Abstract
Pleural lipomas are benign tumours that develop at the expense of adipose tissues, and they never evolve towards liposarcoma. Located usually at the mediastinal, bronchial and pulmonary levels, a pleural situation is extremely rare. Chest X-rays usually detect them and computed tomography scans confirm the diagnosis. As complications occur, a wait-and-see policy is common. We report our pleural lipoma surgical exeresis experience since 1999. We have operated on five cases of pleural lipomas among nearly 1800 cases of thoracic exeresis: three male and two female patients, without obesity (in all cases, body mass index (BMI) < 28). The mean age was 54.6 years (range 35–72 years). Four patients were electively operated and one in emergency, three with video-assisted thoracic surgery (VATS) procedure and two with open chest surgery, without recurrent cases. Advancements in VATS have greatly reduced the morbidity rate of these benign tumours especially if exeresis is performed early on a small, uncomplicated adhesion-free tumour. On the other hand, the operation may be deleterious, complicated by the presence of a large lipoma or in a complicating situation. In our opinion, we should revise the wait-and-see policy when facing these lesions considering their evolutionary potential. We should advise VATS in pleural lipomas.
Keywords: Pleural disease, Lipoma, Thoracic surgery, Video-assisted thoracic surgery
Lipomas are solid, benign tumours that develop at the expense of adipose tissues and distributed ubiquitously in the body. It is the most frequently observed benign tumour in adults. Intrathoracicly, they are located at the mediastinal, bronchial and pulmonary levels. On the other hand, a pleural localization is extremely rare. They are established by the presence of a tumoral adipose tissue originating from the pleura with a pedicle underscored during surgical or pathological examination. Pleural lipomas are observed indifferently on the visceral, parietal, thoracic or mediastinal pleura. In 1781, Fothergill provided the first description of pleural lipoma. Since then, there have been very few data in the literature, mainly as case reports, with the largest series concerning six patients [1]. It is rather difficult to assess pleural lipoma incidence; however, in a review of 3502 cases of thoracic tumours, Jensen reported only three cases of intrathoracic lipoma [2].
When anatomopathological data are the only means to confirm the diagnosis, it is however accepted that it can be established with radiological data, especially scannographic ones. A solid tumour characterizes it with a density comparable with subcutaneous fat between –50 and –150 Hounsfield Unit. In the literature, data available on the subject are sparse and authors described this lesion as a very stable, slow progressing tumour, with no or hardly any surgical management, which is a noteworthy exception concerning intrathoracic solid tumours. The wait-and-see policy is common. We here report our recent experience with surgical exeresis of pleural lipomas.
MATERIALS AND METHODS
During the last decade, we identified in our database five patients who benefited from pleural lipoma exeresis among more than 1800 cases of thoracic exeresis. We established pleural lipoma diagnosis according to the commonly admitted criteria: tumoral adipose tissue originating from the pleura and the presence of a pedicle underscored during surgery or pathological examination. We retrospectively reviewed all clinical and radiological files. Clinical examination and radiological (chest X-ray and computed tomography [CT] scan) follow-up were completed in all patients.
RESULTS
All data are detailed in Table 1. There was a high suspicion of lipoma in three patients. However, we opted for operative management for the first two due to their previous history of carcinoma and for the third because of the compression of a large tumour of the left lower lobe. A patient was operated on due to her lesion size and eventual liposarcoma. The last patient underwent surgery because of her acute symptomatology and due to insufficient material for a definitive anatomo-pathological diagnosis: germinal tumour or lipoma complicated by haemorrhage.
Table 1:
Patient data
| Patient |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age (years) | 35 | 48 | 56 | 62 | 72 |
| Sex | F | F | M | M | M |
| BMI | 22 | 24 | 21 | 19 | 25 |
| History | Pregnancy, 6 months ago | None | Laryngeal, carcinoma, 7 years ago | Thyroid, carcinoma, 12 years ago | Blunt chest trauma, 10 years ago |
| Symptom | None | Chest pain, fever, dyspnoea | None | None | None |
| Aspiration | 0 | 0 | ± | ± | 0 |
| Previous Chest X-ray | None | 7 months ago, normal | 1 year, normal | 1 year, normal | None |
| CRP | <3 | <3 | <3 | <3 | <3 |
| Surgery | Elective, TPL | Emergency, VATS + L | Elective, TPL | Elective, TPL | Elective, TPL |
| Histological | Adenolipoma | Angiolipoma | Adenolipoma | Adenolipoma | Adenolipoma |
| Dimension (cm) | 19 × 16 × 10 | 9 × 7 × 3 | 2 × 3 × 3 | 2 × 2 × 2.8 | 24 × 20 × 15 |
| Weight (g) | 1044 | 187 | 2020 | ||
0: none; ±: realized but negative; CRP: C-reactive protein; TPL: postero-lateral thoracotomy; VATS: video-assisted thoracic surgery.
Operative courses were uneventful. The mean hospital stay was 5 ± 1.4 days. All patients were discharged from the hospital between the 4th and 7th postoperative days and were followed up at 1 month. Neither residual pain nor local recurrence was observed. At a mean follow-up of 5.4 years, there was no recurrence.
DISCUSSION
Solid tumours localized within the pleura are rare, 2.8 cases per 100 000 hospitalizations in North America [3]. In 73% of cases, they are benign, mainly in the form of fibroma (84%), lipomas represent only 8% of cases. Unlike subcutaneous lesions, intrathoracic lipomas are usually isolated, even though some authors have reported cases of multiple intrathoracic lipomas [4]. They are observed indiscriminately from patients' sex and age [2].
Lipoma is a benign mesenchymatous tumour, derived from pleural adipocytes. Various pathological forms have been described such as the fibrous-composed adenolipoma, the angiolipoma of intratumoral vascular composition and the cardiac lipoma that may calcify further to adipose tissue necrosis, and the dysembryoma, all of which constitute a differential diagnosis.
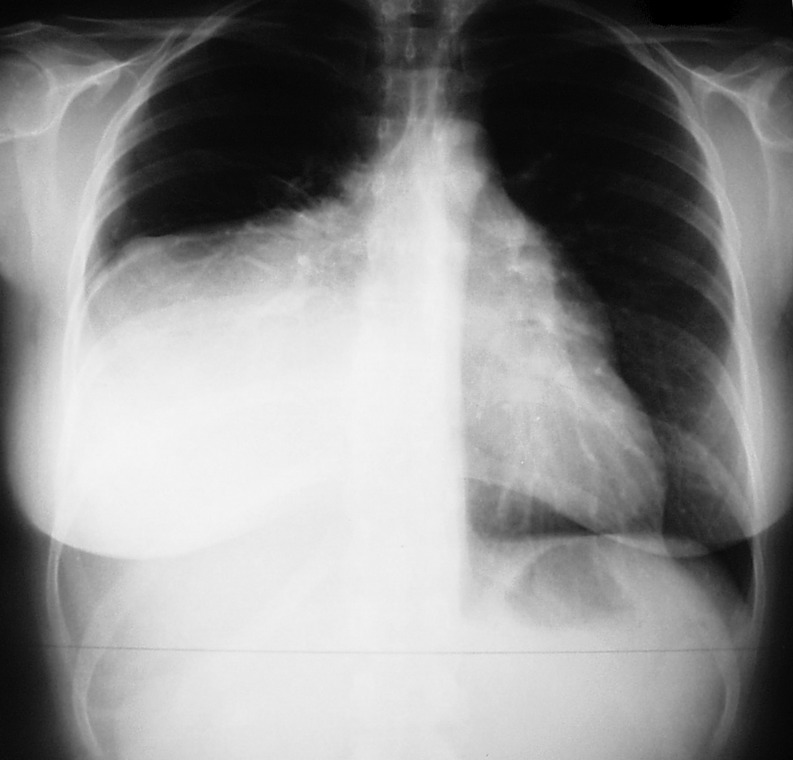
In 8 of 10 times, systematic X-ray examination detected fortuitously these tumours (Fig. 1). In the majority of cases, they are asymptomatic. When symptoms are present, they are non-specific, such as hacking cough, pain, dyspnoea or thoracic tightness sensation. Accompanying signs are occasional. In 11% of cases as part of Pierre Marie's syndrome, clubbing was observed that disappeared after tumour exeresis [5].
Figure 1:
Chest X-ray of a 35-year old female patient with a right basithoracic tumoral process, incidentally discovered, compatible with a lipoma.
Value of chest X-ray is essential in the lipoma discovery mode. While highlighting the tumoral process, it also assesses its consequence notably regarding compression of intrathoracic structure. For tumours of low density, X-ray with a low penetration is better for exposure of the lipoma. As for pedicled tumours, chest fluoroscopy has historically proved its interest to observe tumour motions in accordance with respiratory motion [6].
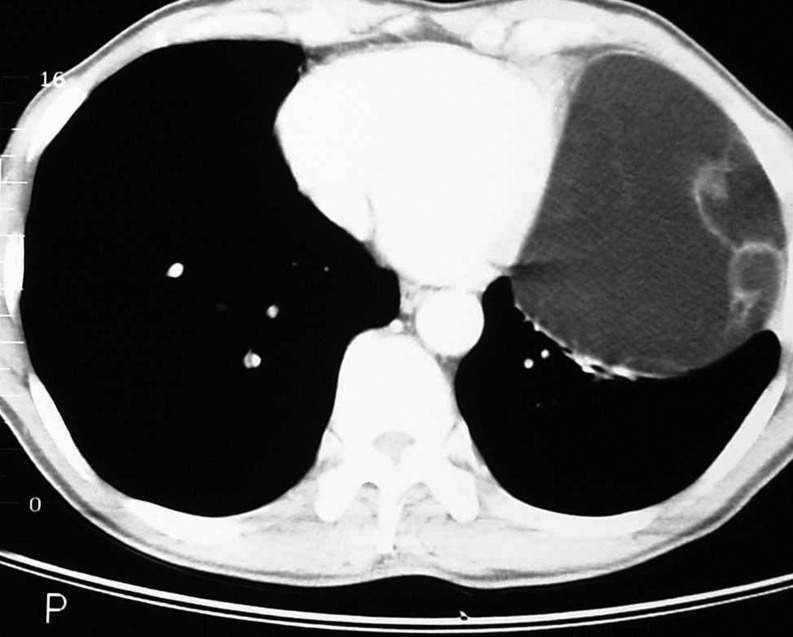
The diagnosis can be solely made upon the CT scan data. Any rounded tumor, originating from the parietal or mediastinal pleura, homogenous, without any calcification, with an adipose density of −50 to −150 UH and not enhanced by an injected contrast medium, is a lipoma [1]. Moreover, the scanner allows the study of connections between lipoma and nearby organs (Fig. 2). Large lipomas can induce lung compression as observed in three of our cases’ ormediastinal deviation. Some authors have even reported parietal evolution with rib lysis [7].
Figure 2:
Sectional CT scan of a left basithoracic tumoral process of fatty density (−127 UH), homogeneous.
If the CT scan is of the utmost utility in lipoma diagnosis, the other imaging modalities are of little importance. Occasionally, thoracic echography comes as a complement, in particular for parietal lipomas by proving the tumour pleural origin and confirming its adipose density and homogeneity. The parietal invasion that characterizes liposarcoma, a differential diagnosis of the lipoma, can be eliminated if the sliding plane between both layers of the pleura is preserved [8].
Magnetic Resonance Imaging (MRI) is only useful if there is a doubt in CT scan diagnosis. It occasionally allows a more refined evaluation of the linkages between both the tumour and the thoracic wall. In our experience, MRI has allowed us to rule out a diaphragmatic hernia and to confirm the strictly intrathoracic location of the pleural lipoma. It is mainly valuable in the lipoma differential diagnosis, the liposarcoma. If this one is enhanced during contrast medium injection, the MRI provides a better analysis of the lipoma fatty density, its heterogeneity and its relationship with contiguous organs [5].
In case of a diagnostic doubt at the scanner or if there is a metastasis evocative context, diagnosis can be confirmed by a fine-needle aspiration [9]. It has no other indication. We performed it in two patients with previous history of cancer. However, it has a limited interest. Only a positive metastasis diagnosis of an unresectable tumour will prevent surgical exeresis. In all other cases, patients underwent surgical exeresis.
There is a triple purpose to surgical exploration of an intrathoracic lipoma. It confirms the diagnosis by a histopathological analysis especially in the case of an inhomogeneous mass. It treats the symptomatology, and finally it limits the compression of adjoining organs.
Being a slow-evolving tumour, surgical exploration of these solid intrathoracic tumoral processes should be limited [1]. In our experience, this evolution was not so slow. Indeed, two of our patients with previously normal chest X-ray at 7 months and at 3 years presented however with lipomas of 9 × 7 × 3 cm and 187 g and 19 × 16 × 10 cm and 1044 g, respectively (Fig. 3). Therefore, evolution of these tumours varies certainly from one patient to another. The fast onset of the tumor led us to operate even with adipose density. On the other hand, the secondary transformation of lipoma into liposarcoma has never been reported in the literature. Therefore, exeresis of the tumour cannot be performed to prevent such an evolution.
Figure 3:
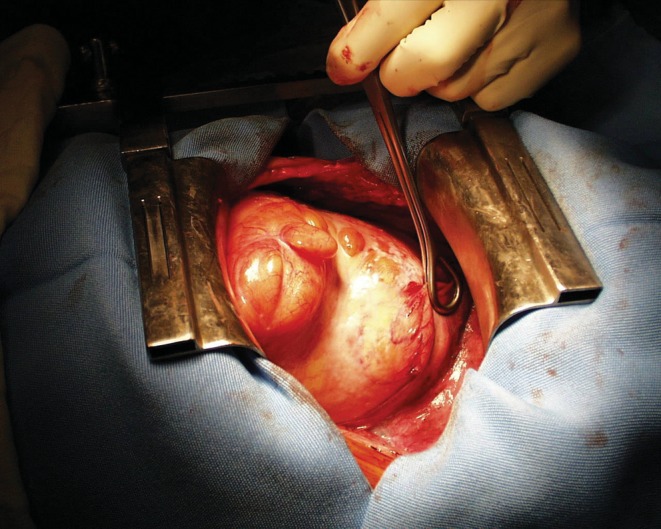
Lipoma weighing 1044 g, 19 × 16 × 10 cm in size, resected through postero-lateral thoracotomy.
Most of the reported cases in the literature mentioned one or two observations. It is difficult to assess the incidence of these pathology-related complications, which nevertheless are not rare in view of the reported number of cases. This is what we report in our experience. The first known complication is adjoining organs compression, these lipomas having significant volumes with an obstructive syndrome. They can invade intercostal spaces and induce rib lysis [7]. As per our experience, intratumoral haemorrhage with pain and fever has already been reported [10]. Surgical exeresis is then inevitable to diagnose and to treat. Nonetheless, this is an emergency surgery usually performed through postero-lateral thoracotomy with its own complications. These complications appear in the case of large lipomas raising the debate of the need for surgery prior to the complication stage.
Finally, some authors have reported cases of patients who were operated on not only because of diagnosed radiologic certainty but also because of patient's anxiety [5]. This anxiety may be worsened in patients with a previous history of treated neoplasia, as with both cases in our series. Despite benign tumour diagnosis, the lack of diagnostic certainty is difficult to accept.
Actually, if the largest series of pleural lipomas (six patients) advocates surgical refraining [1], it was published before videothoracoscopy [11]. This surgery is easier when performed at an early stage. In fact, these tumours may develop adjoining adhesions over time with mediastinum or lung parenchyma (two cases in our series). Furthermore, these tumours present with a variable growing rate and consequently a progressive compression of the lung parenchyma, inducing a restrictive syndrome. Complications may lead to emergency surgery of the tumour. When it is undeniable that thoracotomy indication is to be sparingly decided upon, low risk and good tolerance of video-assisted surgery should not put off surgical exploration [3, 10]. Indeed, complications are not so rare and early exeresis remains the best guarantee to avoid a more extensive, riskier and less-tolerated surgery later on, even more since long-term monitoring can be expensive and difficult to perform.
In conclusion, pleural lipomas may cause complications such as haemorrhage and adjoining organ compression. Despite an increasingly sophisticated radiological exploration, diagnostic uncertainty can exist. Video-assisted surgery improvements have enabled the considerable reduction in surgical morbidity of these benign tumours, especially if there is an early exeresis. On the other hand, in the presence of complications, surgery can be more difficult to perform. In our opinion, a more radical approach should be chosen upon the detection of these lesions.
Conflict of interest: none declared.
REFERENCES
- 1.Epler GR, McLoud TC, Munn CS, Colby TV. Pleural lipoma. Diagnosis by computed tomography. Chest. 1986;90:265–8. doi: 10.1378/chest.90.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MS, Petersen AH. Bronchial lipoma. Scand J Thorac Cardiovasc Surg. 1970;4:131–4. doi: 10.3109/14017437009134253. [DOI] [PubMed] [Google Scholar]
- 3.Furrer M, Inderbitzi R. Case report: endoscopic resection of a 5 cm intrathoracic lipoma. Pneumologie. 1992;46:334–5. [PubMed] [Google Scholar]
- 4.Jones EL, Lucey JJ, Taylor AB. Intrapulmonary lipoma associated with multiple pulmonary hamartomas. Br J Surg. 1973;60:75–8. doi: 10.1002/bjs.1800600123. [DOI] [PubMed] [Google Scholar]
- 5.Pinton F, Brousse D, Lemarié E, Asquier E, Sommerville A. Lipome pleural. Rev Mal resp. 1988;12:169–72. [PubMed] [Google Scholar]
- 6.Gramiak R, Koerner HJ. A roentgen diagnostic observation in subpleural lipoma. Am J Roentgenol Radium Ther Nucl Med. 1966;98:465–7. doi: 10.2214/ajr.98.2.465. [DOI] [PubMed] [Google Scholar]
- 7.Buxton RC, Tan CS, Khane NM, Cuasay NS, Shor MJ, Spigos DG. Atypical transmural thoracic lipoma–CT diagnosis. J Comput Assist Tomogr. 1998;12:196–8. doi: 10.1097/00004728-198803000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Fournier D. Echographie thoracique. Med Hyg. 1993;51:1825–33. [Google Scholar]
- 9.Millward S, Escott N, Masood K. The diagnosis of a pleural lipoma by CT and fine needle biopsy to avoid thoracotomy. Can Assoc Radiol J. 1988;39:57–8. [PubMed] [Google Scholar]
- 10.Geis JR, Russ PD, Adcock KA. Computed tomography of a symptomatic infarcted thoracic lipoma. J Comput Tomogr. 1988;12:54–6. doi: 10.1016/0149-936x(88)90031-8. [DOI] [PubMed] [Google Scholar]
- 11.Roviaro G, Rebuffat C, Varoli F, Vergani C, Maciocco M, Scalambra SM. Videothoracoscopic excision of mediastinal masses : indications and technique. Ann Thorac Surg. 1994;58:1679–83. doi: 10.1016/0003-4975(94)91658-6. [DOI] [PubMed] [Google Scholar]