Abstract
Aortic valve reimplantation has been shown to be a safe procedure. However, evidences of durability in bicuspid aortic valves (BAVs) are limited in the literature. Between 2002 and 2011, 132 patients (mean age 61 ± 12 years) underwent aortic valve reimplantation. In 24 patients (18%), aortic valve was bicuspid. Mean follow-up was 50 ± 26 months (range 1–102 months) and was 99% complete. In-hospital mortality was 0.8% (1 patient). Survival at 1 and 5 years was 99 and 94%, respectively. Overall freedom from aortic valve reoperation at 1 and 5 years was 96 and 90%, respectively, without significant difference between patients with bicuspid and tricuspid aortic valve. Freedom from aortic valve regurgitation >2+/4+, excluding patients reoperated, was at 1 and 5 years of 100 and 99%, respectively. Patients with valve cusp repair showed a higher rate of aortic valve reoperation; however, only postoperative aortic regurgitation >2+/4+ was significant risk factor for redo procedure at multivariate analysis. Aortic valve reimplantation in BAV without cusp repair provides excellent mid-term results. Further observations and longer follow-up are necessary to determine if BAV sparing, even in the presence of cusps alterations, could allow satisfying durability.
Keywords: Aortic valve repair, Aortic root, Heart valve
INTRODUCTION
Aortic valve-sparing operation with valve reimplantation was introduced to treat patients with aortic root aneurysm associated with normal or minimally abnormal aortic cusps [1]. Nowadays, this procedure is considered safe and has a proved durability at long-term follow-up [2], so that indications were extended to patients with severe aortic regurgitation, bicuspid aortic valve (BAV) and aortic cusp prolapse or abnormalities. Cusp repair is performed with great variability among published surgical experiences in up to 55% of the cases [2–4], on the contrary evidences of durability of BAV reimplantation are limited [2, 5, 6].
The aim of this study was to examine our experience with reimplantation valve-sparing operation focusing on influence of preoperative valve characteristics in determining need for reoperation.
MATERIALS AND METHODS
Between 2002 and 2011, 132 consecutive patients underwent aortic valve-sparing operation with valve reimplantation. The indication for operation was the presence of aortic root aneurysm. The Ethics Committee approved the study and waived the need for patient consent. The patients' characteristics are shown in Table 1.
Table 1:
Patient characteristics
| Variable | No. of patients (%) or mean ± SD |
|---|---|
| Gender | |
| Male | 96 |
| Female | 36 |
| Age (years) | 61 ± 12 |
| LVEF > 45% | 116 (88%) |
| Aortic regurgitation degree >2+/4+ | 88 (67%) |
| BAV | 9/24 (38%) |
| TAV | 79/108 (73%) |
| NYHA class III–IV | 25 (19%) |
| Emergent operation | 2 (1.5%) |
| CAD | 17 (13%) |
| COPD | 24 (18%) |
| CRF (creatinine > 200 μmol/l) or preoperative HD | 6 (4.5%) |
| Marfan syndrome | 5 (3.8%) |
| Bicuspid aortic valve | 24 (18%) |
| AAD | 1 (0.8%) |
| Chronic aortic dissection | 5 (4%) |
| Diameter (mm) | |
| Aortic root | 48 ± 9 |
| Ascending aorta | 51 ± 9 |
AAD: acute aortic dissection; BAV: bicuspid aortic valve; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; CRF: chronic renal failure; HD: haemodialysis; LVEF: left ventricular ejection fraction; TAV: tricuspid aortic valve.
Our surgical technique has been previously described in detail [7]. A Gelweave Valsalva™ graft was implanted in all the patients; the graft sizes used were: 26 mm in 2 patients, 28 mm in 13, 30 mm in 42 and 32 mm in 75.
In 13 cases, anatomical or cusp motion abnormalities concurred in determining aortic regurgitation and needed an adjunctive cusp repair. This finding was more frequent in patients with BAV (10/24 patients, 42%) than in tricuspid aortic valve (TAV) (3/108 patients, 3%). Cusp prolapse was found in seven patients with BAV and was corrected with free margin shortening (four patients), central cusp plication along the nodule of Arantius (one case) or cusp triangular resection (two patients). In the remaining three cases, stress fenestration repair with pericardial patch (one patient) and shaving of a calcified raphe (two patients) were performed. Cusp prolapse correction (central plication of right coronary cusp in one case, free margin shortening of right coronary and non-coronary cusps in one case) was necessary in two patients with TAV; in the remaining case, stress fenestration repair with pericardial patch was performed.
Concomitant procedures were mitral valve repair (10 patients, 7.5%), scheduled coronary artery bypass (12 patients, 9%), atrial septal defect repair (3 patients, 2%) and radio frequency ablation for atrial fibrillation (2 patients, 1.5%).
Mean cardiopulmonary bypass time was 134 ± 30 min (range 90–279 min) and the mean duration of aortic cross-clamping time was 114 ± 23 min (range 67–204 min).
Aortic regurgitation was graded as none, trace (1+/4+), mild (2+/4+), moderate (3+/4+) or severe (4+/4+). A transoesophageal echocardiogram (TEE) was carried out intraoperatively in all the patients after weaning from cardiopulmonary bypass in order to evaluate residual aortic valve regurgitation and to characterize cusps' level of coaptation. Transthoracic echocardiography was performed before discharge and at 6 and 12 months and every year thereafter.
Follow-up ranged from 1 to 102 months (mean 50 ± 26 months) and was available in 130 of 131 patients (99% complete).
Statistical analysis
Continuous variables were expressed as the mean ± SD. Categorical variables were compared with the Chi-square or Fisher exact test when appropriate. Six variables (Marfan syndrome, BAV, preoperative cusp prolapse, valve cusp repair, preoperative aortic regurgitation degree>2+/4+, postoperative residual aortic regurgitation degree>2+/4+) were entered into a univariate analysis to determine whether any single variable influenced the risk for reoperation and into a model of Cox regression analysis to study its independent predictability.
Survival rates and freedom from reoperation were calculated using the Kaplan–Meyer method. Univariate comparisons for failure time data were performed using the Wilcoxon test. Statistical analyses were performed using the Stat-View Statistical Software Package 5.0 (SAS Institute, Inc., Cary, NC, USA), NCSS 2001 (Number Chruncher Statistical System, Kaysville, UT, USA).
RESULTS
In-hospital outcome
There were no intra-operative deaths. The mortality pre-discharge was 0.8% (one patient). Early reoperation for excessive bleeding or tamponade was necessary in 15 patients (11%) and in 3 cases (2.2%) for sternal dehiscence/mediastinitis. Perioperative myocardial infarction occurred in two patients (1.6%). Cerebrovascular accidents were registered in three patients (2.2%); stroke in one and transient ischaemic attack in two. A permanent pacemaker implantation was necessary in three patients (2.2%) for complete AV block.
Seven patients were discharged with a residual aortic regurgitation degree >2+/4+; nevertheless in last 4 years, all patients were discharged with residual aortic regurgitation degree <2+/4+.
Survival
There were eight late deaths during follow-up; in three cases because of cancer, one had acute bowel ischaemia after descending thoracic aorta endovascular procedure, two patients had worsening heart failure and in two cases sudden death occurred. The cumulative 1-year and 5-year survival rates (excluding hospital mortality) were 99 and 94%, respectively.
Freedom from aortic valve endocarditis was 100 and 99% at 1 and 5 years. Thromboembolic events occurred in two patients during follow-up with a rate of freedom from cerebrovascular ischaemic events at 1 and 5 years of 99 and 97%, respectively.
Reoperation
Eleven patients underwent aortic valve reoperation during follow-up for recurrent aortic insufficiency, in one case because of aortic valve endocarditis. All the patients underwent successful aortic valve replacement and the survival rate was 100% at 38 ± 27 months. Table 2 reports data about reoperation. At univariate analysis, preoperative cusp prolapse (P = 0.013), valve cusp repair (P = 0.023) and postoperative aortic regurgitation >2+/4+ (P < 0.001) were significant risk factors for aortic valve reoperation. Preoperative aortic regurgitation degree >2+/4+ (P = 0.08), Marfan syndrome (P = 0.06) and bicuspidy (P = 0.32) showed no significance. At the multivariate analysis, postoperative aortic regurgitation degree >2+/4+ (P = 0.032) was the only significant independent risk factor for aortic valve reoperation.
Table 2:
Aortic valve characteristics and mechanisms of recurrent aortic regurgitation in patients who underwent aortic valve reoperation
| Patient | AV | Preop AVR | AV repair | Reason for recurrent AVR | Interval time (months) |
|---|---|---|---|---|---|
| 1 | TAV | Trivial | – | Three cusps prolapse, technical problem | 1 |
| 4 | TAV | Severe | – | RC and LC retraction, normal annulus | 79 |
| 7 | BAV | Severe | Free margin shortening NC-RC cusp | NC-RC cusp retraction | 7 |
| 23 | TAV | Severe | – | NC cusp prolapse, no cusps abnormalities | 34 |
| 30 | TAV | Severe | – | NC cusp prolapse, no cusps abnormalities | 15 |
| 45 | TAV | Moderate | – | Not well defined | 34 |
| 54 | TAV | Severe | – | LC cusp prolapse, no cusps abnormalities | 31 |
| 67 | TAV | Moderate | – | Endocarditis | 48 |
| 72 | BAV | Severe | NC cusp triangular resection and central plication | NC cusp prolapse | 9 |
| 91 | TAV | Moderate | Fenestration repair | Not well defined | 4 |
| 105 | BAV | Moderate | NC cusp triangular resection and central plication | NC cusp tear (suture dehiscence?) | 6 |
BAV: bicuspid aortic valve; LC: left coronary; NC: non-coronary; RC: right coronary; TAV: tricuspid aortic valve.
Overall freedom from reoperation for aortic valve regurgitation was 96% at 1 year and 90% at 5 years.
Cumulative freedom from aortic valve reoperation and from residual aortic regurgitation degree >2+/4+ was 96 and 89% at 1- and 5-year follow-up, respectively.
Patients with preoperative none or trace aortic regurgitation (AR) had a freedom from reoperation at 1 and 5 years of 98%; in patients with AR degree >2+/4+, freedom from aortic valve redo was at 1 and 5 years of 95 and 87%, respectively (AR ≤ 2+/4+ vs. AR > 2: P = 0.11).
Patients with BAV showed freedom from aortic valve reoperation of 86% after 1 year with stable results in the first 5 years. There was no difference comparing the durability between TAV (freedom from reoperation of 98 and 92% at 1 and 5 years, respectively) and BAV (P = 0.26) (Fig. 1).
Figure 1:
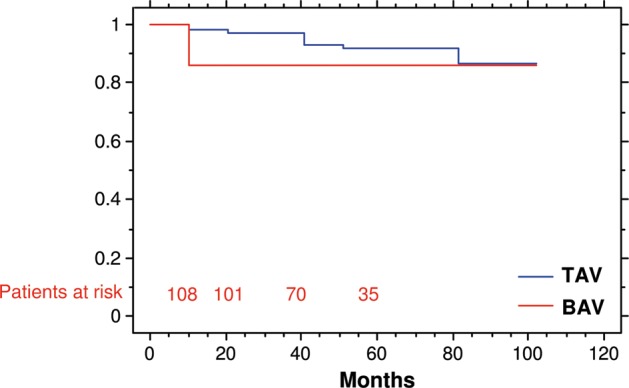
Freedom from aortic valve reoperation in patients with tricuspid and BAV (P = 0.26).
Overall freedom from reoperation in patients with preoperative normal or minimally abnormal aortic valve cusps was 99 and 93% at 1 and 5 years, respectively (Fig. 2). Patients needing cusp repair showed freedom from aortic valve redo of 69% at 5 years (P < 0.001). No difference between BAV and TAV was found in durability after stratification for adjunctive cusp repair (Fig. 3). BAV and TAV reimplantation without cusp repair provided a freedom from aortic valve reoperation at 1 year of 100 and 99%, respectively, at 5 years of 100 and 93%, respectively. Freedom from reoperation at 5 years in patients who underwent associated cusps repair was 72% in TAV and 67% in BAV.
Figure 2:
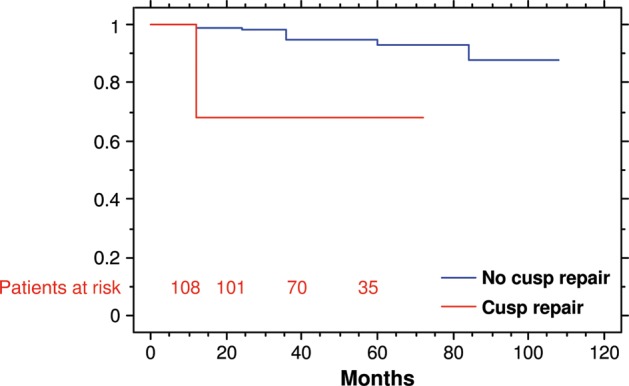
Freedom from aortic valve reoperation according to the adjunct of cusp repair (P < 0.001).
Figure 3:
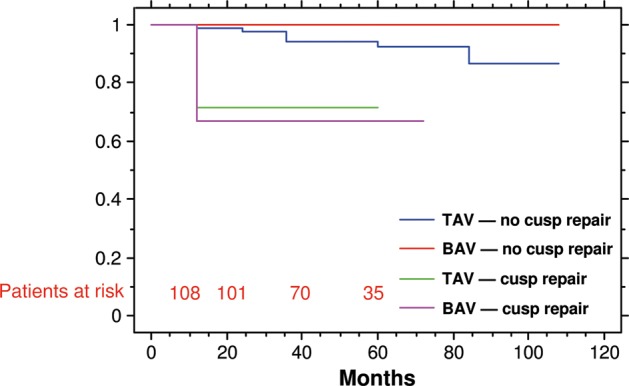
Freedom from aortic valve reoperation in patients with tricuspid or BAV associated or not with valve cusp repair.
DISCUSSION
The BAV annular asymmetry is considered to be better preserved with root remodelling [6, 8]. Previous experiences [3, 8] showed no differences in durability between preserved BAV and TAV. These results were supported by a recent work by Schäfers et al. [9] with excellent results and freedom from aortic valve reoperation of 97% at 10 years.
Annular dilatation, together with cusp injuries or retraction, stress tears and fenestration, developing fibrosis and, of course, failure of suboptimal surgical correction [8, 10–12], are possible mechanisms leading to aortic regurgitation recurrence [10, 13, 14]. Valve-sparing reimplantation is the only operative approach able to stabilize the size of a dilated aortoventricular junction thus preventing future dilatation [2, 14]. This characteristic could be more effective in preventing recurrence of valve regurgitation in BAV. However, despite the widespread of this technique, few reports focused on BAV [5, 12]; even in experiences with large population, the number of BAV patients is limited [2, 4, 15] or the results not discussed [13].
In our experience, a favourable anatomical and functional cusp condition allowed satisfying mid-term durability with a global freedom from reoperation of 94% at 5 years; in particular, excellent results were registered in the BAV subgroup. However, especially in BAV, cusp abnormalities are not uncommonly associated [12]. We were led to add valve cusp repair in only 10% of the cases, but this rate is lower than in several other experiences reporting cusp repair incidences ranging from 20 to 60% [2, 3, 10, 12, 15]. Even if bicuspidy did not emerge as a risk factor for aortic valve reoperation [2–6, 12, 15], there is no general agreement about the durability of BAV sparing with adjunctive cusp repair [2, 3, 10, 12] and our data are supportive for a less satisfying durability in this subgroup of patients. However, a very recent paper from de Kerchove et al. [14], focusing on patients with BAV reimplantation with adjunctive cusp repair in 93% of the cases, has reported a superb freedom from reoperation: 100% at 6 years.
We found a tendency in early failure (less than 1 year from valve-sparing procedure) in patients with BAV who needed valve cusp repair (Figs 2 and 3). Interestingly, similar results were reported in literatures with most of the reoperations (up to 80%) performed during the first year of the follow-up [3, 8, 11, 12, 15]. Cusp lesions and technical failure were reported as the main cause for reoperation in these cases. Furthermore, many of these patients were discharged with a residual mild insufficiency degree that abruptly hesitated in moderate or severe regurgitation. Postoperative degree of aortic regurgitation is recognized to be an independent risk factor for recurrent aortic regurgitation [3] and our data confirm this finding. In the early phase of our experience, seven patients were discharged with a suboptimal result and four of these underwent aortic valve reoperation during the first year after the valve-sparing repair. Concerning intraoperative TEE, a residual aortic regurgitation ≥2+/4+ and a level of coaptation>2 mm below the lower border of the Dacron graft is considered inacceptable nowadays. Our policy allowed us to register no early failure in last 3 years and will probably provide an even better mid- and long-term outcome in the future.
Limitations
The main limitation of the study was the small number of patients with BAV; however, the presented subgroup is one of the most representative single-centre series in the literature. This is a retrospective analysis even if performed on a prospectively collected database. Data about residual aortic regurgitation or development of significant aortic insufficiency were extrapolated in up to 20% of the cases from echocardiography evaluation performed in peripheral centres.
CONCLUSION
If a BAV does not present cusp calcification or some stenosis degree, for which the indication for valve replacement is clear, a valve-sparing procedure should be considered as a primary approach. We are familiar with the reimplantation technique since 2002 and we agree with the potential role in annular stabilization provided by valve reimplantation.
Preoperative aortic regurgitation degree is not an independent risk factor for recurrent aortic insufficiency in BAV sparing. Although bicuspidy could present as a complex disease-causing aortic regurgitation, it did not emerge as a risk factor for reoperation after valve-sparing procedures. Several pathogenetic mechanisms could lead to recurrent aortic regurgitation along annular or root dilatation and therefore to the need for further procedures. BAV preservation is particularly interesting in patients developing aortic regurgitation or undergoing aortic root surgery who deny mechanical prostheses and could suffer from suboptimal durability of biologic prostheses.
Sparing BAVs with minimal cusp alterations provide excellent mid-term results. A proper intraoperative assessment, a close postoperative follow-up and the limited risk during redo procedure in these young patients could allow experienced surgeons to make efforts in sparing BAV even in the presence of cusp abnormalities. However, further evidences and longer follow-up are required to state if this particular subgroup of patients could benefit from a satisfying durability.
Conflict of interest: none declared.
REFERENCES
- 1.David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1992;103:617–22. [PubMed] [Google Scholar]
- 2.David TE, Feindel CM, Webb GD, Colman JM, Armstrong S, Maganti M. Aortic valve preservation in patients with aortic root aneurysm: results of the reimplantation technique. Ann Thorac Surg. 2007;83:S732–5. doi: 10.1016/j.athoracsur.2006.10.080. [DOI] [PubMed] [Google Scholar]
- 3.de Kerchove L, Boodwhwani M, Glineur D, Poncelet A, Verhelst R, Astarci P, et al. Effects of preoperative aortic insufficiency on outcome after aortic valve-sparing surgery. Circulation. 2009;120(Suppl. 1):S120–6. doi: 10.1161/CIRCULATIONAHA.108.841445. [DOI] [PubMed] [Google Scholar]
- 4.De Paulis R, Scaffa R, Nardella S, Maselli D, Welter L, Bertoldo F, et al. Use of the Valsalva graft and long-term follow-up. J Thorac Cardiovasc Surg. 2010;140:S23–7. doi: 10.1016/j.jtcvs.2010.07.060. [DOI] [PubMed] [Google Scholar]
- 5.Sareyyupoglu B, Suri RM, Schaff HV, Dearani JA, Daly RC, Orszulak TA, et al. Survival and reoperation risk following bicuspid aortic valve-sparing root replacement. J Hear Valve Dis. 2009;18:1–8. [PubMed] [Google Scholar]
- 6.Svensson LG, Batizy LH, Blackstone EH, Gillinov AM, Moon MC, D'Agostino RS, et al. Results of matching valve and root repair to aortic valve and root pathology. J Thorac Cardiovasc Surg. 2011;142:1491–8. doi: 10.1016/j.jtcvs.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Settepani F, Ornaghi D, Barbone A, Citterio E, Eusebio A, Manasse E, et al. Aortic valve-sparing operations in patients with aneurysms of the aortic root or ascending aorta: preliminary results. Interact CardioVasc Thorac Surg. 2005;4:137–9. doi: 10.1510/icvts.2004.095422. [DOI] [PubMed] [Google Scholar]
- 8.Aicher D, Langer F, Kissinger A, Lausberg H, Fries R, Schäfers H. Valve-sparing aortic root replacement in bicuspid aortic valves: a reasonable option? J Thorac Cardiovasc Surg. 2004;128:662–8. doi: 10.1016/j.jtcvs.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Schäfers H, Aicher D, Langer F, Lausberg H. Preservation of the bicuspid aortic valve. Ann Thorac Surg. 2007;83:S740–5. doi: 10.1016/j.athoracsur.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Hanke T, Charitos EI, Stierle U, Robinson D, Gorski A, Sievers HH, et al. Factors associated with the development of aortic valve regurgitation over time after two different techniques of valve-sparing aortic root surgery. J Thorac Cardiovasc Surg. 2009;137:314–9. doi: 10.1016/j.jtcvs.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Oka T, Okita Y, Matsumori M, Okada K, Minami H, Munakata H, et al. Aortic regurgitation after valve-sparing aortic root replacement: modes of failure. Ann Thorac Surg. 2011;92:1639–44. doi: 10.1016/j.athoracsur.2011.06.080. [DOI] [PubMed] [Google Scholar]
- 12.Badiu CC, Bleizzifer S, Eichinger W, Zaimova I, Hutter A, Mazzitelli D, et al. Are bicuspid aortic valves a limitation for aortic valve repair? Eur J Cardiothorac Surg. 2011;40:1097–104. doi: 10.1016/j.ejcts.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 13.David TE, Maganti M, Armstrong S. Aortic root aneurysm: principles of repair and long-term follow-up. J Thorac Cardiovasc Surg. 2010;140:S14–9. doi: 10.1016/j.jtcvs.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 14.de Kerchove L, Boodhwani M, Glineur D, Vandyck M, Vanoverschelde JL, Noirhomme P, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg. 2011;142:1430–8. doi: 10.1016/j.jtcvs.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Kallenbach K, Karck M, Pak D, Salcher R, Khaladj N, Leyh R, et al. Decade of aortic valve sparing reimplantation. Are we pushing the limits too far? Circulation. 2005;112(Suppl. I):I-253–9. doi: 10.1161/01.CIRCULATIONAHA.104.525907. [DOI] [PubMed] [Google Scholar]


