Abstract
We assessed the prognostic value of the ‘Zone-classification’ which has been proposed by the Japanese Association for Lung Cancer (JALC) for mediastinal nodal metastases in non-small cell lung cancer (NSCLC). Among 357 NSCLC patients who underwent curative surgery, 46 patients with pathological (p) N2 disease were divided into two groups as follows: 32 patients in whom the nearer zone was involved were classified as the pN2a-1 group, and 14 patients in whom the further mediastinal node station was involved were classified as the pN2a-2 group. The proportions of patients with non-adenocarcinoma histology, with multiple station metastases with the involvement of four or more nodes, and who underwent pneumonectomy, were higher in the pN2a-2 group. The ‘Zone-classification’ proved to be a significant prognostic factor in a univariate analysis (the 5-year overall survival rate, 7.1% for pN2a-2 versus 21.9% for pN2a-1; P < 0.01). A multivariate analysis confirmed that pN2a-2 sub-classification (hazard ratio 2.77; P = 0.03) and undergoing pneumonectomy (hazard ratio 4.86; P < 0.01) were independent and significant factors in predicting a poor prognosis. In pN2 NSCLC patients, the involved mediastinal zone according to the primary tumour site was important in prediction of survival.
Keywords: Lung cancer surgery, Lymph nodes (mediastinal), Statistics, Survival analysis
INTRODUCTION
Pathological N (pN) 2 NSCLC is heterogeneous, and many studies have evaluated the validity of various prognostic factors among pN2 non-small cell lung cancer (NSCLC) patients in order to identify a more accurate classification system [1–4].
The seventh edition of the TNM classification revised by the International Association for the Study of Lung Cancer (IASLC) has started to be applied [4]. Following the revision of IASLC-TNM, the Japan Lung Cancer Society (JLCS) proposed a new TNM classification. In the JLCS-TNM classification, both the TNM staging and the lymph node map are the same as those of the IASLC; however, it also described a new classification (‘Zone-classification’) for mediastinal nodal metastases according to the site of the primary tumour [5].
The relationship between ‘Zone-classification’ and prognosis has not been explored so far. Thus, we assessed the prognostic value of the ‘Zone-classification’ in resected NSCLC cancer.
PATIENTS AND METHODS
Patients
Data were collected from a total of 357 lung cancer patients who underwent surgery at the University of Occupational and Environmental Health, Japan, between January 1997 and December 2002. Among them, 15 patients with double primary lung cancer were excluded. There were 252 pN 0 patients and 44 pN1 patients. Forty-six of those patients who underwent completely resected NSCLC and were diagnosed as pN 2, were retrospectively analysed. The patients who had received preoperative therapy were excluded. All patients, except one, who underwent hilar lymph-node dissection and removal of the representative mediastinal lymph nodes (ND1b), had systemically dissected regional lymph nodes (ND2a).
The lymph node classification of Naruke's map was used. Lymph node stations 1–4 of Naruke's map were grouped into the upper zone, stations 5 and 6 into the aortopulmonary zone and 8 and 9 into the lower zone, while station 7 was designated as the subcarinal zone. Patients with pN2 status were divided into two groups based on the location of the primary site and the zone of the metastatic mediastinal nodes, as shown in Table 1. The patients with N2a-2 involvement were categorized as pN2a-2 in this study. The patients in whom N2a-2 nodes were free from metastasis but had involvement of the N2a-1 zone were categorized as pN2a-1. A single-station pN2 was defined as one station of the mediastinal lymph node involved, and a multiple-station pN2 was defined as more than one mediastinal station involved. The number of metastatic nodes both in the regional hilar and mediastinal nodes was used as the number of metastatic nodes.
Table 1:
pN2 subgroups based on the primary tumour site and the involved mediastinal zone
| Location of primary cancer | Categorization |
|
|---|---|---|
| pN2a-1 | pN2a-2 | |
| Rt. upper lobe | Rt. upper zonea | Subcarinal and Rt. lower zonee |
| Rt. middle lobe | Rt. upper and subcarinal zoneb | Rt. lower zone |
| Rt. lower lobe | Subcarinal and Rt. lower zone | Rt. upper zone |
| Lt. upper division | Lt. upperc and aortopulmonary zoned | Subracinal and Lt. lower zone |
| Lt. lingular division | –f | –f |
| Lt. lower lobe | Lt. subcarinal and lower zone | Lt. upper and aortopulmonary zone |
aMediastinal nodes in the rt. upper and/or lower paratracheal were pathologically involved.
bSub-carinal mediastinal nodes were the pathologically involved.
cMediastinal nodes in the lt. paratracheal region were pathologically involved.
dMediastinal nodes in the para- and sub aortic arch were pathologically involved.
eIpsilateral mediastinal nodes in the para-oesophageal and/or pulmonary ligament were pathologically involved.
fThere were no patients with a primary lesion located in the lingular division in this study.
All resected specimens underwent a pathological examination, and the pathological T factor was classified according to the 7th edition of the TNM classification described by the IASLC. The patients were followed-up every month during the first postoperative year and at ∼2–4-month intervals thereafter.
Statistical analysis
The overall survival (OS) was calculated from the day of the surgery to the known date of death according to hospital medical records. Disease-free survival (DFS) was defined as the time from the operation to the first event of either recurrence of disease or death. The patient was censored for DFS on the last date on which the medical records were available if the medical records did not show any evidence of recurrence or death. The OS and DFS were measured for each patient from the day of surgical treatment. The survival curves were estimated using the Kaplan–Meier method, and differences among them were evaluated by the log-rank test. The univariate and multivariate analyses were performed using the Cox proportional hazard model. Independent t-tests were used for two-group comparisons of continuous variables. The categorical data of the cross-tabulation tables were compared using Fisher's exact test. A value of P < 0.05 was considered to be significant. We used the R statistical package (www.r-project.org) for all of the analyses.
RESULTS
Thirty-two patients were classified into the pN2a-1 group, and 14 into the pN2a-2 group, as shown in Table 1. The mean follow-up time was 43.9 months (range; 2–142 months).
Comparisons between the status of subgroups and the clinical characteristics in the patients are presented in Table 2. The comparison between the present categorization (pN2a-1/pN2a-2) and other subgroups categorized by the number of involved stations and the number of metastatic nodes is shown in Table 3. These subgroups did not differ significantly in terms of age, gender, clinical N factor, location of the primary cancer, type of lymph node dissection, pathological T factor or status of adjuvant chemotherapy. There were no postoperative mortalities among these cases, and three patients with pN2a-1 status underwent adjuvant chemotherapy using concomitant carboplatin and paclitaxel. The proportions of patients with non-adenocarcinoma histology (P = 002), with multiple-station metastases (P < 0.01) with the involvement of 4 or more nodes (P < 0.01) and who underwent pneumonectomy (P < 0.01) were higher in the pN2a-2 group than in the pN2a-1 group.
Table 2:
Patient characteristics by subgroup
| pN2a-1 (n = 32) | pN2a-2 (n = 14) | P-value | |
|---|---|---|---|
| Age | |||
| Mean (range) | 67.6 (47–84) | 68.3 (54–85) | 0.8; NS |
| Gender | |||
| Male/female | 24/8 | 11/3 | 1.0; NS |
| Clinical N factor | |||
| N0 | 16 | 5 | 0.7; NS |
| N1 | 3 | 1 | |
| N2 | 13 | 8 | |
| Location of primary cancer | |||
| Rt. upper lobe | 10 | 2 | 0.5; NS |
| Rt. middle lobe | 2 | 0 | |
| Rt. lower lobe | 5 | 3 | |
| Lt. upper division | 10 | 4 | |
| Lt. lingular division | 0 | 0 | |
| Lt. lower lobe | 5 | 5 | |
| Type of pulmonary resection | |||
| Lobectomy | 29 | 8 | <0.01 |
| Pneumonoectomy | 3 | 6 | |
| Type of lymph-node dissection | |||
| ND1b+ sampling | 1 | 0 | 1.0; NS |
| ND2a | 31 | 14 | |
| Number of dissected nodes | |||
| Mean (range) | 23.5 (9–49) | 17.9 (5–34) | 0.07; NS |
| Pathological T factor | |||
| T1 (1a/1b) | 7 (3/4) | 3 (0/3) | 0.08; NS |
| T2 (2a/2b) | 16 (11/5) | 3 (1/2) | |
| T3 | 9 | 6 | |
| T4 | 0 | 2 | |
| Histology | |||
| Adenocarcinoma | 21 | 3 | 0.02 |
| Squamous cell ca. | 8 | 8 | |
| Large cell ca. | 2 | 3 | |
| Adenosquamous cell ca. | 1 | 0 | |
| Adjuvant therapy | |||
| Chemotherapy | 3 | 0 | 0.5; NS |
| None | 29 | 14 | |
NS: not significant.
Table 3:
The relationship between the present categorization, the number of metastatic nodes and the number of involved stations
| Number of involved pN2 station |
P-value | Number of metastatic nodes |
P-value | |||
|---|---|---|---|---|---|---|
| Single station pN2 | Multiple stations pN2 | <4 | ≥4 | |||
| pN2a-1 | 22 | 10 | <0.01 | 19 | 13 | <0.01 |
| pN2a-2 | 3 | 11 | 1 | 13 | ||
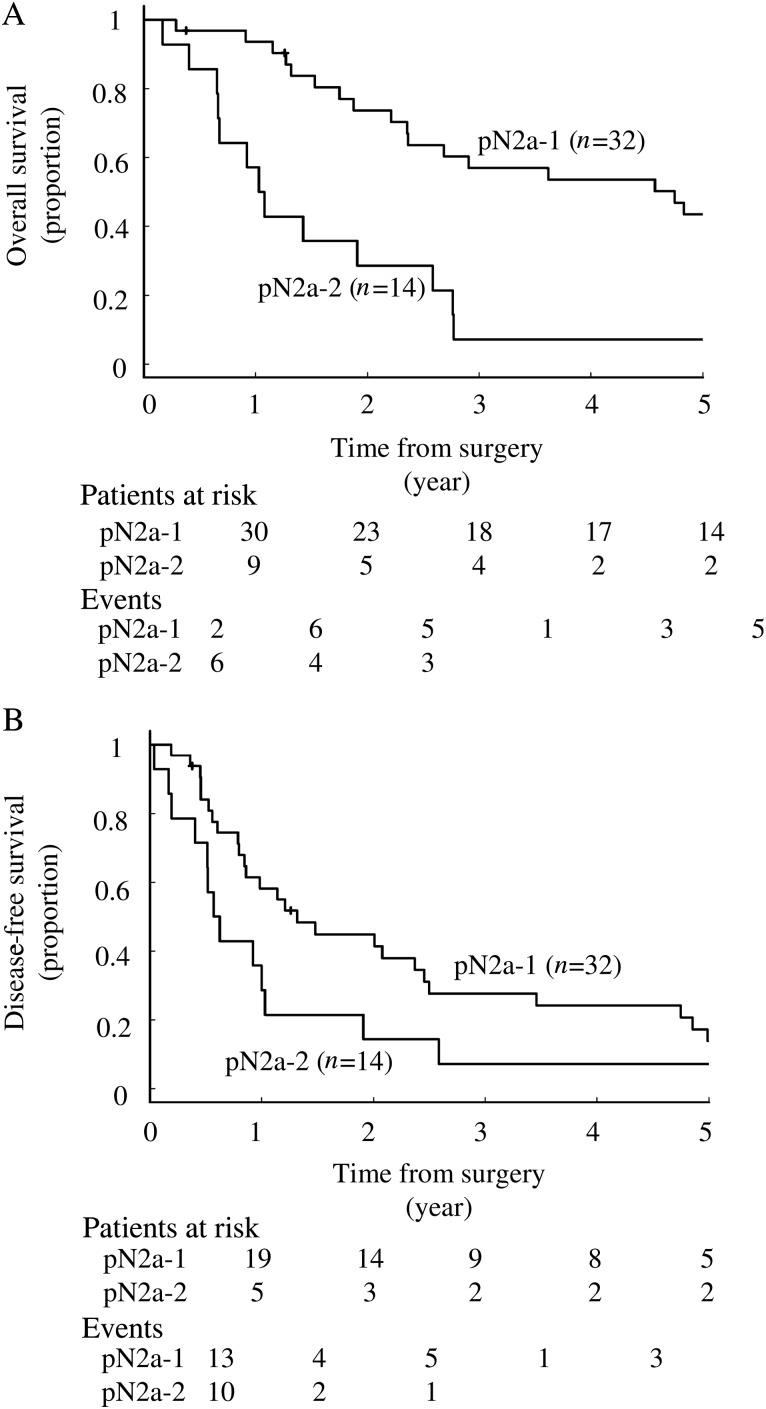
The 5-year OS rate and median survival time after surgery in all pN2 patients were 32% and 32.7 months (95% CI, 22.8–58.8), respectively, and those of DFS were 11.8% and 12.2 months, respectively (95% CI, 9.6–25.3). Figure 1 demonstrates the survival curves of the OS (Fig. 1A) and DFS (Fig. 1B) between the pN2a-1 and pN2a-2 subgroups. The 5-year OS rate and the median survival duration after surgery in the patients with pN2a-1 status were 21.9% and 57.7 months (95% CI, 28.8–99.8) and those in patients with pN2a-2 status were 7.1% and 12.8 months (95% CI, 8.2–33.7), respectively. Both the 5-year OS rate and the median survival after surgery were statistically significantly worse in the pN2a-2 patients (P < 0.01).
Figure 1:
(A) The overall and (B) DFS curves between the pN2a-1 and pN2a-2 groups. The overall survival was significantly better in pN2a-1 than pN2a-2 patients (P < 0.01), but there were no significant differences in the DFS (P = 0.06). In analysis of the overall survival, events occurred in 35 patients (22 in pN2a-1 and 13 in pN2a-2). In DFS analysis, there were 39 events (26 in pN2a-1 and 13 in pN2a-2).
The 5-year DFS rate and the median survival duration of the patients with pN2a-1 status were 13.8% and 16 months (95% CI, 10.3–30.4), respectively, and those of pN2a-2 patients were 0.71% and 7.2 months (95% CI, 6.3–31.4), respectively, which were not significantly different (P = 0.06). Twenty-five (78%) of the pN2a-1 and 8 (57%) of pN2a-2 patients relapsed after surgery. With regard to the recurrence pattern, local recurrence, including pleuritis carcinomatosa and lymph node recurrence, occurred in 8 (32%) and 3 (38%) patients in the pN2a-1 and pN2a-2 groups, and distant metastasis occurred in 19 (76%) and 5 (62%) patients in these groups.
The joint effects of age, the pathological T factor, the type of pulmonary resection, the present categorization, number of metastatic stations and number of involved nodes were examined using a Cox regression analysis. Univariate analysis did not demonstrate that the number of involved mediastinal stations or the number of metastatic nodes was a significant prognostic factor (Table 4). On the other hand, pN2a-2 classification and undergoing pneumonectomy were significantly associated with a shorter OS (hazard ratio, 3.11; P < 0.01 and hazard ratio 5.75; P < 0.01, respectively). Regarding the DFS, only undergoing pneumonectomy was a significant poor prognostic factor (hazard ratio, 2.84; P < 0.01). A multivariate analysis confirmed that the present categorization and type of pulmonary resection were the independent and significant factors predicting the OS (hazard ratio, 2.77; P = 0.03 and hazard ratio, 4.86; P < 0.01, respectively) and that pneumonectomy was an independent poor factor predicting the DFS (hazard ratio, 2.77; P = 0.02) (Table 5).
Table 4:
The results of the univariate survival analyses of patients with pN2 disease
| Overall survival |
Disease-free survival |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Age | ||||
| Under 75/75 and over | 1.10 (0.51–2.37) | 0.8; NS | 1.35 (0.66–2.77) | 0.42; NS |
| Pathological T factor | ||||
| pT1,2/pT3,4 | 1.19 (0.62–2.36) | 0.6; NS | 1.06 (0.55–2.05) | 0.87; NS |
| Type of pulmonary resection | ||||
| Pneumonectomy/lobectomy | 5.75 (2.40–13.8) | <0.01 | 2.84 (1.33–6.10) | <0.01 |
| Histology | ||||
| Non-adenoca./adenoca. | 2.02 (1.03–3.98) | 0.04 | 1.00 (0.53–1.88) | 1.0; NS |
| Present categorization | ||||
| pN2a-2/pN2a-1 | 3.11 (1.53–6.32) | <0.01 | 1.88 (0.96–3.69) | 0.06; NS |
| Number of metastatic nodes | ||||
| 4 and more/3 and less | 1.51 (0.76–2.97) | 0.24; NS | 1.12 (0.59–2.12) | 0.73; NS |
| Number of involved mediastinal stations | ||||
| Multiple/single | 1.65 (0.85–3.23) | 0.14; NS | 1.33 (0.71–2.50) | 0.63; NS |
NS: not significant.
Table 5:
The results of the multivariate survival analyses of patients with pN2 disease
| Overall survival |
Disease-free survival |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Age | ||||
| Under 75/75 and over | 0.96 (0.42–2.17) | 0.91; NS | 1.34 (0.63–2.85) | 0.45; NS |
| Pathological T factor | ||||
| pT1,2/pT3,4 | 1.11 (0.53–2.33) | 0.79; NS | 1.31 (0.63–2.85) | 0.46; NS |
| Type of pulmonary resection | ||||
| Pneumonectomy/lobectomy | 4.86 (1.86–12.69) | <0.01 | 2.77 (1.15–6.68) | 0.02 |
| Histology | ||||
| Non-adenoca./adenoca. | 1.19 (0.45–3.15) | 0.83; NS | 0.60 (0.28–1.31) | 0.2; NS |
| Present categorization | ||||
| pN2a-2/pN2a-1 | 2.77 (1.09–7.08) | 0.03 | 2.12 (0.88–5.09) | 0.09; NS |
| Number of metastatic nodes | ||||
| 4 and more/3 and less | 1.02 (0.42–2.48) | 0.97; NS | 0.87 (0.43–2.11) | 0.73; NS |
| Number of involved mediastinal station | ||||
| Multiple/single | 0.81 (0.30–2.14) | 0.67; NS | 1.05 (0.47–2.32) | 0.9; NS |
NS: not significant.
The OS and DFS of pN2a-1 patients were compared with those of pN1 (n = 44) patients during the same period. The 5-year OS and DFS rate of the pN1 patients were 51.9 and 43.5%, respectively. Regarding the OS, the prognostic difference between pN2a-1 and pN1 was not significant (P = 0.58). On the other hand, the DFS of pN2a-1 patients was significantly poorer than that of the pN1 patients (P = 0.035).
DISCUSSION
The prognosis of N2 NSCLC patients has remained poor, and most oncologists believe that surgery alone is not sufficient to cure patients with N2 disease. During the past decade, most patients with N2 have received perioperative chemotherapy to improve their survival. However, pN2 NSCLC is a heterogeneous population [6], and in our analysis the 5-year OS rate of pN2 patients was 32%. It suggested that some cases can be cured by surgery alone, while others should be considered for more intensive treatments in order to potentially achieve a cure.
Some studies have shown that a single station of mediastinal-node metastasis was an acceptable prognostic predictor [7], and another study demonstrated that the highest lymph node involvement, skip metastasis and single station involvement were associated with the prognosis [6]. Other reports have shown that single and skip mediastinal lymph-node involvement were predictors of better survival than multiple or both N1 and N2 metastases [8, 9], and another suggested that an increased number of positive nodes was an indicator of a worse prognosis [10]. In this study, neither the number of mediastinal stations (single/multiple) nor the number of involved nodes (less than 4/4 or more) was an independent prognostic factor in the univariate and multivariate analyses.
Following the revision of the TNM classification proposed by the IASLC, a relationship between the involved zone and the patient prognosis has been reported recently. Zheng et al. [11] reviewed 413 cases with single-zone involvement and 207 with multiple-zone involvement, and reported that the number of metastatic nodes and the ratio of nodal metastasis were related to the prognosis in a multivariate analysis. Kim et al. categorized 217 patients with ipsilateral mediastinal metastasis divided by ‘nodal zone’ and reported that the single-zone metastasis group had a better OS and DFS than the multiple-zone group, even when multiple stations within a single zone had metastases [13].
Regarding the relationship between the prognosis and the combination of the primary site and involved stations, Ichinose et al. [3] demonstrated that single-station metastasis was associated with a favourable prognosis when the primary cancer was located in the upper lobe, and the OS in N2 NSCLC patients was associated with the location of the primary tumour.
In this analysis, 59% of the pN2a-1 and 43% of the pN2a-2 patients had not been suspected to have mediastinal lymphadenopathy before surgery. Recently, some devices have improved the rate of preoperative detection of occult N2 involvement [14, 15]. However, some patients are still diagnosed as N2 after surgery. A recent meta-analysis of cisplatin-based adjuvant chemotherapy for NSCLC showed that the 5-year survival benefit in favour of chemotherapy was 5.3% [15]. Therefore, pN2 NSCLC patients, especially those with pN2a-2 status, whose estimated 5-year OS is only 7.1%, should be considered for more intensive therapeutic strategies after surgery.
The limitations of this study were: (i) the node dissection and pathological diagnosis were based on Naruke's map, not the new nodal map proposed by the IASLC and JLCS and (ii) the sample size was limited, so we could not confirm the prognostic significance of the difference between pN2a-1 and pN2a-2 with regard to DFS. However, our findings indicate that ‘Zone categorization’ may be a useful tool to revise the sub-classification of pN2 NSCLC, and that it can contribute to the adequate selection of the optimal postoperative therapeutic strategy.
FUNDING
Tetsuro Baba is currently receiving a postdoctral fellowship from the Uehara Memorial Foundation.
Conflict of interest: none declared.
REFERENCES
- 1.Keller SM, Vangel MG, Wagner H, Schiller JH, Herskovic A, Komaki R, et al. Prolonged survival in patients with resected non-small cell lung cancer and single-level N2 disease. J Thorac Cardiovasc Surg. 2004;128:130–7. doi: 10.1016/j.jtcvs.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Casali C, Stefani A, Natali P, Rossi G, Morandi U. Prognostic factors in surgically resected N2 non-small cell lung cancer: the importance of patterns of mediastinal lymph nodes metastases. Eur J Cardiothorac Surg. 2005;28:33–8. doi: 10.1016/j.ejcts.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Ichinose Y, Kato H, Koike T, Tsuchiya R, Fujisawa T, Shimizu N, et al. Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung Cancer. 2001;34:29–36. doi: 10.1016/s0169-5002(01)00207-0. [DOI] [PubMed] [Google Scholar]
- 4.Rusch VW, Crowley J, Giroux DJ, Goldstraw P, Im JG, Tsuboi M, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:603–12. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- 5.The Japan Lung Cancer Society. Japan Lung Cancer Society; 2010. The 7th Edition of General Rule for Clinical and Pathological Reccord of Lung Cancer. [Google Scholar]
- 6.Vansteenkiste JF, De Leyn PR, Deneffe GJ, Lerut TE, Demedts MG. Clinical prognostic factors in surgical treated stage IIIA-N2 non-small cell lung cancer: analysis of the literature. Lung Cancer. 1998;19:3–13. doi: 10.1016/s0169-5002(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 7.Xie L, Ugnat AM, Morriss J, Semenciw R, Mao Y. Histology-related variation in the treatment and survival of patients with lung carcinoma in Canada. Lung Cancer. 2003;42:127–39. doi: 10.1016/s0169-5002(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Dai CH, Shi SB, Chen P, Yu LC, Wu JR. Prognostic factors and long term results of neo adjuvant therapy followed by surgery in stage IIIA N2 non-small cell lung cancer patients. Ann Thorac Med. 2009;4:201–7. doi: 10.4103/1817-1737.56010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Dai CH, Yu LC, Chen P, Li XQ, Shi SB, et al. Results of trimodality therapy in patients with stage IIIA (N2-bulky) and stage IIIB non-small-cell lung cancer. Clin Lung Cancer. 2009;10:353–9. doi: 10.3816/CLC.2009.n.048. [DOI] [PubMed] [Google Scholar]
- 10.Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1:120–5. [PubMed] [Google Scholar]
- 11.Zheng H, Wang LM, Bao F, Jiang GN, Xie HK, Ding JA, et al. Re-appraisal of N2 disease by lymphatic drainage pattern for non-small-cell lung cancers: by terms of nodal stations, zones, chains, and a composite. Lung Cancer. 2011 doi: 10.1016/j.lungcan.2011.03.020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Lee HS, Lee JM, Zo JI, Lee GK, Nam BH. Prognostic value of single nodal zone metastasis in non-small-cell lung cancer. Eur J Cardiothorac Surg. 2010;38:491–7. doi: 10.1016/j.ejcts.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Wallace MB, Ravenel J, Block MI, Fraig M, Silvestri G, Wildi S, et al. Endoscopic ultrasound in lung cancer patients with a normal mediastinum on computed tomography. Ann Thorac Surg. 2004;77:1763–8. doi: 10.1016/j.athoracsur.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Billé A, Pelosi E, Skanjeti A, Arena V, Errico L, Borasio P, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg. 2009;36:440–5. doi: 10.1016/j.ejcts.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Chhatwani L, Cabebe E, Wakelee HA. Adjuvant treatment of resected lung cancer. Proc Am Thorac Soc. 2009;6:194–200. doi: 10.1513/pats.200807-068LC. [DOI] [PubMed] [Google Scholar]