Abstract
We evaluated the diagnostic value of high-resolution computed tomography (HRCT) images generated from 64 detector multi-slice CT scanners (HRCT64-MSCT imaging) in relation to primary graft dysfunction (PGD) after lung-transplantation (LUTX) in a pilot study. PGD has mortality rates ranging from 17 to 50% over a 90-day period. Detailed HRCT lung images, reconstructed using 64-MSCT, may aid diagnostic and therapeutic efforts in PGD. Thirty-two patients were scanned four times within a year post-LUTX, in a single-centre prospective study. HRCT lung images were reviewed, evaluated and scored by two observers, for ground-glass (GG) opacities, consolidation, septal thickening (ST) and pulmonary embolism. Image and PGD scores were compared in each patient. GG and consolidation changes were largely present up until 2 weeks post-LUTX, and markedly reduced by the 12th week. ST was predominantly found in patients with PGD. There were no vascular changes found at CT angiographies. The most severe cases of GG opacities and consolidation were found in patients with PGD. ST seems to be an important indicator of PGD. HRCT64-MSCT imaging may be a useful tool for the identification of pathological features of PGD not detected by classical evaluation in patients undergoing LUTX.
Keywords: Education, Lung, Lung transplantation
INTRODUCTION
Lung-transplantation (LUTX) patients may develop diffuse infiltrates visible on chest X-ray images which may be accompanied by an impaired graft function with impaired oxygenation in the first 1–3 days after the transplantation, defined as primary graft dysfunction (PGD) [1]. The International Society of Heart and Lung Transplantation (ISHLT) Working Group on primary Lung Graft Dysfunction (PGD) has recommended a definition of PGD based on radiological findings at chest X-ray examination, and the gas exchange impairment measured by the PaO2: FiO2 ratio (ratio of the partial pressure of oxygen in arterial blood to the fraction of inspired oxygen, also termed P/F ratio) [2–5], an operational definition that pertains to the symptoms of PGD only. This is the definition of PGD we have used in this paper.
PGD occurs in 11–60% of lung-transplanted patients depending on the diagnostic criteria applied and reporting different degrees of severity [6]. Development of PGD in the early post-operative period, negatively affects long-term survival of lung-transplanted patients, even in those patients who do not develop bronchiolitis obliterans syndrome [1, 6, 7]. Transplant recipients with PGD are at significantly higher risk for early mortality, longer intensive care unit stays and increased hospital staying with mortality rates ranging between 17 and 60% over a 90-day period [1, 6].
Evaluation and grading of the disease using high-resolution computed tomography (HRCT) scans have not been incorporated in the definition of PGD. HRCT of the lung has proved to be a sensitive method for detecting and following lung disease, and visual scoring systems have correlated with disease severity as determined by clinical scoring systems, radiographic scoring systems and pulmonary function tests [8]. Several visual scoring systems have been made to characterize other lung disease such as cystic fibrosis and pulmonary fibrosis from HRCT images [9–13]. There are studies on scoring systems for acute respiratory distress syndrome, which is closely related to PGD [14], but none for PGD.
Suggestions for a PGD scoring system where a 64 detector multi-slice CT (64-MSCT) scanner is used, have been made by Belmaati et al. [15]. A more detailed description of PGD obtained from HRCT imaging would possibly help refine diagnostic and therapeutic efforts in future lung-transplant patients. Possibilities for non-invasive characterization of lung tissue have improved with current development in MSCT. In lung imaging, HRCT64-MSCT has the advantage of visualizing the whole lung and reconstructing images in several planes as opposed to classic HRCT, where chosen sections of the lung are visualized. Advantages of HRCT are the fine detail available and the ability to distinguish areas of lung parenchyma showing different disease patterns. A potential disadvantage of this technique is the considerably greater radiation dose that patients could be exposed to, compared with X-rays. New developments have, however, been made, which lower peak kilo voltage (kVp) settings that results in dose savings of over 40% and doses as low as 2 mSv are now possible [16–18].
The aim of this study was to describe radiographic patterns in PGD after LUTX with the use of HRCT64-MSCT.
METHODS
This is a single-centre prospective study over a period of 18 months (August 2007–February 2009), including all patients having LUTX in this period. All patients on the waiting list at the start of the study and all patients entered on the waiting list in the study period were offered participation in the study and all except one gave informed consent. The Copenhagen Committee on Biomedical Research Ethics approved the study protocol. The MSCT plan, the definitions of parameters used in the scoring system and an example of the scoring sheets used are explained.
Study plan
The standard treatment protocol for this centre has been published in detail previously [19], but briefly patients received induction with 500 mg of methylprednisolone and received anti-thymocyte globulin (thymoglobulin®) in a dose of 1.5 mg/kg for 3 days and started on cyclosporine (initial target level 200–250 ng/ml) and azathioprine 2 mg/kg on first post-operative day.
Patients were scanned four times over a period of a year and two observers reviewed CT images of the transplanted lung (M.I. and E.O.B.).
– First MSCT. Third day post-LUTX: Maximal changes in the lung are expected at this time. Most patients (>90%) will be extubated at this time, which means that there are fewer practical problems compared with an earlier scan.
– Second MSCT. Fourteenth day post-LUTX: Most patients with PGD will have a normal chest X-ray, whereas significant changes in the lung tissue are expected with MSCT at this stage.
– Third MSCT. Three months post-LUTX: Most patients have achieved stable lung function, close to the maximum obtainable after transplantation.
– Fourth MSCT. Twelve months post-LUTX: We expect that patients are quite stabile and that any lung changes found at this time will most likely be chronic.
MSCT scans
The CT scan images were obtained using a Toshiba Aquilion 64 scanner (64 detectors of 0.5 mm). All lung parenchyma was included in the scan region ranging from the apex of the lungs, to below the costophrenic angle. The scans were obtained at 120 kVp using milliampere modulation (varies according to patient size), and a pitch of 53 mm/rotation (or 0.828 = fraction of detector breadth per rotation). Images were reconstructed in a 512 × 512 matrix with 1 mm non-overlapping slices, applying a standard lung reconstruction algorithm FC52. Images were viewed at lung window settings (window width 1600 HU; window level −600 HU). All patients underwent scanning in the supine position, and intravenous contrast material [Visipaque (320 mg iodine/ml)] was used during HRCT angiographies, which were performed at the third day, and 12th week scan. Patients had to have a creatinine measure of <140 μmol/l to determine whether contrast was given or not. The radiation dose for CT scan with angiography was 10 mSv, and the dose without angiography was 7 mSv.
The scan was helical after inspiration and expiration.
Scoring system
Based on previous experience with MSCT in lung-transplanted patients at this centre, a scoring system was defined based on the assessment of each transplanted lung at a lobal level, both centrally and peripherally. The lung was evaluated in lobes, for example, RU: right upper lobe, RM: right middle lobe, RL: right lower lobe, LU: left upper lobe and LL: left lower lobe. Each lobe was divided into two, a peripheral and central part; the central part of the lobe is defined as the anatomical half of the lobe closest to the hilus, whereas the peripheral part of the lobe is defined as the outer half of the lobe around the central part of the lobe. Some patients had double LUTX and others had either right or left single LUTX and had as such 10, 6 or 4 evaluation areas, respectively. Each lobe was evaluated for several parameters such as ground-glass pattern (GG), consolidation (Cons), septal thickening (ST), peribronchial thickening, arterial lung embolism, venous lung embolism, mosaic attenuation, air trapping and bronchiectasies. The central and peripheral lobe were each given a score of 1 or 2 or 9 according to whether changes were present, absent or unknown, respectively. The score sheet set-up, and the definitions of parameters used in this study were outlined in an earlier study by Belmaati et al. [15].
To calculate the score for the transplanted lung and make the results comparable for those with a double, right or left lung, the number of positive scores (that is number of score of 1) was divided with the number of evaluation areas for the lung and a fraction was obtained. For example, in a left lung with a total score of 3 in ground glass, the affected area is calculated by dividing 3/4 = 0.75. In another example, a score of 3 in a double-transplanted lung gives 3/10 = 0.3. The mean value of the scores from the two observers was used in this study.
PGD—imaging with chest X-ray within 72 h
To determine the presence or absence of PGD, chest X-ray images were evaluated by two observers, (M.I. and E.O.B.), and consensus was obtained. The chest X-ray images were graded according to the recommendation by ISHLT for grading PGD, where scores are based on the presence or absence of infiltrates and the corresponding PaO2: FiO2 score (ratio of the partial pressure of oxygen in arterial blood to the fraction of inspired oxygen, also termed P/F ratio). X-ray images with no infiltrates received a score of 0; images with infiltrates were given a score from 1 to 3 depending on the P/F ratio. Score 1 = P/F ratio of >300, 2 = P/F ratio 200–300, 3 ≤ 200. Patients extubated immediately after operation (n = 4) and managed on nasal oxygen supplement were given a PGD score of 0 if they had no infiltrates, and a score of 1 if an infiltrate was present. The highest PGD value obtained in the first 72 h was recorded for use in the analysis.
RESULTS
There were 32 patients included in the study. Twenty-eight patients were scanned in the first scan, 29 patients in the second and third scan, and 23 patients only in the fourth scan. Missed scans were due to technical difficulties (logistical problems), unstable patient condition, dropouts or death.
The mean patient age at the time of transplantation was 51.6 years. There were 16 males and 16 females. Eight patients had right LUTXs, 8 patients had left LUTXs and 16 had a double-sided transplantation (Table 1).
Table 1:
Demographics for transplanted patients
| Patient | Age | Gender | Type of lung transplantation | Main lung disease | Vital status within the first year |
|---|---|---|---|---|---|
| 1 | 61 | F | L.SLTX | COPD | Alive |
| 2 | 51 | M | R.SLTX | COPD | Alive |
| 3 | 61 | F | R.SLTX | COPD | Alive |
| 4 | 64 | M | DLUTX | COPD | Dead by first year |
| 5 | 56 | F | R.SLTX | COPD | Alive |
| 6 | 61 | F | L.SLTX | COPD | Alive |
| 7 | 59 | M | DLUTX | FIBR | Alive |
| 8 | 58 | M | L.SLTX | COPD | Alive |
| 9 | 59 | M | L.SLTX | COPD | Alive |
| 10 | 40 | M | R.SLTX | COPD | Alive |
| 11 | 50 | M | R.SLTX | FIBR | Alive |
| 12 | 48 | M | DLUTX | FIBR | Dead by 12th week |
| 13 | 31 | F | DLUTX | CF | Alive |
| 14 | 61 | F | DLUTX | COPD | Alive |
| 15 | 59 | M | DLUTX | COPD | Alive |
| 16 | 62 | F | L.SLTX | COPD | Dead by first year |
| 17 | 57 | M | DLUTX | COPD | Alive |
| 18 | 59 | F | R.SLTX | COPD | Alive |
| 19 | 57 | F | DLUTX | FIBR | Alive |
| 20 | 58 | M | DLUTX | FIBR | Alive |
| 21 | 35 | F | DLUTX | CF | Alive |
| 22 | 53 | F | R.SLTX | COPD | Alive |
| 23 | 62 | F | L.SLTX | COPD | Alive |
| 24 | 31 | F | DLTX | CF | Alive |
| 25 | 57 | M | L.SLUTX | COPD | Alive |
| 26 | 60 | M | DLTX | SARC | Alive |
| 27 | 41 | F | L.SLTX | COPD | Alive |
| 28 | 58 | M | DLUTX | COPD | Dead by first year |
| 29 | 24 | F | DLUTX | CF | Dead by first year |
| 30 | 60 | M | R.SLTX | FIBR | Alive |
| 31 | 46 | M | DLUTX | Bronchiectasis | Alive |
| 32 | 64 | F | DLUTX | FIBR | Dead by 12th week |
Prefix R. or L. is for right or left lung single lung transplantation.
DLUTX, double lung transplantation; SLTX, single lung transplantation; COPD, chronic obstructive pulmonary disease; CF, cystic fibrosis; SARC, sarcoidosis; FIBR, lung fibrosis.
The actual numbers of patients counted in each score category for the parameters, GG opacities, consolidation, ST and peribronchial thickening over a period of time are shown in Table 2. Very low incidences of peribronchial thickening, air trapping, mosaic attenuation and bronchiectasis were found. The percentage of affection of the transplanted lung by mosaic attenuation and air trapping fell <10%. Bronchiectasis was not seen until the 12th week, and only one patient had a 65% affection of the transplanted lung affected by bronchiectasis after 12 weeks; all the other patients had lung affection values of between 0 and 10%. After a year, two patients had developed bronchectasis; one had a 65% affection of the transplanted lung, while the other had 85% affection. All the other patients had values <10%. There were no vascular changes found at CT angiographies.
Table 2:
The distribution of the number of patients with each lung parameter categorized in MSCT score categories at different times within a year
| Third day | 14th day | 12th week | 1 year | |
|---|---|---|---|---|
| Ground glass | ||||
| 0.00–0.10 | 7 | 10 | 17 | 14 |
| 0.10–0.25 | 3 | 6 | 8 | 2 |
| 0.26–0.50 | 8 | 7 | 1 | 3 |
| 0.51–0.75 | 6 | 4 | 1 | 2 |
| 0.76–1.00 | 4 | 2 | 2 | 2 |
| Total number of patients | 28 | 29 | 29 | 23 |
| Consolidation | ||||
| 0.00–0.10 | 5 | 6 | 17 | 11 |
| 0.10–0.25 | 9 | 8 | 8 | 4 |
| 0.26–0.50 | 11 | 12 | 3 | 6 |
| 0.51–0.75 | 2 | 2 | 0 | 1 |
| 0.76–1.00 | 1 | 1 | 1 | 1 |
| Total number of patients | 28 | 29 | 29 | 23 |
| Septal thickening | ||||
| 0.00–0.10 | 14 | 14 | 17 | 17 |
| 0.10–0.25 | 4 | 4 | 5 | 4 |
| 0.26–0.50 | 5 | 5 | 2 | 0 |
| 0.51–0.75 | 2 | 0 | 0 | 0 |
| 0.76–1.00 | 3 | 6 | 3 | 2 |
| Total number of patients | 28 | 29 | 29 | 23 |
| Peri-bronchial thickening | ||||
| 0.00–0.10 | 23 | 25 | 18 | 17 |
| 0.10–0.25 | 4 | 4 | 7 | 4 |
| 0.26–0.50 | 1 | 0 | 2 | 2 |
| 0.51–0.75 | 0 | 0 | 2 | 0 |
| 0.76–1.00 | 0 | 0 | 0 | 0 |
| Total number of patients | 28 | 29 | 29 | 23 |
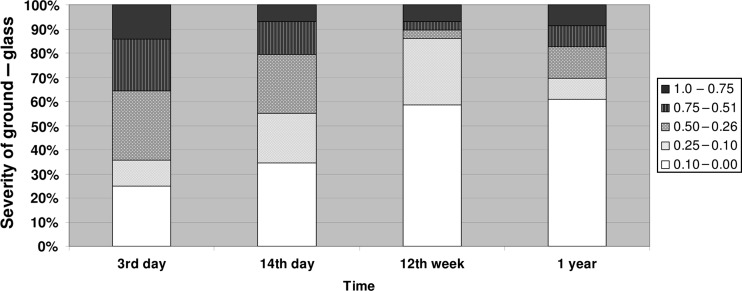
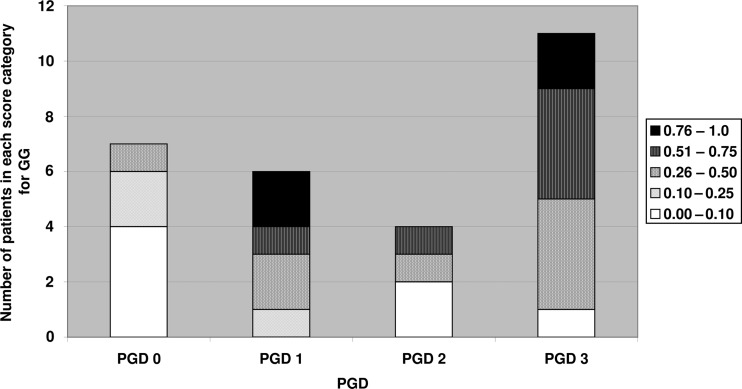
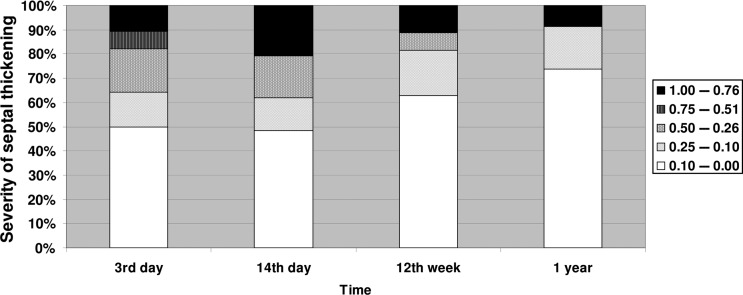
GG opacity changes were seen to be largely present up until 2 weeks and were markedly reduced by the 12th week (Fig. 1). Consolidation was seen in small areas, it increased in some patients by the 14th day. It decreased over time but had a tendency to persist over time. Figure 3 ST was seen in small amounts that increased by the 14th day and tended to persist over time (see Fig. 2).
Figure 1:
Change in prevalence of GG in the transplanted lung over time.
Figure 3:
GG severity in the transplanted lung at different PGD scores.
Figure 2:
Change in prevalence of ST in the transplanted lung over time.
The same patients who had GG opacity changes at 14 days post transplantation also had them at 3 days. However, a single patient had no GG at 3 days but developed it at 14 days. The general picture tended towards a decrease in GG with time (see Fig. 1).
Eight patients had an increased consolidation by the 14th day. MSCT scores were compared with PGD scores in Table 3. Of 32 patients included in the study, 13 patients had PGD 3 (2 missed scans), 4 patients had PGD 2, 6 patients had PGD 1 and 9 patients had PGD 0 (2 missed scans). We found that in MSCT scores of both GG opacities and consolidation, scores higher than 0.51 were not found in patients without PGD (see Fig. 3).
Table 3:
Number of patients in MSCT score categories compared with their PGD scores
| PGD 0 | PGD 1 | PGD 2 | PGD 3 | |
|---|---|---|---|---|
|
Ground-glass third day scores | ||||
| 0.00–0.10 | 4 | 0 | 2 | 1 |
| 0.10–0.25 | 2 | 1 | 0 | 0 |
| 0.26–0.50 | 1 | 2 | 1 | 4 |
| 0.51–0.75 | 0 | 1 | 1 | 4 |
| 0.76–1.0 | 0 | 2 | 0 | 2 |
| Consolidation third day scores | ||||
| 0.00–0.10 | 2 | 1 | 1 | 2 |
| 0.10–0.25 | 3 | 2 | 1 | 3 |
| 0.26–0.50 | 2 | 2 | 2 | 5 |
| 0.51–0.75 | 0 | 0 | 0 | 1 |
| 0.76–1.0 | 0 | 1 | 0 | 0 |
| Septal thickening third day scores | ||||
| 0.00–0.10 | 6 | 2 | 2 | 4 |
| 0.10–0.25 | 1 | 1 | 1 | 1 |
| 0.26–0.50 | 0 | 2 | 0 | 3 |
| 0.51–0.75 | 0 | 0 | 1 | 1 |
| 0.76–1.0 | 0 | 1 | 0 | 2 |
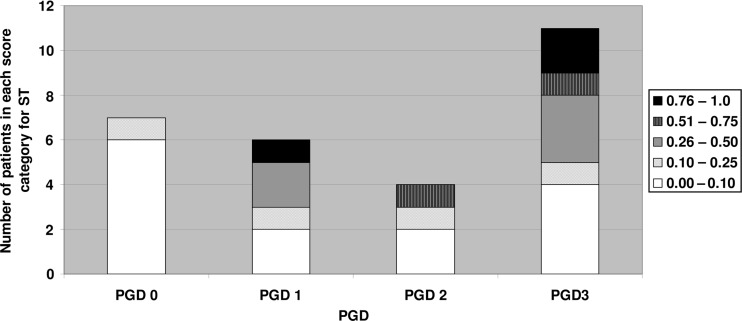
Close to 85% of patients without PGD (PGD 0) appear to have very little ST, <0.1. The remaining 15% without PGD lie in the range of 0.10–0.25 (see Fig. 4).
Figure 4:
ST severity in the transplanted lung at different PGD scores.
DISCUSSION
The definition of PGD recommended by the ISHLT Working Group on Primary Lung Graft Dysfunction (PGD) is based on radiological findings at chest X-ray examination, and the gas exchange impairment measured by the PaO2: FiO2 ratio (ratio of the partial pressure of oxygen in arterial blood to the fraction of inspired oxygen, also termed P/F ratio) It is an operational definition that pertains to the symptoms of PGD only, and does not show the heterogeneity of PGD. From our results, we found that 71% of patients had PGD, 40% had PGD grade 3, 12.5% had PGD grade 2, 18.8% had PGD grade 1 and 28% had no PGD. These high incidences may be caused by an over-representation of PGD cases in a small sample.
Earlier studies have documented the higher sensitivity of HRCT [8, 13, 20, 21] and we have studied HRCT images of the transplanted lung that were generated by a 64-MSCT scanner, in relation to PGD and found that GG opacities, consolidation and ST changes were largely present up until 2 weeks, and reduced by the 12th week. Fluctuations seen in GG and consolidation after a year may be caused by acute disease. Consolidation was seen in small areas, which had increased in severity in several patients by the 14th day. It decreased over time but also had a tendency to persist. ST was seen in smaller amounts in several patients, and also seemed to persist over time. We found that the same patients with GG opacities and/or consolidation at 3 days post-LUTX were also the same patients that had changes at 14 days post-LUTX. There was only a single case of a patient without GG opacities at 3 days developing it at 14 days. Several of the same patients with consolidations have GG changes but often to a lesser extent than the affected area by GG. ST does not consistently seem to occur with GG opacities or consolidation.
Only the most severe cases of GG opacity scores (0.51–1.0) were found in patients with PGD. GG opacity scores higher than 0.51 are not found in patients without PGD. However, GG opacities scores ranging from 0.0 to 0.5 were seen in all patients, both in those with and without PGD. Two patients with PGD 2, and one patient with PGD 3 had low GG opacities scores of <0.10. PGD appears to be a heterogeneous syndrome, as patients with PGD display all grades of GG opacities scores. However, the largest number of patients with low GG MSCT scores <0.10 were found in the group, PGD 0, which indicates that patients without PGD do not score as highly as do those with PGD, which is what we expected. Higher GG scores in the PGD 0 group may represent actual cases of PGD that were missed due to the conventional mode of defining PGD, which involves the use of X-ray images that have a lower sensitivity compared with HRCT.
We also found that the highest consolidation scores ranging from 0.5 to 1, were only seen in patients with PGD. Like with GG opacities, all consolidation score categories were seen in patients with PGD; however, patients with PGD 3 displayed the highest numbers of patients with a consolidation score range of 0.25–5.0. GG opacities changes seem to affect a larger area of the lung than do consolidations, and may as such appear more severe. However, GG opacities and consolidation are often present in the same patient and both conditions peak around the 14th day after transplantation.
Approximately, 85% of patients without PGD (PGD 0) appear to have very little ST, <0.1. The remaining 15% lie in the range of 0.10–0.25. The most severe cases of ST 0.26–1.0 are only found in patients with PGD. ST was rare in patients without PGD and only one patient without PGD had ST at a level of 0.10–0.25.
There were no vascular changes found at CT angiography and this examination may not be justified as routine follow-up. All patients received prophylactic treatment with low-molecular heparine until fully mobilized, on average 7–10 days, which could explain that no vascular changes were seen. Also, the sensitivity for detecting small peripheral emboli is low. Very low incidences of peribronchial thickening, air trapping, mosaic attenuation and bronchiectasis were found in our results; these parameters do not seem to be a useful in evaluating PGD. All patients had scheduled bronchoscopies at 2, 4, 6, 12, 26 and 52 weeks and no patient had bronchial dehiscence or bronchial stenosis.
The study is the first of its kind and was carried out at a single national centre representing a population of 5.5 million people. The study was prospective and included almost all patients transplanted at the centre over a period of 24 months starting in September 2007, which represent an unbiased sample of lung transplant patients from our centre. The two observers were blinded to the clinical PGD grade at the time of the HRCT image evaluations. The limited number of patients and a possible bias in this centre compared with other centres are the limitations of the study. The most severely ill patients did not receive HRCT scan due to unstable condition and represent a serious bias in evaluating PGD.
A potential disadvantage of the method used in this study is the greater radiation dose that patients are exposed to. Based on the specifications of the MSCT scanner producers, the effective radiation dose for all four scans is estimated to be 34 mSv. It is a theoretical calculation and such a radiation dose will give a risk of developing radiation-induced cancer of 1–1000, which can be compared with the risk of developing cancer in the general public increasing from 25 to 25.1%. Since all patients are adults, the estimated risk is even lower as the risk of developing radiation-induced cancer depends on age. It should be noted that these scans might have been carried out as a part of optimizing treatment after transplantation anyway.
Suppression of cell-mediated immunity following irradiation has been reported by several studies [22, 23] and this is an added risk to immuno-suppressed post-transplant patients. The dose of 7–10 mSv for each CT scan is, however very low compared with radiation doses used in total lymphoid irradiation in lung transplant patients where the most common protocols deliver 8 Gy in 10 sessions (800 mSv per treatment) in order to obtain a clinically significant immunosuppression in addition to the usual immunosuppression. Even if immunosuppression enhances susceptibility to radiation, it is unlikely that the doses used should have caused any additional acute immunosuppression.
The renal function of the patient is of concern when administering contrast agents, which are nephrotoxic; however, patients were not given Visipaque unless their serum creatinine levels were between 45 and 90 µmol/l for females and between 60 and 105 µmol/l for men. Likewise glomerular filtration rate (GFR) had to be over 60 ml/min/1.73 m2. To be sure of this, new values of the serum creatinine and GFR were always obtained within a week of a new administration of Visipaque. Nephrotoxic medications were temporarily suspended prior to the use of contrast in radiological examinations. It was ensured that patients were well hydrated or well informed about the necessity of being well hydrated before and after the examination.
This study was primarily focused on PGD; additional research on acute rejection and pneumonia is important and interesting but requires further research and data, which are unavailable at this time.
CONCLUSION
HRCT images of the transplanted lung generated by 64-MSCT scanners show that GG opacities, consolidation and ST changes were largely present up until 2 weeks post transplantation in most patients and were markedly reduced by the 12th week. Consolidations appear to be the result of severe GG opacities and are seen to accompany GG opacities in the same patient. Many more changes were seen on HRCT than on chest X-ray on Day 3.
Only the most severe cases of GG opacities and consolidation, with a lung affection of up to 50%, were found in patients with PGD; however, patients with PGD were also found to have low levels of GG opacities and consolidation, and as such PGD appears to be a heterogeneous syndrome. There were no vascular changes found at CT angiographies, and this study suggests that arterial or venous changes do not play any significant role in PGD.
Most patients without PGD have less than a 10% affection of the lung by ST. ST does not consistently seem to occur with GG opacities or consolidation. ST may be an important indicator of PGD.
This study did not address the question of clinical usefulness of routine HRCT scan and this has still to be determined. However, this was a descriptive study, which found that HRCT64-MSCT imaging might be a useful tool for the identification of pathological features of difficult cases of PGD in patients after LUTX.
Conclusions: (i) Changes on HRCT are much more widespread than on chest X-ray in the first 72 h and at later time points. Most changes undergo near-total resolution within the first 3 months, and few patients exhibit residual changes at 12 months. (ii) Different HRCT patterns exist for PGD defined by chest X-ray but the clinical significance of this has yet to be determined.
Funding
This research project has received a grant from the Danish Lung Association (Danmarks Lungeforening).
Conflict of interest: none declared.
REFERENCES
- 1.Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–11. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Oto T, Levvey BJ, Snell GI. Potential refinements of the International Society for Heart and Lung Transplantation primary graft dysfunction grading system. J Heart Lung Transplant. 2007;26:431–6. doi: 10.1016/j.healun.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Christie J, Keshavjee S, Orens J, Arcasoy S, DePerrot M, Barr M, et al. Potential refinements of the International Society for Heart and Lung Transplantation primary graft dysfunction grading system. J Heart Lung Transplant. 2008;27:138. doi: 10.1016/j.healun.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1231–9. doi: 10.1016/j.healun.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter YM, Davis RD. Primary graft dysfunction in lung transplantation. Semin Respir Crit Care Med. 2006;27:501–7. doi: 10.1055/s-2006-954608. [DOI] [PubMed] [Google Scholar]
- 7.Woodrow JP, Shlobin OA, Barnett SD, Burton N, Nathan SD. Comparison of bronchiolitis obliterans syndrome to other forms of chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1159–64. doi: 10.1016/j.healun.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Malinen A, Erkinjuntti-Pekkanen R, Partanen K, Rytkonen H, Vanninen R. Reproducibility of scoring emphysema by HRCT. Acta Radiol. 2002;43:54–9. doi: 10.1080/028418502127347448. [DOI] [PubMed] [Google Scholar]
- 9.Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, McCauley DI, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991;179:783–8. doi: 10.1148/radiology.179.3.2027992. [DOI] [PubMed] [Google Scholar]
- 10.Brody AS. Scoring systems for CT in cystic fibrosis: who cares? Radiology. 2004;231:296–8. doi: 10.1148/radiol.2312032097. [DOI] [PubMed] [Google Scholar]
- 11.Brody AS, Kosorok MR, Li Z, Broderick LS, Foster JL, Laxova A, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging. 2006;21:14–21. doi: 10.1097/01.rti.0000203937.82276.ce. [DOI] [PubMed] [Google Scholar]
- 12.Lynch DA, David GJ, Safrin S, Starko KM, Hormel P, Brown KK, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172:488–93. doi: 10.1164/rccm.200412-1756OC. [DOI] [PubMed] [Google Scholar]
- 13.Robinson TE. High-resolution CT scanning: potential outcome measure. Curr Opin Pulm Med. 2004;10:537–41. doi: 10.1097/01.mcp.0000142924.38801.45. [DOI] [PubMed] [Google Scholar]
- 14.Nobauer-Huhmann IM, Eibenberger K, Schaefer-Prokop C, Steltzer H, Schlick W, Strasser K, et al. Changes in lung parenchyma after acute respiratory distress syndrome (ARDS): assessment with high-resolution computed tomography. Eur Radiol. 2001;11:2436–43. doi: 10.1007/s003300101103. [DOI] [PubMed] [Google Scholar]
- 15.Belmaati E, Jensen C, Kofoed KF, Iversen M, Steffensen I, Nielsen MB. Primary graft dysfunction; possible evaluation by high resolution computed tomography, and suggestions for a scoring system. Interact CardioVasc Thorac Surg. 2009;9:859–67. doi: 10.1510/icvts.2009.207852. [DOI] [PubMed] [Google Scholar]
- 16.Arthurs OJ, Yates SJ, Set PA, Gibbons DA, Dixon AK. Evaluation of image quality and radiation dose in adolescent thoracic imaging: 64-slice is preferable to 16-slice multislice CT. Br J Radiol. 2009;82:157–61. doi: 10.1259/bjr/52970138. [DOI] [PubMed] [Google Scholar]
- 17.Szucs-Farkas Z, Kurmann L, Strautz T, Patak MA, Vock P, Schindera ST. Patient exposure and image quality of low-dose pulmonary computed tomography angiography: comparison of 100- and 80-kVp protocols. Invest Radiol. 2008;43:871–6. doi: 10.1097/RLI.0b013e3181875e86. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz LM, Yoshizumi TT, Goodman PC, Nelson RC, Toncheva G, Nguyen GB, et al. Radiation dose savings for adult pulmonary embolus 64-MDCT using bismuth breast shields, lower peak kilovoltage, and automatic tube current modulation. Am J Roentgenol. 2009;192:244–53. doi: 10.2214/AJR.08.1066. [DOI] [PubMed] [Google Scholar]
- 19.Burton CM, Andersen CB, Jensen AS, Iversen M, Milman N, Boesgaard S, et al. The incidence of acute cellular rejection after lung transplantation: a comparative study of anti-thymocyte globulin and daclizumab. J Heart Lung Transplant. 2006;25:638–47. doi: 10.1016/j.healun.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Biederer J, Schnabel A, Muhle C, Gross WL, Heller M, Reuter M. Correlation between HRCT findings, pulmonary function tests and bronchoalveolar lavage cytology in interstitial lung disease associated with rheumatoid arthritis. Eur Radiol. 2004;14:272–80. doi: 10.1007/s00330-003-2026-1. [DOI] [PubMed] [Google Scholar]
- 21.Tiddens HA, de Jong PA. Imaging and clinical trials in cystic fibrosis. Proc Am Thorac Soc. 2007;4:343–6. doi: 10.1513/pats.200611-174HT. [DOI] [PubMed] [Google Scholar]
- 22.Wara WM, Phillips TL, Wara DW, Ammann AJ, Smith V. Immunosuppression following radiation therapy for carcinoma of the nasopharynx. Am J Roentgenol Radium Ther Nucl Med. 1975;123:482–5. doi: 10.2214/ajr.123.3.482. [DOI] [PubMed] [Google Scholar]
- 23.Wara WM. Immunosuppression associated with radiation therapy. Int J Radiat Oncol Biol Phys. 1977;2:593–6. doi: 10.1016/0360-3016(77)90174-2. [DOI] [PubMed] [Google Scholar]