and pneumothorax than in the Nuss procedure. Also in terms of patient satisfaction, no differences could be found between the different surgical techniques [46]. In a further survey by Antonoff et al. [38], the comparison between Ravitch, Nuss and Leonard operation procedures clearly demonstrated the highest levels for length of hospitalization, fees, analgesic needs and complication rates within the Nuss group, whereas in the patients treated with the Leonard operation the lowest values in these parameters could be found. Regarding the health-related quality-of-life (HRQL) outcomes, no significant differences were found between the Nuss and Ravitch procedure [47].
Taking these data of different treatment options together, an early long-term follow-up by Lacquet et al. [48] revealed that the subjective physical improvement after operation could not be explained by changes in static lung volumes or in cardiorespiratory function at exercise, but should be due to other unexplained factors.
FUTURE PERSPECTIVES FOR THE CORRECTION OF PECTUS EXCAVATUM
Most PE operation techniques are based on a stabilizing metal material which has to be removed in a second intervention, a fact which implicates several complications. First of all, a second intervention has to be performed [49]. In addition, the metal devices can shift and migrate into neighbouring tissue [49]. Furthermore, the introduced material can also cause problems such as postoperative chronic pain [32, 50]. In this context, the so-called Strasbourg Thorax Osteosynthesis System (STRATOS®), using titanium implants, reduced the risks of material shifting and allergy [51]. With a view to new biomedical material developments, the use of absorbable material is of interest, since a second intervention would not be necessary. Several approaches using absorbable material for the correction of chest wall deformities were performed [20, 50, 52, 53]. Torre et al. [53] described the use of stabilizers made of poly-L-lactic-polyglycolic polymer. However, Pilegaard and Licht [54] published unsatisfying results using this device: they found a higher complication rate of the absorbable stabilizers for the Nuss technique.
Luzzi et al. [20] retrospectively analysed the treatment of PE using a bioabsorbable mesh instead of a metallic device in the Robiscek technique after a 10-year experience. They highlighted a lower inflammatory reaction, avoiding complications of retrosternal device dislocation, the reduction of re-operations to remove the metal devices and reduced postoperative chest pain allowing an earlier mobilization of the patient [20]. Länsman et al. [50] used bioabsorbable plates for the correction of PE in children and also demonstrated good results in the surgical correction. In addition, Gürkök et al. [52] reported the use of a resorbable copolymer plaque stating the advantage of a single operation. However, by using biodegradable materials, problems such as mechanical instability or a relevant pH shift due to the degradation with consecutive relevant inflammatory response should be taken into account. In this context, long-term experiments analysing the biocompatibility and potential recurrence of PE by use of different materials are needed to obtain information about the optimal material. Furthermore, trials are needed to define in which cases biodegradable materials should be preferred and in which cases metals would be the better choice. In this context, the systematic analyses of the biomechanics and the various forces acting in the thoracic cage would be very helpful in further optimizing or developing new material approaches using either biodegradable or non-biodegradable materials or new shape-memory metal devices. New approaches like these could open innovative perspectives for the rational use of different biomaterials for the correction of chest wall deformities.
Conflict of interest: none declared.
REFERENCES
- 1.Bauhinus J. Johannes Observatorium medicarum, rararum, novarum, admirabilium, et montrosarum, liber secundus. Frankfurt: De partibus vitalibus, thorace contentis; 1609. Observatio; p. 322. In: Ioannis Schenckii a Grafenberg, ed. [Google Scholar]
- 2.Woillez . Paris: Rap Soc Med d'Hop; 1860. Sur un cas de deformitée thoracique considérable avec deplacement inoffensif de plusieur organes et signes sthetoscopiaques particulières; p. 3. [Google Scholar]
- 3.von Luschka H. Die Anatomie der Brust des Menschen. Tübingen: Laupp; 1863. Die Anatomie des Menschen in Rücksicht auf die Bedürfnisse der praktischen Heilkunde; p. 23. [Google Scholar]
- 4.Eggel. Eine seltene Mißbildung des Thorax. Virchows Arch Path Anat. 1870;49:230. [Google Scholar]
- 5.Williams CT. Congenital malformation of the thorax great depression of the sternum. Trans Path Soc. 1872;24:50. [Google Scholar]
- 6.Flesch M. Über eine seltene Missbildung des Thorax. Virchows Arch Path Anat. 1873;75:289. [Google Scholar]
- 7.Hagmann. Selten vorkommende Abnormität des Brustkastens. Jb Kinderheilkunde. 1888;15:455. [Google Scholar]
- 8.Langer E. Zuckerkandel: Untersuchungen über den mißbildeten Brustkorb des. Herrn JW Wiener med Zeit. 1880;49:515. [Google Scholar]
- 9.Meyer L. Zur chirurgischen Behandlung der angeborenen Trichterbrust. Berl Klin Wschr. 1911;48:1563–66. [Google Scholar]
- 10.Kelly RE, Jr, Lawson ML, Paidas CN, Hruban RH. Pectus excavatum in a 112-year autopsy series: anatomic findings and the effect on survival. J Pediatr Surg. 2005;40:1275–8. doi: 10.1016/j.jpedsurg.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Ravitch MM. Repair of pectus excavatum in children under 3 years of age: a twelve-year experience. Ann Thorac Surg. 1977;23:301. doi: 10.1016/s0003-4975(10)64129-x. [DOI] [PubMed] [Google Scholar]
- 12.Kelly RE, Jr, Shamberger RC, Mellins RB, Mitchell KK, Lawson ML, Oldham K, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg. 2007;205:205–16. doi: 10.1016/j.jamcollsurg.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Fonkalsrud EW, Dunn JC, Atkinson JB. Repair of pectus excavatum deformities: 30 years of experience with 375 patients. Ann Surg. 2000;231:443–8. doi: 10.1097/00000658-200003000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotzot D, Schwabegger AH. Etiology of chest wall deformities—a genetic review for the treating physician. J Pediatr Surg. 2009;44:2004–11. doi: 10.1016/j.jpedsurg.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Colombani PM. Preoperative assessment of chest wall deformities. Semin Thorac Cardiovasc Surg. 2009;21:58–63. doi: 10.1053/j.semtcvs.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Beiser GD, Epstein SE, Stampfer M, Goldstein RE, Noland SP, Levitsky S. Impairment of cardiac function in patients with pectus excavatum, with improvement after operative correction. N Engl J Med. 1972;287:267–72. doi: 10.1056/NEJM197208102870602. [DOI] [PubMed] [Google Scholar]
- 17.Kubiak R, Habelt S, Hammer J, Hacker FM, Mayr J, Bielek J. Pulmonary function following completion of Minimally Invasive Repair for Pectus Excavatum (MIRPE) Eur J Pediatr Surg. 2007;17:255–60. doi: 10.1055/s-2007-965060. [DOI] [PubMed] [Google Scholar]
- 18.Malek MH, Berger DE, Housh TJ, Marelich WD, Coburn JW, Beck TW. Cardiovascular function following surgical repair of pectus excavatum: a metaanalysis. Chest. 2006;130:506–16. doi: 10.1378/chest.130.2.506. [DOI] [PubMed] [Google Scholar]
- 19.Coln E, Carrasco J, Coln D. Demonstrating relief of cardiac compression with the Nuss minimally invasive repair for pectus excavatum. J Pediatr Surg. 2006;41:683–6. doi: 10.1016/j.jpedsurg.2005.12.009. discussion 683–6. [DOI] [PubMed] [Google Scholar]
- 20.Luzzi L, Voltolini L, Zacharias J, Campione A, Ghiribelli C, Di Bisceglie M, et al. Ten year experience of bioabsorbable mesh support in pectus excavatum repair. Br J Plast Surg. 2004;57:733–40. doi: 10.1016/j.bjps.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Malek MH, Berger DE, Marelich WD, Coburn JW, Beck TW, Housh TJ. Pulmonary function following surgical repair of pectus excavatum: a meta-analysis. Eur J Cardiothorac Surg. 2006;30:637–43. doi: 10.1016/j.ejcts.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Guntheroth WG, Spiers PS. Cardiac function before and after surgery for pectus excavatum. Am J Cardiol. 2007;99:1762–4. doi: 10.1016/j.amjcard.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JN, Hartman TK, Pianosi PT, Driscoll DJ. Cardiorespiratory function after operation for pectus excavatum. J Pediatr. 2008;153:359–64. doi: 10.1016/j.jpeds.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 24.Aronson DC, Bosgraaf RP, Merz EM, van Steenwijk RP, van Aalderen WM, van Baren R. Lung function after the minimal invasive pectus excavatum repair (Nuss procedure) World J Surg. 2007;31:1518–22. doi: 10.1007/s00268-007-9081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly RE, Jr, Cash TF, Shamberger RC, Mitchell KK, Mellins RB, Lawson ML, et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics. 2008;122:1218–22. doi: 10.1542/peds.2007-2723. [DOI] [PubMed] [Google Scholar]
- 26.Brown AL. Pectus excavatum (funnel chest) J Thorac Surg. 1939;9:164–184. [Google Scholar]
- 27.Sweet RH. Pectus excavatum: report of two cases successfully operated upon. Ann Surg. 1944;119:922–34. doi: 10.1097/00000658-194406000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fokin AA, Robicsek F, Watts LT. Genetic analysis of connective tissue in patients with congenital thoracic abnormalities. Interact CardioVasc Thorac Surg. 2008;7:56. [Google Scholar]
- 29.Nakaoka T, Uemura S, Yano T, Nakagawa Y, Tanimoto T, Suehiro S. Does overgrowth of costal cartilage cause pectus excavatum? A study on the lengths of ribs and costal cartilages in asymmetric patients. J Pediatr Surg. 2009;44:1333–6. doi: 10.1016/j.jpedsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Geisbe H, Buddecke E, Flach A, Muller G, Stein U. 88. Biochemical, morphological and physical as well as animal experimental studies on the pathogenesis of funnel chest. Langenbecks Arch Chir. 1967;319:536–41. doi: 10.1007/BF02659333. [DOI] [PubMed] [Google Scholar]
- 31.Rupprecht H, Hummer HP, Stoss H, Waldherr T. Pathogenesis of chest wall abnormalities—electron microscopy studies and trace element analysis of rib cartilage. Z Kinderchir. 1987;42:228–9. doi: 10.1055/s-2008-1075590. [DOI] [PubMed] [Google Scholar]
- 32.Feng J, Hu T, Liu W, Zhang S, Tang Y, Chen R, et al. The biomechanical, morphologic, and histochemical properties of the costal cartilages in children with pectus excavatum. J Pediatr Surg. 2001;36:1770–6. doi: 10.1053/jpsu.2001.28820. [DOI] [PubMed] [Google Scholar]
- 33.Chen JM. Studies on the morphogenesis of the mouse sternum. II. Experiments on the origin of the sternum and its capacity for self-differentiation in vitro. J Anat. 1952;86:387–401. [PMC free article] [PubMed] [Google Scholar]
- 34.Ravitch MM. The operative treatment of pectus excavatum. Ann Surg. 1949;129:429–44. doi: 10.1097/00000658-194904000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehbein F, Wernicke HH. The operative treatment of the funnel chest. Arch Dis Child. 1957;32:5–8. doi: 10.1136/adc.32.161.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuss D, Kelly RE, Jr, Croitoru DP, Katz ME. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545–52. doi: 10.1016/s0022-3468(98)90314-1. [DOI] [PubMed] [Google Scholar]
- 37.Ohno K, Nakamura T, Azuma T, Yamada H, Hayashi H, Masahata K. Modification of the Nuss procedure for pectus excavatum to prevent cardiac perforation. J Pediatr Surg. 2009;44:2426–30. doi: 10.1016/j.jpedsurg.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Antonoff MB, Erickson AE, Hess DJ, Acton RD, Saltzman DA. When patients choose: comparison of Nuss, Ravitch, and Leonard procedures for primary repair of pectus excavatum. J Pediatr Surg. 2009;44:1113–8. doi: 10.1016/j.jpedsurg.2009.02.017. discussion 118–9. [DOI] [PubMed] [Google Scholar]
- 39.Robicsek F, Watts LT, Fokin AA. Surgical repair of pectus excavatum and carinatum. Semin Thorac Cardiovasc Surg. 2009;21:64–75. doi: 10.1053/j.semtcvs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Weber PG, Huemmer HP, Reingruber B. Forces to be overcome in correction of pectus excavatum. J Thorac Cardiovasc Surg. 2006;132:1369–73. doi: 10.1016/j.jtcvs.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Harrison MR, Estefan-Ventura D, Fechter R, Moran AM, Jr, Christensen D. Magnetic mini-mover procedure for pectus excavatum: I. Development, design, and simulations for feasibility and safety. J Pediatr Surg. 2007;42:81–5. doi: 10.1016/j.jpedsurg.2006.09.042. discussion 85–6. [DOI] [PubMed] [Google Scholar]
- 42.Lacquet LK. Surgical treatment of anterior chest wall deformities by sternochondroplasty and internal fixation without prosthesis. Acta Chir Belg. 1982;82:541–9. [PubMed] [Google Scholar]
- 43.Coelho Mde S, Silva RF, Bergonse Neto N, Stori Wde S, Jr, dos Santos AF, Mendes RG, et al. Pectus excavatum surgery: sternochondroplasty versus Nuss procedure. Ann Thorac Surg. 2009;88:1773–9. doi: 10.1016/j.athoracsur.2009.07.051. [DOI] [PubMed] [Google Scholar]
- 44.Schier F, Bahr M, Klobe E. The vacuum chest wall lifter: an innovative, nonsurgical addition to the management of pectus excavatum. J Pediatr Surg. 2005;40:496–500. doi: 10.1016/j.jpedsurg.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 45.Haecker FM. The vacuum bell for conservative treatment of pectus excavatum: the Basle experience. Pediatr Surg Int. 2011;27:623–7. doi: 10.1007/s00383-010-2843-7. [DOI] [PubMed] [Google Scholar]
- 46.Nasr A, Fecteau A, Wales PW. Comparison of the Nuss and the Ravitch procedure for pectus excavatum repair: a meta-analysis. J Pediatr Surg. 2010;45:880–6. doi: 10.1016/j.jpedsurg.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Lam MW, Klassen AF, Montgomery CJ, LeBlanc JG, Skarsgard ED. Quality-of-life outcomes after surgical correction of pectus excavatum: a comparison of the Ravitch and Nuss procedures. J Pediatr Surg. 2008;43:819–25. doi: 10.1016/j.jpedsurg.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 48.Lacquet LK, Morshuis WJ, Folgering HT. Long-term results after correction of anterior chest wall deformities. J Cardiovasc Surg (Torino) 1998;39:683–8. [PubMed] [Google Scholar]
- 49.Stefani A, Morandi U, Lodi R. Migration of pectus excavatum correction metal support into the abdomen. Eur J Cardiothorac Surg. 1998;14:434–6. doi: 10.1016/s1010-7940(98)00190-0. [DOI] [PubMed] [Google Scholar]
- 50.Länsman S, Serlo W, Linna O, Pohjonen T, Törmälä P, Waris T, et al. Treatment of pectus excavatum with bioabsorbable polylactide plates: preliminary results. J Pediatr Surg. 2002;37:1281–6. doi: 10.1053/jpsu.2002.34983. [DOI] [PubMed] [Google Scholar]
- 51.Wihlm JM, Grosdidier G, Chapelier A. Thoracic osteosyntheses for chest wall malformations, traumas and tumors using the STRATOS ™ titanium system. Interact CardioVasc Thorac Surg. 2007;6:273. [Google Scholar]
- 52.Gürkök S, Genc O, Dakak M, Balkanli K. The use of absorbable material in correction of pectus deformities. Eur J Cardiothorac Surg. 2001;19:711–2. doi: 10.1016/s1010-7940(01)00661-3. [DOI] [PubMed] [Google Scholar]
- 53.Torre M, Jasonni V, Asquasciati C, Costanzo S, Romanini MV, Varela P. Absorbable stabilisation of the bar in minimally invasive repair of pectus excavatum. Eur J Pediatr Surg. 2008;18:407–9. doi: 10.1055/s-2008-1039176. [DOI] [PubMed] [Google Scholar]
- 54.Pilegaard HK, Licht PB. Can absorbable stabilizers be used routinely in the Nuss procedure? Eur J Cardiothorac Surg. 2009;35:561–4. doi: 10.1016/j.ejcts.2008.10.049. [DOI] [PubMed] [Google Scholar]