Abstract
Organic-solvent-free cross-couplings between benzylic and alkenyl halides have been developed. Various alkenyl halides can be effciently benzylated by combining the precursor halides in the presence of Zn dust and a Pd catalyst at room temperature, in water as the only medium.
Recently, we described a new process for effecting sp2–sp3 cross-coupling reactions that avoids prior preparation of organozinc reagents. Zinc-mediated, palladium-catalyzed cross-couplings between two organic halides were accomplished in water using micellar catalysis,1 and on water2 at room temperature.
Allylated aromatics (I) are important subunits in organic synthesis.3 Classically, compounds of general formula I (Scheme 1) have been accessed via Friedel–Crafts allylation4 of aromatic precursors. More recent strategies involve transition metal-catalyzed cross-couplings of sp2-organometallics with activated halides, or allylic organometallics with sp2-halides/triflates (Scheme 1).5 The corresponding organometallic reagents, therefore, require preparation in a separate step.6 Based on our previous work2 it was anticipated that direct ‘on water’ cross-couplings of benzylic halides with alkenyl halides would provide a 1-step synthesis of functionalized allylated aromatics (Scheme 1). The important issue of olefin geometry would also need to be addressed.
Scheme 1.
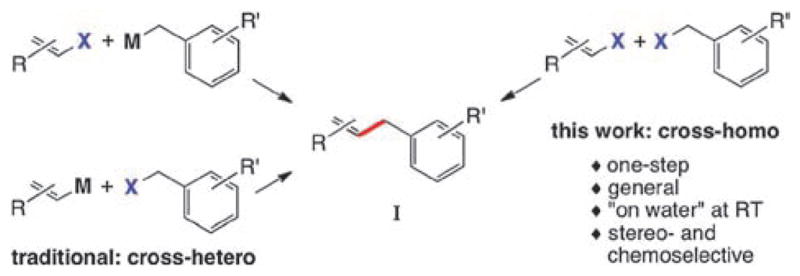
Synthetic approaches to benzylic-alkenyl cross-couplings.
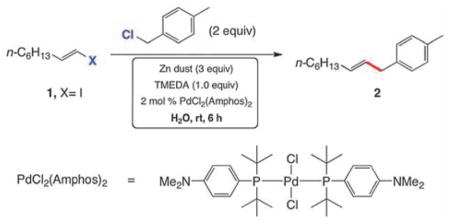
Initially, 1-(chloromethyl)-4-methylbenzene (2 equiv) was coupled with (E)-1-iodooctene (1) to afford unsymmetrically substituted allylated toluene 2 (Table 1). Under optimized conditions, 1 coupled within six hours, on water at room temperature, using 2 mol % PdCl2(Amphos)27 along with Zn dust (3 equiv) and N,N,N′,N′-tetramethylethylenediamine (TMEDA; 1 equiv).1,2 Modifications (entries 2–5), including use of a lesser excess of benzylic chloride (entry 2), less TMEDA (entry 3) or Pd catalyst (entry 4), Zn powder in place of Zn dust (entry 5), or alkenyl bromide (entry 6), suggested some flexibility in reagents and/or the standard stoichiometry. Use of a surfactant for purposes of generating nanomicelles1 in this reaction type neither enhanced the rate of reaction, nor improved the overall yield of products.
Table 1.
Optimization of the reaction between 1-(chloromethyl)-4-methylbenzene (1) and (E)-1-iodooct-1-enea
 | ||
|---|---|---|
| Entry | Modification of original conditions | Conversion, %b |
| 1 | None | 100 |
| 2 | 1.5 equiv of benzylic chloride | 98 |
| 3 | TMEDA (0.5) | 77 |
| 4 | 1 mol% PdCl2(Amphos)2 | 88 |
| 5 | Zn powder | 96 |
| 6 | X = Br | 90 |
Conditions: benzyl chloride (2 mmol), (E)-1-iodooct-1-ene (1 mmol), zinc dust (3 mmol), PdCl2(Amphos)2 (0.02 mmol), degassed water (3 mL).
By GC.
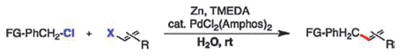
As illustrated in Table 2, the substrates that participate in these cross-couplings leading to allylated aromatics are of broad scope. In all representative cases examined, >98% retention of olefin stereochemistry was observed, regardless of starting olefin geometry. Three equivalents of electron-poor benzylic chlorides were needed to achieve good isolated yields due to competitive Zn-insertion and subsequent protio-quenching by water8 of the in situ-formed benzylic zinc halides. Unlike cross-couplings with aryl halides,2 switching to zinc powder instead of zinc dust did not impact yields. Attempts to control the rate of Zn-insertion by employing only 0.25 equivalents of TMEDA with two equivalents of an electron-poor benzylic chloride led to full consumption of starting alkenyl halide; however, 10–30% of diene formation occurred, leading to poor isolated yields of final products. This coupling does tolerate β,β-disubstituted alkenyl iodides (entry 11), as well as secondary benzylic chlorides (entries 13 and 14). Nevertheless, sterically crowded 2-(chloromethyl)-1,3,5-tri-methylbenzene did not afford the desired product, and under a variety of conditions, no product was detected using benz-hydryl chloride. α-Benzylated styrene 15 could be efficiently prepared starting from commercially available α-bromostyrene (entry 14). A highly lipophilic, solanesol-derived alkenyl halide was successfully coupled on water with a functionalized benzyl chloride at room temperature (entry 15).
Table 2.
Representative benzylic-alkenyl cross-couplingsa
 | |||
|---|---|---|---|
| Entry | Product (Yield)b | Entry | Product (Yield)b |
| 1 |
2 (82%) |
2 |
3 (91%) |
 |
|||
| 3 | 4 R = CF3 (84%) | 5 | 6 R = H (95%) |
| 4 | 5 R = Cl (89%) | 6 | 7 R = OMe (81%) |
| 7 |
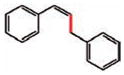 8 (90%) |
8 |
9 (85%) E/Z 83/17c |
| 9 |
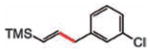 10 (77%) E/Z 92/8d |
10 |
 11 (95%) |
| 11 |
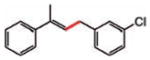 12 (92%) |
12 |
13 (96%) |
| 13 |
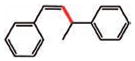 14 (83%) |
14 |
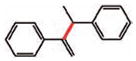 15 (79%)e |
| 15 |
16 (82%) |
||
Conditions: benzylic chloride (2–3 mmol), alkenyl halide (X = I, for entries 1–6, 10, 12, 15, and X = Br for entries 7–9, 11, 13–14; 1 mmol), zinc dust (3–4 mmol), PdCl2(Amphos)2 (0.02 mmol), degassed water (3 mL), rt, 6 h.
Isolated yield.
From commercially available β-bromo-styrene, E/Z = 87/13.
From commercially available (2-bromovinyl)trimethylsilane, E/Z = 93/7.
From commercially available 90% technical grade a-bromostyrene.
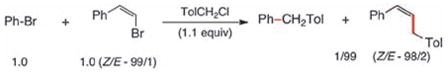 |
(1) |
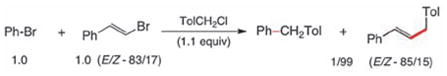 |
(2) |
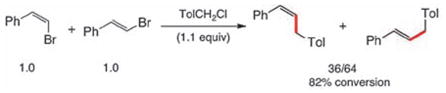 |
(3) |
To probe for potential differences in reactivity in these cross-couplings between sp2-aryl and sp2-alkenyl halides, several competition experiments were conducted (Scheme 2). The representative case of unfunctionalized bromobenzene and β-bromostyrene was chosen. Remarkably, both Z-and E-alkenyl bromides reacted far more rapidly, and with complete stereocontrol, than did bromobenzene with 1-(chloromethyl)-4-methylbenzene (equations 1, 2). Additionally, the E-isomer reacted faster than did Z-β-bromostyrene (equation 3), as expected.9 These results suggested that selective, sequential cross-couplings on dibromide 17 should be possible. In the event, use of just 1.1 equivalents of 1-(chloromethyl)-4-methylbenzene led to cross-coupled product 18 with very good chemoselectivity and in good isolated yield. Hence, the corresponding 1-pot, stepwise dibenzylation of 17 could also be achieved to form the unsymmetrically derivatized product 19, without introduction of additional Pd(II) catalyst in the second step.
Scheme 2.
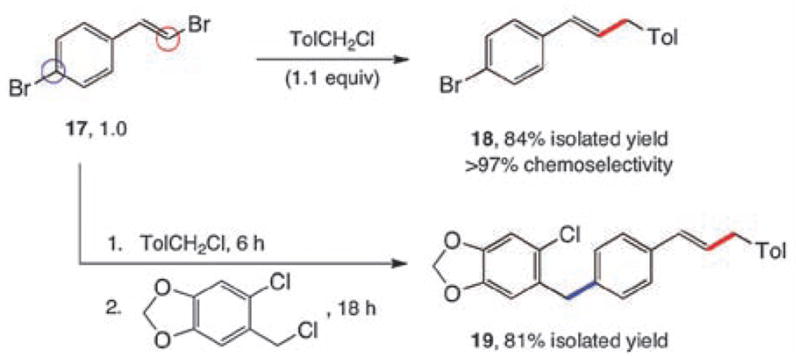
Competition experiments (rt, 6 h), and a 1-pot, double benzylation (1st step 6 h, 2nd step 18 h at rt).
In summary, new technology for preparing functionalized allylated benzenes has been developed. These reactions proceed in a green chemistry sense: in the absence of organic solvents, and without heating or cooling; i.e., in water only, and at room temperature, to afford the targeted products without erosion of olefin geometries and in high chemical yields.
We are grateful to the NIH for financial support (GM 86485), and to Johnson Matthey for generously supplying palladium catalyst PdCl2(Amphos)2 (Pd-132, catalog #C4138).
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available: Experimental section and spectroscopic data.
Notes and references
- 1.Krasovskiy A, Duplais C, Lipshutz BH. J Am Chem Soc. 2009;131:15592. doi: 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]; Krasovskiy A, Lipshutz BH. Org Lett. 2010;12:4742. doi: 10.1021/ol101885t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duplais C, Krasovskiy A, Wattenberg A, Lipshutz BH. Chem Commun. 2010:562. doi: 10.1039/b922280d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma JC, Dougherty DA. Chem Rev. 1997;97:1303. doi: 10.1021/cr9603744. [DOI] [PubMed] [Google Scholar]
- 4.Mitsuo K, Satoru N, Tadahiro M. J Chem Soc, Chem Commun. 1994;18:1895. [Google Scholar]
- 5.Negishi E, King AO, Okukado N. J Org Chem. 1977;42:1821. [Google Scholar]; Miyaura N, Yano T, Suzuki A. Tetrahedron Lett. 1980;21:2865. [Google Scholar]; Chowdhury S, Georghiou PE. Tetrahedron Lett. 1999;40:7599. [Google Scholar]; Botella L, Nájera C. J Organomet Chem. 2002;663:46. [Google Scholar]; Yamada YMA, Takeda K, Takahashi H, Ikegami S. J Org Chem. 2003;68:7733. doi: 10.1021/jo034354v. [DOI] [PubMed] [Google Scholar]; Chahen L, Doucet H, Santelli M. Synlett. 2003:1668. [Google Scholar]; Langle S, Abarbri M, Duchene A. Tetrahedron Lett. 2003;44:9255. [Google Scholar]; Nájera C, Gil-Moltó J, Karlström S. Adv Synth Catal. 2004;346:1798. [Google Scholar]; Bandgar BP, Bettigeri SV, Phopase J. Tetrahedron Lett. 2004;45:6959. [Google Scholar]; Nobre SM, Monteiro AL. Tetrahedron Lett. 2004;45:8225. [Google Scholar]; Kuwano R, Yokogi M. Org Lett. 2005;7:945. doi: 10.1021/ol050078q. [DOI] [PubMed] [Google Scholar]; Singh R, Viciu MS, Kramareva N, Navarro O, Nolan SP. Org Lett. 2005;7:1829. doi: 10.1021/ol050472o. [DOI] [PubMed] [Google Scholar]; McLaughlin M. Org Lett. 2005;8:4875. doi: 10.1021/ol0517271. [DOI] [PubMed] [Google Scholar]; Flaherty A, Trunkfield A, Barto W. Org Lett. 2005;8:4975. doi: 10.1021/ol051929x. [DOI] [PubMed] [Google Scholar]; Kuwano R, Yokogi M. Chem Commun. 2005:5899. doi: 10.1039/b513372f. [DOI] [PubMed] [Google Scholar]; Burns JM, Fairlamb IJS, Kapdi AR, Sehnal P, Taylor JRK. Org Lett. 2007;9:5397. doi: 10.1021/ol702291r. [DOI] [PubMed] [Google Scholar]; Molander GA, Elia MD. J Org Chem. 2006;71:9198. doi: 10.1021/jo061699f. [DOI] [PMC free article] [PubMed] [Google Scholar]; Henry N, Enguehard-Gueiffer C, Thery I, Guieffier A. Eur J Org Chem. 2008:4824. [Google Scholar]; Alacid E, Náceja C. J Org Chem. 2009;74:2321. doi: 10.1021/jo802356n. [DOI] [PubMed] [Google Scholar]; Onuma K, Hashimoto H. Bull Chem Soc Jpn. 1972;45:2582. [Google Scholar]; Ku YY, Patel RR, Sawick DP. Tetrahedron Lett. 1996;37:1949. [Google Scholar]; Dohle W, Lindsay DM, Knochel P. Org Lett. 2001;3:2871. doi: 10.1021/ol0163272. [DOI] [PubMed] [Google Scholar]; Kofink CC, Knochel P. Org Lett. 2006;8:4121. doi: 10.1021/ol0616790. [DOI] [PubMed] [Google Scholar]; Park K, Kang M, Yie JE, Kim JM, Lee LM. Tetrahedron Lett. 2005;46:2849. [Google Scholar]; Bedford RB, Huwe M, Wilkinson MC. Chem Commun. 2009:600. doi: 10.1039/b818961g. [DOI] [PubMed] [Google Scholar]; Betzemeier B, Knochel P. Angew Chem, Int Ed Engl. 1997;36:2623. [Google Scholar]; Hargreaves SL, Pilkington BL, Russel SE, Worthington PA. Tetrahedron Lett. 2000;41:1653. [Google Scholar]; Angiolelli ME, Casalnuovo AL, Selby TP. Synlett. 2000;6:905. [Google Scholar]; Utas JE, Olofsson B, Åkermark B. Synlett. 2006;12:1965. [Google Scholar]; Hossain KM, Takagi K. Chem Lett. 1999:1241. [Google Scholar]; Shade MA, Metzger A, Hug S, Knochel P. Chem Commun. 2008:3046. doi: 10.1039/b803072c. [DOI] [PubMed] [Google Scholar]
- 6.Rare exceptions: Amatore M, Gosmini C. Chem Commun. 2008:5019. doi: 10.1039/b809626k.Czaplik WM, Mayer M, von Wangelin AJ. Angew Chem, Int Ed. 2009;48:607. doi: 10.1002/anie.200804434.
- 7.PdCl2(Amphos)2 (CAS #887919-35-9) obtained from Johnson Matthey (Amphos=p-dimethylaminophenyl-di-t-butylphosphine). Guram AS, King AO, Allen JG, Wang X, Schenkel LB, Chan J, Bunel EE, Faul MM, Larsen RD, Martinelli MJ, Reider PJ. Org Lett. 2006;8:1787. doi: 10.1021/ol060268g.Guram AS, Wang X, Bunel EE, Faul MM, Larsen RD, Martinelli MJ. J Org Chem. 2007;72:5104. doi: 10.1021/jo070341w.
- 8.For pKa tolerance of organozinc reagents, see: Manolikakes G, Muñoz Hernandez C, Schade MA, Metzger A, Knochel P. J Org Chem. 2008;73:8422. doi: 10.1021/jo8015852.
- 9.Minato A, Suzuki K, Tamao K. J Am Chem Soc. 1987;109:1257. [Google Scholar]; Zeng X, Hu Q, Qian M, Negishi E. J Am Chem Soc. 2003;125:13636. doi: 10.1021/ja0304392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


