Abstract
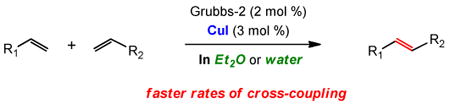
Copper iodide has been shown to be an effective co-catalyst for the olefin cross metathesis reaction. In particular, it has both a catalyst stabilizing effect due to iodide ion, as well as copper(I)-based phosphine-scavenging properties that apply to use of the Grubbs-2 catalyst. A variety of Michael acceptors and olefinic partners can be cross-coupled under mild conditions in refluxing diethyl ether that avoid chlorinated solvents. This effect has also been applied to chemistry in water at room temperature using the new surfactant TPGS-750-M.
The formation of carbon-carbon double bonds by olefin metathesis is among the most powerful and broadly applicable synthetic tools of modern organic chemistry.1 In particular, cross metathesis (CM) reactions promoted by ruthenium-based catalysts have been widely utilized by synthetic organic as well as polymer chemists in the construction of higher olefins from simple alkene precursors.2 N-Heterocyclic carbene (NHC) ligand-containing catalysts,3 such as the second-generation Grubbs catalyst 14 (Figure 1), have emerged as especially promising in selective CM.5 For some conjugated olefins, however, reactions can be rather challenging, most notably with vinyl ketones,6 acrylic acid,7 and acrylonitrile,8 oftentimes requiring higher loadings of catalyst and heat. Although CuCl serves as phosphine scavenger to assist with formation of Grubbs-Hoveyda-19 or Grubbs-Hoveyda-210 ruthenium carbene complexes, use of copper salts to enhance rates of metathesis reactions themselves is rare.8,11 In this note we describe a new procedure for carrying out CM reactions under the beneficial impact of a copper(I) salt, CuI, which not only leads to faster rates of cross-couplings but avoids chlorinated solvents as well.
FIGURE 1.
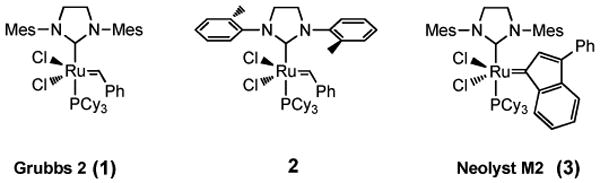
Structures of Ru-based catalysts used for olefin metathesis.
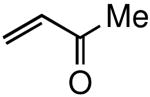
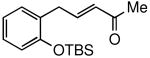
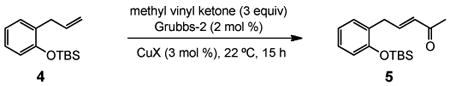
Several conditions and copper salts were screened utilizing a TBS-protected allylphenol 4 as a representative substrate (Table 1). Moving from CuCN (entry 1) to counter ions with increased solubility in organic solvents (entries 2-8) showed little effect on turnover enhancement. An increase from 4% to 6 mol % of CuI showed no effect (entry 13), while an increase in the Grubbs-2 catalyst loading showed only modest improvement (entry 14). Changing the solvent to toluene (entry 15) had no impact, while an improvement was noted in both THF (entry 16) and in diethyl ether (entry 17). Better results were obtained by diluting the latter ethereal mixture from 0.5 m to 0.1 m (entry 19). Ultimately, running the reaction at 35 °C in refluxing Et2O for three hours gave a nearly quantitative yield (entry 21), while in the absence of CuI, the yield was only 57%. The corresponding control reactions, each run in CH2Cl2 and Et2O in the absence of CuI, clearly show an effect on the rate and extent of reaction (entries 12, 20).
TABLE 1. Optimizing Reaction Conditions.
 | |||
|---|---|---|---|
| entry | CuX | solventa | conversion (%)b |
| 1 | CuCN | CH2CI2 | 24 |
| 2 | CuOAc | CH2CI2 | <5 |
| 3 | Cu(OTf)2 | CH2CI2 | 0 |
| 4 | Cu(BF4)2 | CH2CI2 | 15 |
| 5 | Cu(NO3)2 | CH2CI2 | 0 |
| 6 | Cu(CIO4)2 | CH2CI2 | 22 |
| 7 | Cu(CF3COO)2 | CH2CI2 | 0 |
| 8 | Cu(CH3CN)4PF6 | CH2CI2 | 27 |
| 9 | CuCI | CH2CI2 | 35 |
| 10 | CuBr | CH2CI2 | 43 |
| 11 | CuI | CH2CI2 | 64 |
| 12 | - | CH2CI2 | 45 |
| 13c | CuI | CH2CI2 | 63 |
| 14d | CuI | CH2CI2 | 68 |
| 15 | CuI | Toluene | 64 |
| 16 | CuI | THF | 70 |
| 17 | CuI | Et2O | 71 |
| 18e | CuI | Et2O | 76 |
| 19f | CuI | Et2O | 85 |
| 20f | - | Et2O | 43 |
| 21g | CuI | Et2O | >99 (98)h |
Reaction run in 0.5 m solution.
Based on 1H NMR.
Using 6 mol % CuI.
Using 3 mol % Grubbs-2 and 6 mol % CuI.
Reaction run in 0.2 m solution.
Reaction run in 0.1 m solution.
At 35 °C for 3 h; without CuI; yield = 57%
Isolated yield.
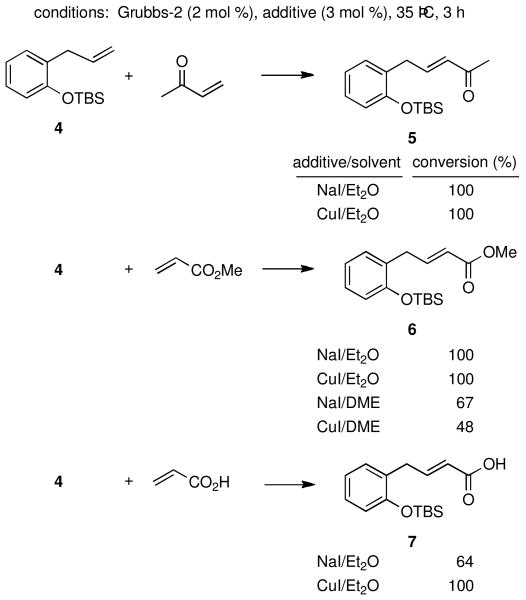
Figure 2 illustrates graphically the effect of CuI. The data suggests that iodide ion may be serving an important role as a stabilizing ligand on ruthenium, thereby extending the lifetime of the original Grubbs-2 catalyst.11 To test this, an identical coupling with MVK was performed with NaI, which in fact led to the same result as that seen with CuI (Scheme 1). Replacing MVK with methyl acrylate to afford product 6 in the presence of NaI alone led to the same positive outcome. However, the NaI effect was not operative in the corresponding metathesis reactions involving acrylic acid en route to product 7 (or acrylonitrile; vide infra). Thus, while CuI gave complete conversion after three hours, NaI afforded only 64% consumption of educt 4. Curiously, switching solvents from ether to DME in which NaI is especially soluble, the level of conversion for MVK dropped to 70% (from 79%) and that for methyl acrylate to 67% (from 100%). Hence, unlike previously studied additives and solvents, it appears that CuI in ether provides both the ligand stabilizing effect of iodide on ruthenium,12 as well as presumably a phosphine sequestering effect by copper(I) from ruthenium.8
FIGURE 2.
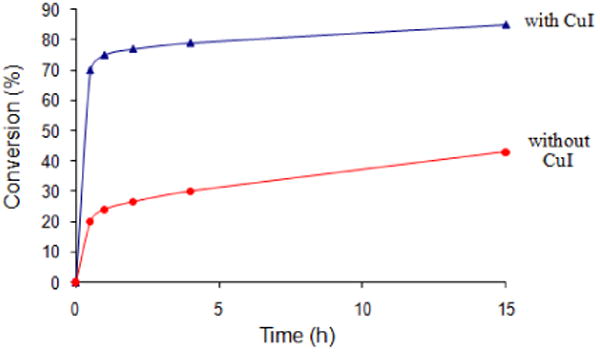
Conversion versus time profile for the CM reaction between olefin 4 and MVK with Grubbs-2 catalyst in refluxing Et2O, as measured by 1H NMR. [conditions: 0.1 M, 2 mol% Grubbs-2, with and without 3 mol % CuI at room temperature.]
SCHEME 1. Comparisons of NaI vs. CuI in Olefin Cross-metathesis Reactions.
Other olefin metathesis catalysts bearing phosphines were also tested for reactivity in the presence of catalytic amounts of CuI (Table 2). As noted previously, the Grubbs-2 catalyst showed a remarkable near doubling in turnover when tested with CuI at room temperature for fifteen hours (entry 1; see also Table 1, entries 19 vs. 20). The less sterically encumbered, more reactive Grubbs catalyst 213 (entry 2), as well as the Neolyst M2 catalyst14 (3, entry 3), showed similar increases in reactivity, but were not pursued further due to the lower levels of conversions seen relative to those noted using catalyst 1.
TABLE 2. Screening of Alternative Ruthenium Catalysts.
 | |||
|---|---|---|---|
| entry | catalyst | conversion (%)a | |
| with CuI | without CuI | ||
| 1 | 1 | 85 | 43 |
| 2 | 2 | 59 | 22 |
| 3 | 3 | 71 | 32 |
Based on 1H NMR.
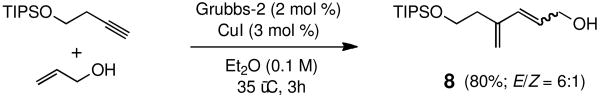
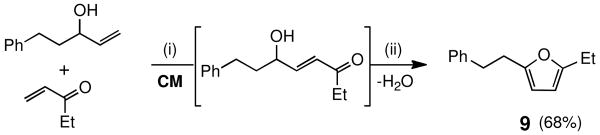
A series of olefins were screened under these newly developed conditions (Table 3). As these examples indicate, a variety of olefinic partners can be successfully cross-coupled in refluxing ether at 0.1 M concentration within three hours. Challenging cases, such as acrylic acid and methyl vinyl ketone readily participated and led to high isolated yields, as did both methyl and t-butyl acrylate derivatives. An olefinic tetrazole15 and p-methoxy-substituted allylbenzene each coupled smoothly with high E/Z selectivities. Prospects for enyne cross-metathesis16 also look encouraging, with improved stereoselectivity due to the reduced temperatures involved, as well as a reduced catalyst loading all being used in a more attractive, non-chlorinated solvent (8, Scheme 2).17 Substituted furans 9 are also accessible via sequential CM/acid-catalyzed cyclization, with lower levels of Grubbs-2 catalyst loadings (Scheme 3).18
TABLE 3. CuI Assisted Olefin Metathesis Reactionsa.
Reactions were conducted at 0.5 mmol scale.
Isolated yields.
E/Z ratio was determined by 1H NMR.
SCHEME 2. Cross Enyne Metathesis with Allyl Alcohol.
SCHEME 3. One-pot Synthesis of a Substituted Furana.
a Reagents and conditions: (i) Grubbs-2 (2 mol %), CuI (3 mol %), Et2O, 35 °C, 3 h. (ii) PPTS (2.5 mol %), CH2Cl2, 40 °C, 12 h.
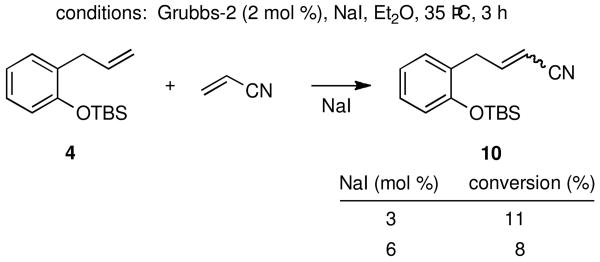
Selective and high-yielding CM using acrylonitrile remains a challenge in olefin metathesis chemistry, presumably due to competitive complexation of Ru by the nitrile group.19 Blechert has studied this coupling reaction and finds that CuCl in the presence of a Grubbs-2 precatalyst in refluxing CH2Cl2 leads to somewhat improved results.8d Heating reaction mixture, especially under microwave conditions, can also be very effective in such reactions.20 An initial attempt using, typically, an excess of the nitrile under optimized conditions in refluxing ether led to a discouraging 35% conversion to 10, even after extended reaction times (Table 4, entry 1). Reducing the nitrile concentration led to an increase in homocoupling with the type I olefinic partner, as well as a slight increase in the nitrile CM products (E + Z; entry 2). To maximize the production of the desired unsaturated nitrile and minimize any decomposition of the highly reactive, phosphine-free Ru complex, the catalyst was added over time. Best results were obtained under these conditions (entries 4 and 5). As before, increasing the amount of copper did not improve the conversion to product (entry 6); leaving copper out of the reaction entirely cut the conversion in half (entry 7). Using NaI alone gave poor levels of conversion using three, or even six, mole percent of this additive (Scheme 4).
TABLE 4. Effect of CuI on CM with Acrylonitrile.
 | |||
|---|---|---|---|
| entry | acrylonitrile (equiv) | time (h) | conversion (%)a |
| 1 | 5.0 | 24 | 35 |
| 2 | 1.5 | 3 | 38b |
| 3c | 1.5 | 1 | 53 |
| 4c | 1.5 | 3 | 60 |
| 5d | 1.5 | 3 | 64 (51)e |
| 6d,f | 1.5 | 3 | 61 |
| 7d,g | 1.5 | 3 | 30 |
Based on 1H NMR.
Significant amount of starting material dimer formed.
Grubbs-2 catalyst added as a solution over 30 minutes.
Grubbs-2 catalyst added portionwise as a solid over 2 hours.
Isolated yield.
8 mol % CuI.
No CuI added.
SCHEME 4. Effect of NaI on CM with Acrylonitrile.
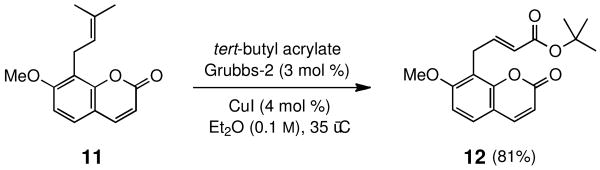
Opportunities to apply the CuI effect to cross metathesis reactions involving trisubstituted olefins of the isopropylidene variety also exist, and while uncommon in general, could prove especially useful.21 A challenging reaction between this Type III olefin and a Type II acrylate was attempted on the natural product osthole, 11, an antiplatelet agent that inhibits phosphoinositide breakdown.22 As shown in Scheme 5, the desired t-butyl acrylate 12 (all E) was formed in good yield (81%, quant. brsm) under standard conditions in refluxing ether over 24 hours.
SCHEME 5. Copper-assisted Cross Metathesis on Osthole.
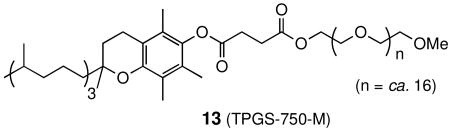
Lastly, we have recently reported that the amphiphile “TPGS-750-M” (13),23 present only to the extent of ca. 2.5% (by weight), allows for cross-metathesis to take place within nanometer micelles in water as the gross reaction medium.24 As illustrated by several examples in Table 5, the benefits ascribed to the presence of CuI can be realized as well under conditions of micellar catalysis25 using this new nonionic surfactant.
TABLE 5. CuI Assisted Olefin Metathesis Reactions in Water at Room Temperaturea.
 | ||||
|---|---|---|---|---|
| entry | product | time (h) | yield (%)b | |
| without CuI | with CuIc | |||
| 1 | 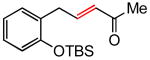 |
12 | 74 | 93 |
| 2 | 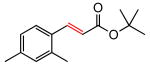 |
15 | 74 | 84 |
| 3 | 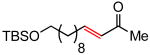 |
12 | 76 | 90 |
| 4 | 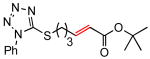 |
20 | 55 | 80 |
| 5 | 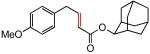 |
12 | 82 | 89 |
Reactions were conducted at 0.5 mmol scale.
Isolated yields.
3 mol % CuI.
In summary, the positive impact of catalytic quantities of CuI in cross-metathesis reactions mediated by the Grubbs-2 catalyst has been demonstrated, in particular where more challenging Type II and Type III olefinic reaction partners are involved (e.g., MVK, acrylic acid, acrylonitrile, and isopropylidene derivatives). These couplings are done in ethereal solvent, rather than chlorinated media, as the former is preferred both insofar as the chemistry is concerned, and from the environmental perspective.26 It has also been shown that equally effective couplings can be achieved using micellar catalysis conditions, where CM within nanoparticles takes place in water at room temperature.
Experimental Section
General Procedure for Cross Metathesis Reactions in Et2O
A flame dried pear shaped flask with a rubber septum containing a stir bar was charged with alkene (0.50 mmol), acrylate/ketone (1.50 mmol), Grubbs-2 catalyst (8.5 mg, 10.0 μmol), and CuI (2.9 mg, 15.0 μmol) under an Ar atmosphere. Freshly distilled ethyl ether (5.0 mL) was added, and the rubber septum was then replaced with a reflux condenser. The solution was heated at 40 °C (oil bath temperature) for 3 hours. After cooling to room temperature, the reaction mixture was concentrated in vacuo and the residue was purified by column chromatography, under the conditions noted, to afford the corresponding metathesis adduct.
(E)-4-(2-(tert-Butyldimethylsilyloxy)phenyl)but-2-enoic acid (Table 3, entry 1)
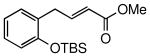
The general procedure was above followed using tert-butyl(2-allylphenoxy)dimethylsilane (124 mg, 0.50 mmol), acrylic acid (72.1 mg, 1.0 mmol), Grubbs-2 catalyst (8.5 mg, 10.0 μmol), and CuI (2.9 mg, 15.0 μmol). Column chromatography on silica gel (eluting with 30% EtOAc/hexanes) afforded the product as a white solid (133 mg, 82%). mp 81-83 °C; IR (thin-film): 3022, 2955, 2930, 2859, 1697, 1649, 1492, 1263, 926 cm-1; 1H NMR (400 MHz, CDCl3): δ 7.23 (dt, J = 15.6, 6.4 Hz, 1H), 7.14 (dt, J = 7.5, 1.8 Hz, 1H), 7.10 (dd, J = 7.5, 1.8 Hz, 1H), 6.91 (dt, J = 7.5, 1.1 Hz, 1H), 6.83 (dd, J = 8.1, 1.1 Hz, 1H), 5.76, (dt, J = 15.6, 1.8 Hz, 1H), 3.54, (dd, J = 6.4, 1.6 Hz, 2H), 1.01 (s, 9H), 0.26 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 172.5, 153.7, 150.5, 130.7, 128.2, 128.1, 121.5, 121.4, 118.6, 33.4, 26.0, 18.4, -3.9; ESI-MS m/z: 315 (M + Na); HRMS (ESI) calcd for C16H24O3SiNa [M + Na]+ 315.1392, found 315.1395.
(E)-5-(2-(tert-Butyldimethylsilyloxy)phenyl)pent-3-en-2-one (Table 3, entry 2)
The general procedure above was followed using tert-butyl(2-allylphenoxy)dimethylsilane (124 mg, 0.50 mmol), methyl vinyl ketone (106 mg, 1.50 mmol), Grubbs-2 catalyst (8.5 mg, 10.0 μmol), and CuI (2.9 mg, 15.0 μmol). Column chromatography on silica gel (eluting with 3% EtOAc/hexanes) afforded the product as a colorless oil (142 mg, 98%). IR (neat): 3062, 3034, 2932, 2894, 2859, 1699, 1676, 1626, 1599, 1582, 1492, 1472, 1452, 1422, 1390, 1361, 1254, 1182, 1108, 1043, 982, 929 cm-1; 1H NMR (400 MHz, CDCl3): δ 7.15 (td, J = 7.6, 1.6 Hz, 1H), 7.11 (dd, J = 7.6, 1.6 Hz, 1H), 6.95 (dt, J = 16.0, 6.4 Hz, 1H), 6.92 (td, J = 7.6, 1.2 Hz, 1H), 6.84 (dd, J = 7.6, 1.2 Hz, 1H), 6.03 (dt, J = 16.0, 1.6 Hz, 1H), 3.54 (dd, J = 6.4, 1.6 Hz, 2H), 2.24 (s, 3H), 1.01 (s, 9H), 0.26 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 198.8, 153.7, 146.8, 132.0, 130.7, 128.4, 128.1, 121.4, 118.6, 33.7, 26.9, 25.9, 18.4, -4.0; EI-MS m/z (%): 275 (M – CH3+, 2), 233 (M – C4H9+, 100), 215 (20), 151 (8), 75 (42); HRMS (EI) calcd for C13H17O2Si [M – C4H9]+ 233.0998, found 233.1006.
(E)-tert-Butyl 4-(2-(tert-butyldimethylsilyloxy)phenyl)-2-butenoate (Table 3, entry 3)
The general procedure above was followed using tert-butyl(2-allylphenoxy)dimethylsilane (124 mg, 0.50 mmol), tert-butyl acrylate (192 mg, 1.50 mmol), Grubbs-2 catalyst (8.5 mg, 10.0 μmol), and CuI (2.9 mg, 15.0 μmol). Column chromatography on silica gel (eluting with 3% EtOAc/hexanes) afforded the product as a pale yellow oil (162 mg, 93%). 1H NMR (400 MHz, CDCl3): δ 7.15-7.10 (m, 2H), 7.00 (dt, J = 15.6, 6.4 Hz, 1H), 6.91 (dt, J = 7.6, 1.2 Hz, 1H), 6.82 (dd, J = 8.0, 0.8 Hz, 1H), 5.68 (dt, J = 15.6, 1.6 Hz, 1H), 3.48 (dd, J = 6.4, 1.6 Hz, 2H), 1.46 (s, 9H), 1.01 (s, 9H), 0.25 (s, 6H).8a
(E)-Methyl 4-(2-(tert-butyldimethylsilyloxy)phenyl)but-2-enoate (Table 3, entry 4)
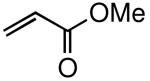
The general procedure above was followed using tert-butyl(2-allylphenoxy)dimethylsilane (124 mg, 0.50 mmol), methyl acrylate (129 mg, 1.50 mmol), Grubbs-2 catalyst (8.5 mg, 10.0 μmol), and CuI (2.9 mg, 15.0 μmol). Column chromatography on silica gel (eluting with 3% EtOAc/hexanes) afforded the product as a pale yellow oil (143 mg, 93%). IR (neat): 3063, 3023, 2953, 2931, 2859, 1726, 1656, 1492, 1265, 1161, 930; 1H NMR (400 MHz, CDCl3): δ 7.16-7.08 (m, 3H), 6.90 (dt, J = 7.2, 1.1 Hz, 1H), 6.82 (dd, J = 8.2, 1.1 Hz, 1H), 5.77 (dt, J = 15.6, 1.8 Hz, 1H), 3.72 (s, 3H), 3.51 (dd, J = 6.3, 1.5 Hz, 2H), 1.01 (s, 9H), 0.25 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 167.1, 153.6, 147.8, 130.6, 128.5, 128.0, 122.0, 121.4, 119.0, 51.5, 33.2, 25.9, 18.4, -4.0; FI-MS m/z: 306 [M]+, 249 [M – C4H9]+; HRFIMS calcd for C17H26O3Si [M]+ 306.1651, found 306.1645.
(E)-tert-Butyl 6-(1-phenyl-1H-tetrazol-5-ylthio)-2-hexenoate (Table 3, entry 5)
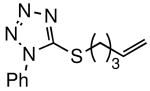
The general procedure above was followed using 5-(pent-4-en-1-ylthio)-1-phenyl-1H-tetrazole5-(pent-4-en-1-ylthio)-1-phenyl-1H-tetrazole8a (123 mg, 0.50 mmol), tert-butyl acrylate (192 mg, 1.50 mmol), Grubbs-2 catalyst (8.5 mg, 10.0 μmol), and CuI (2.9 mg, 15.0 μmol). Column chromatography on silica gel (eluting with 10% EtOAc/hexanes) afforded the product as a colorless oil (145 mg, 84%). 1H NMR (400 MHz, CDCl3): δ 7.57-7.53 (m, 5H), 6.80 (dt, J = 15.6, 7.2 Hz, 1H), 5.77 (dt, J = 15.6, 1.6 Hz, 1H), 3.39 (t, J = 7.2 Hz, 2H), 2.33 (qd, J = 6.8, 1.6 Hz, 2H), 2.01 (quintet, J = 7.6 Hz, 2H), 1.46 (s, 9H).8a
(E)-Methyl 4-(4-methoxyphenyl)-2-butenoate (Table 3, entry 6)
The general procedure above was followed using 4-allylanisole (74 mg, 0.50 mmol), methyl acrylate (129 mg, 1.50 mmol), Grubbs-2 catalyst (8.5 mg, 10.0 μmol), and CuI (2.9 mg, 15.0 μmol). Column chromatography on silica gel (eluting with 2.5% EtOAc/hexanes) afforded the product as a colorless oil (97 mg, 94%). 1H NMR (400 MHz, CDCl3): δ 7.13-7.06 (m, 3H), 6.86 (d, J = 8.8 Hz, 2H), 5.80 (dt, J = 15.6, 1.6 Hz, 1H), 3.79 (s, 3H), 3.72 (s, 3H), 3.47 (d, J = 6.8 Hz, 2H).8a
(E)-4-Methylene-6-(triisopropylsilyloxy)hex-2-en-1-ol (8)
The general procedure above was followed using (but-3-yn-1-yloxy)triisopropylsilane (106.2 mg, 0.50 mmol), prop-2-en-1-ol (174.2 mg, 3.00 mmol), Grubbs-2 catalyst (8.5 mg, 10.0 μmol), and CuI (2.9 mg, 15.0 μmol). Column chromatography on silica gel (eluting with 20% EtOAc/hexanes) afforded the product as a pale yellow oil (106 mg, 80%, E/Z = 6:1). IR (neat): 3337, 3083, 2943, 2867, 1684, 1607, 1464, 1384, 1104, 883 cm-1; 1H NMR (400 MHz, CDCl3): δ 6.27 (d, J = 16.1 Hz, 1H), 5.89 (dt, J = 15.8, 5.8 Hz, 1H), 5.07 (s, 1H), 5.03 (s, 1H), 4.22 (br t, J = 4.0 Hz, 2H), 3.81 (t, J = 7.0 Hz, 2H), 2.49 (dt, J = 7.4, 0.9 Hz, 2H) 1.07-1.05 (m, 21H). 13C NMR (100 MHz, CDCl3): δ 142.5, 133.5, 128.1, 117.4, 63.7, 62.8, 35.9, 18.2, 12.2; FI-MS m/z: 284 [M]+, 241 [M – C3H7]+; HRFIMS calcd for C16H32O2Si [M]+ 284.2172, found 284.2179.
2-Ethyl-5-phenethylfuran (9)
A flame dried pear shaped flask with a rubber septum containing a stir bar was charged with 5-phenylpent-1-en-3-ol (41 mg, 0.25 mmol), ethyl vinyl ketone (53 mg, 0.63 mmol), Grubbs-2 catalyst (4.2 mg, 5.0 μmol), and CuI (1.4 mg, 7.5 μmol) in Et2O (2.5 mL) under an argon atmosphere. The rubber septum was then replaced with a reflux condenser and the solution was heated at 40 °C (oil bath temperature) for 3 hours. After cooling to room temperature, the reaction mixture was concentrated in vacuo. PPTS (1.6 mg, 6.3 μmol) and CH2Cl2 (1 mL) were added and the reaction was allowed to stir for an additional 12 hours at 40 °C. After cooling to room temperature, the reaction mixture was concentrated in vacuo. Column chromatography on silica gel (eluting with 2% EtOAc/hexanes) afforded the product as a colorless oil (34 mg, 68%). 1H NMR (400 MHz, CDCl3): δ 7.32–7.27 (m, 2H), 7.23-7.18 (m, 3H), 5.88-5.85 (m, 2H), 2.98-2.93 (m, 2H), 2.92-2.86 (m, 2H), 2.63 (q, J = 7.5 Hz, 2H), 1.23 (t, J = 7.5 Hz, 3H).17
(Z)-4-(2-(tert-Butyldimethylsilyloxy)phenyl)but-2-enenitrile (10)
The general procedure above was followed using tert-butyl(2-allylphenoxy)dimethylsilane (124 mg, 0.50 mmol), acrylonitrile (39.8 mg, 0.75 mmol), Grubbs-2 catalyst (8.5 mg, 10.0 μmol), and CuI (2.9 mg, 15.0 μmol). Column chromatography on silica gel (eluting with 3% EtOAc/hexanes) afforded the product as a colorless oil (70 mg, 51%, Z/E = 3.3:1.0). IR (neat): 3067, 3035, 2956, 2931, 2896, 2859, 2222, 1683, 1620, 1599, 1583, 1492, 1472, 1454, 1390, 1362, 1258, 1184, 1109, 1045, 1108, 928, 838 cm-1; 1H NMR (400 MHz, CDCl3): δ 7.17-7.14 (m, 2H), 6.93 (t, J = 7.6 Hz, 1H), 6.84 (d, J = 7.6 Hz, 1H), 6.65 (dt, J = 11.2, 7.2 Hz, 1H), 5.38 (d, J = 11.2 Hz, 1H), 3.74 (d, J = 7.2 Hz, 2H), 1.03 (s, 9H), 0.27 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 153.3, 130.8, 130.4, 128.6, 128.4, 121.6, 118.7, 116.3, 99.7, 34.4, 33.2, 26.0, -3.9; FI-MS m/z: 273 [M]+, 216 [M – C4H9]+; HRFIMS calcd for C16H23NOSi [M]+ 273.1549, found 273.1557.
(E)-tert-Butyl 4-(7-methoxy-2-oxo-2H-chromen-8-yl)but-2-enoate (12)
The general procedure above was followed using osthole 11) (40 mg, 0.16 mmol), tert-butyl acrylate (63 mg, 0.49 mmol), Grubbs-2 catalyst (4.2 mg, 4.91 μmol), and CuI (1.4 mg, 7.37 μmol). The catalyst and acrylate were added over time and the solution was allowed to stir for 24 hours at 40 °C (oil bath temperature). Column chromatography on silica gel (eluting with 10% EtOAc/hexanes) afforded the product as a white solid (42 mg, 81%). mp 137-140 °C; IR (thin-film): 3071, 2979, 2931, 2843, 1734, 1713, 1605, 1287, 1253, 1119, 1103 cm-1; 1H NMR (400 MHz, CDCl3): δ 7.63 (d, J = 9.2 Hz, 1H), 7.36 (d, J = 8.6 Hz, 1H), 6.94 (dt, J = 15.4, 6.6 Hz, 1H), 6.85 (d, J = 8.5 Hz, 1H), 6.22 (d, J = 9.3 Hz, 1H), 5.67 (dt J = 15.6, 1.7 Hz, 1H), 3.91 (s, 3H), 3.71 (d, J = 1.7 Hz, 2H) 1.44 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 166.3, 161.3, 160.5, 153.1, 144.3, 143.9, 127.5, 123.7, 114.1, 113.3, 113.1, 107.5, 80.3, 56.3, 28.3, 25.3; ESI-MS m/z: 355 (M + K), 339 (M + Na); HRMS (ESI) calcd for C18H20O5Na [M + Na]+ 339.1208, found 339.1195.
General Procedure for Cross Metathesis Reactions in Water
Alkene (0.50 mmol), acrylate (1.00 mmol)/ketone (1.50 mmol), CuI (2.9 mg, 15.0 μmol) and Grubbs-2 catalyst (8.5 mg, 10.0 μmol) were sequentially added into a Teflon-coated-stir-bar-containing Biotage 2-5 mL microwave reactor vial at rt, and then sealed with a septum. An aliquot of TPGS-750-M/H2O (1.0 mL; 2.5% TPGS-750-M by weight; all cross-coupling reactions were conducted at 0.5 m unless stated otherwise) was added, via syringe, and the resulting solution was allowed to stir at rt for 12-20 h. The homogeneous reaction mixture was then diluted with EtOAc (2 mL), filtered through a bed of silica gel, and the bed further washed (3 × 3 mL) with EtOAc to collect all of the cross-coupled material. The volatiles were removed in vacuo to afford the crude product that was subsequently purified by flash chromatography on silica gel.
(E)-5-(2-(tert-Butyldimethylsilyloxy)phenyl)pent-3-en-2-one (Table 5, entry 1)
The general procedure above was followed using tert-butyl(2-allylphenoxy)dimethylsilane (124 mg, 0.50 mmol), methyl vinyl ketone (106 mg, 1.50 mmol), CuI (2.9 mg, 15.0 μmol) and Grubbs-2 catalyst (8.5 mg, 10.0 μmol). Column chromatography on silica gel (eluting with 3% EtOAc/hexanes) afforded the product as a colorless oil (135 mg, 93%). 1H NMR (400 MHz, CDCl3): δ 7.15 (td, J = 7.6, 1.6 Hz, 1H), 7.11 (dd, J = 7.6, 1.6 Hz, 1H), 6.95 (dt, J = 16.0, 6.4 Hz, 1H), 6.92 (td, J = 7.6, 1.2 Hz, 1H), 6.84 (dd, J = 7.6, 1.2 Hz, 1H), 6.03 (dt, J = 16.0, 1.6 Hz, 1H), 3.54 (dd, J = 6.4, 1.6 Hz, 2H), 2.24 (s, 3H), 1.01 (s, 9H), 0.26 (s, 6H).
(E)-tert-Butyl 3-(2,4-dimethylphenyl)acrylate (Table 5, entry 2)
The general procedure above was followed using 2,4-dimethyl-1-vinylbenzene (66 mg, 0.50 mmol), tert-butyl acrylate (128 mg, 1.00 mmol), CuI (2.9 mg, 15.0 μmol) and Grubbs-2 catalyst (8.5 mg, 10.0 μmol). Column chromatography on silica gel (eluting with 2% EtOAc/hexanes) afforded the product as a colorless oil (97 mg, 84%). 1H NMR (400 MHz, CDCl3): δ 7.87 (d, J = 15.6 Hz, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.03-7.01 (m, 2H), 6.29 (d, J = 15.6 Hz, 1H), 2.42 (s, 3H), 2.34 (s, 3H), 1.55 (s, 9H).8a
(E)-13-(tert-Butyldimethylsilyloxy)tridec-3-en-2-one (Table 5, entry 3)
The general procedure above was followed using tert-butyldimethyl(undec-10-enyloxy)silane10a (144 mg, 0.50 mmol), methyl vinyl ketone (106 mg, 1.50 mmol), CuI (2.9 mg, 15.0 μmol) and Grubbs-2 catalyst (8.5 mg, 10.0 μmol). Column chromatography on silica gel (eluting with 3% EtOAc/hexanes) afforded the product as a colorless oil (146 mg, 90%). 1H NMR(400MHz, CDCl3): δ 6.80 (dt, J = 16.0, 6.8 Hz, 1H), 6.06 (d, J = 16.0 Hz, 1H), 3.59 (t, J = 6.8 Hz, 2H), 2.24 (s, 3H), 2.21 (q, J = 7.2 Hz, 2H), 1.53–1.42 (m, 4H), 1.28 (br s, 10H), 0.89 (s, 9H), 0.04 (s, 6H).10a
(E)-tert-Butyl 6-(1-phenyl-1H-tetrazol-5-ylthio)-2-hexenoate (Table 5, entry 4)
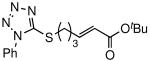
The general procedure above was followed using 5-(pent-4-en-1-ylthio)-1-phenyl-1H-tetrazole8a (123 mg, 0.50 mmol), tert-butyl acrylate (192 mg, 1.50 mmol), CuI (7.2 mg, 38.0 μmol) and Grubbs-2 catalyst (21.2 mg, 25.0 μmol). Column chromatography on silica gel (eluting with 10% EtOAc/hexanes) afforded the product as colorless oil (138 mg, 80%). 1H NMR (400 MHz, CDCl3): δ 7.59-7.55 (m, 5H), 6.82 (dt, J = 15.6, 7.2 Hz, 1H), 5.79 (dt, J = 15.6, 1.6 Hz, 1H), 3.41 (t, J = 7.2 Hz, 2H), 2.36 (qd, J = 7.2, 1.6 Hz, 2H), 2.03 (quintet, J = 7.2 Hz, 2H), 1.48 (s, 9H).8a
(E)-2-Adamantyl 4-(4-methoxyphenyl)-2-butenoate (Table 5, entry 5)
The general procedure above was followed using 4-allylanisole (74 mg, 0.50 mmol), 2-adamantyl acrylate (206 mg, 1.00 mmol), CuI (2.9 mg, 15.0 μmol) and Grubbs-2 catalyst (8.5 mg, 10.0 μmol). Column chromatography on silica gel (eluting with 5% EtOAc/hexanes) afforded the product as a colorless oil (145 mg, 89%). 1H NMR (400 MHz, CDCl3): δ 7.16-7.08 (m, 3H), 6.88 (d, J = 8.8 Hz, 2H), 5.84 (dt, J = 15.2, 1.2 Hz, 1H), 4.99 (br s, 1H), 3.80 (s, 3H), 3.47 (dd, J = 6.8, 1.2 Hz, 2H), 2.05-2.01 (m, 4H), 1.90-1.74 (m, 8H), 1.58-1.56 (m, 2H).8a
Supplementary Material
Acknowledgments
Financial support provided by the NSF (CHE 0948479) and NIH (GM 86485) is warmly acknowledged. We also thank Dr. Richard Pederson (Materia) and Dr. Oliver Briel (Umicore) for supplying the Grubbs and Neolyst catalysts, respectively, used in this study.
Footnotes
Supporting Information Available. Copies of 1H and 13C NMR spectra of all new compounds and copies of 1H NMR spectra of all known compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Chauvin Y. Angew Chem, Int Ed. 2006;45:3740–3747. doi: 10.1002/anie.200601234. [DOI] [PubMed] [Google Scholar]; (b) Schrock RR. Angew Chem, Int Ed. 2006;45:3748–3759. doi: 10.1002/anie.200600085. [DOI] [PubMed] [Google Scholar]; (c) Grubbs RH. Angew Chem, Int Ed. 2006;45:3760–3765. doi: 10.1002/anie.200600680. [DOI] [PubMed] [Google Scholar]; (d) Grubbs RH. Tetrahedron. 2004;60:7117–7140. [Google Scholar]; (e) Grubbs RH, editor. Handbook of Metathesis. Wiley-VCH; Weinheim, Germany: 2003. three-volume set. [Google Scholar]
- 2.For a review, see Schrodi Y, Pederson RL. Aldrichimica Acta. 2007;40:45–52.
- 3.(a) Fürstner A, Thiel OR, Lehmann CW. Organometallics. 2002;21:331–335. [Google Scholar]; (b) Jafarpour L, Hillier AC, Nolan SP. Organometallics. 2002;21:442–444. [Google Scholar]; (c) Wakamatsu H, Blechert S. Angew Chem, Int Ed. 2002;41:2403–2405. doi: 10.1002/1521-3773(20020703)41:13<2403::AID-ANIE2403>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]; (d) Grela K, Harutyunyan S, Michrowska A. Angew Chem, Int Ed. 2002;41:4038–4040. doi: 10.1002/1521-3773(20021104)41:21<4038::AID-ANIE4038>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 5.(a) Morgan JP, Morrill C, Grubbs RH. Org Lett. 2002;4:67–70. doi: 10.1021/ol016918s. [DOI] [PubMed] [Google Scholar]; (b) Chatterjee AK, Sanders DP, Grubbs RH. Org Lett. 2002;4:1939–1942. doi: 10.1021/ol0259793. [DOI] [PubMed] [Google Scholar]; (c) Lera M, Hayes CJ. Org Lett. 2001;3:2765–2768. doi: 10.1021/ol016366d. [DOI] [PubMed] [Google Scholar]; (d) Stragies R, Voigtmann U, Blechert S. Tetrahedron Lett. 2000;41:5465–5468. [Google Scholar]
- 6.(a) Ettari R, Micale N. J Organomet Chem. 2007;692:3574–3576. [Google Scholar]; (b) Michrowska A, Bujok R, Harutyunyan S, Sashuk V, Dolgonos G, Grela K. J Am Chem Soc. 2004;126:9318–9325. doi: 10.1021/ja048794v. [DOI] [PubMed] [Google Scholar]
- 7.(a) Choi TL, Chatterjee AK, Grubbs RH. Angew Chem, Int Ed. 2001;40:1277–1279. doi: 10.1002/1521-3773(20010401)40:7<1277::aid-anie1277>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]; (b) Lipshutz BH, Ghorai S, Bošković ZV. Tetrahedron. 2008;64:6949–6954. [Google Scholar]
- 8.(a) Lipshutz BH, Aguinaldo GT, Ghorai S, Voigtritter K. Org Lett. 2008;10:1325–1328. doi: 10.1021/ol800028x. [DOI] [PubMed] [Google Scholar]; (b) Lipshutz BH, Ghorai S, Leong WWY. J Org Chem. 2009;74:2854–2857. doi: 10.1021/jo900012z. [DOI] [PubMed] [Google Scholar]; (c) Boddaert T, Coquerel Y, Rodriguez J. C R Chimie. 2009;12:872–875. [Google Scholar]; (d) Rivard M, Blechert S. Eur J Org Chem. 2003:2225–2228. [Google Scholar]
- 9.(a) Lipshutz BH, Ghorai S. Org Lett. 2009;11:705–708. doi: 10.1021/ol8027829. [DOI] [PubMed] [Google Scholar]; (b) Kingsbury JS, Harrity JPA, Bonitatebus PJ, Jr, Hoveyda AH. J Am Chem Soc. 1999;121:791–799. [Google Scholar]
- 10.(a) Lipshutz BH, Ghorai S. Tetrahedron. 2010;66:1057–1063. [Google Scholar]; (b) Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J Am Chem Soc. 2000;122:8168–8179. [Google Scholar]
- 11.(a) Morgan JP, Grubbs RH. Org Lett. 2000;2:3153–3155. doi: 10.1021/ol0063510. [DOI] [PubMed] [Google Scholar]; (b) Dias EL, Nguyen ST, Grubbs RH. J Am Chem Soc. 1997;119:3887–3897. [Google Scholar]; (c) Sanford MS, Henling LM, Grubbs RH. Organometallics. 1998;17:5384–5389. [Google Scholar]
- 12.(a) Funk TW, Berlin JM, Grubbs RH. J Am Chem Soc. 2006;128:1840–1846. doi: 10.1021/ja055994d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wappel J, Urbina-Blanco CA, Abbas M, Albering JH, Saf R, Nolan SP, Slugovc C. Beilstein J Org Chem. 2010;6:1091–1098. doi: 10.3762/bjoc.6.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart IC, Ung T, Pletnev AA, Berlin JM, Grubbs RH, Schrodi Y. Org Lett. 2007;9:1589–1592. doi: 10.1021/ol0705144. [DOI] [PubMed] [Google Scholar]
- 14.(a) Hurley PB, Dake GR. J Org Chem. 2008;73:4131–4138. doi: 10.1021/jo800336k. [DOI] [PubMed] [Google Scholar]; (b) Verpoort F, Opstal T. New J Chem. 2003;27:257–262. [Google Scholar]
- 15.A numerous attempts (by varying reaction temperature, and the amount of catalyst) to drive this coupling beyond the 55% reported previously had not been successful See ref 8a.
- 16.(a) Poulsen CS, Madsen R. Synthesis. 2003:1–18. [Google Scholar]; (b) Mori M. Ene-Yne Metathesis. In: Grubbs RH, editor. Handbook of Metathesis. Vol. 2. Wiley-VCH; Weinheim, Germany: 2003. pp. 176–204. [Google Scholar]; (c) Diver ST, Giessert AJ. Chem Rev. 2004;104:1317–1382. doi: 10.1021/cr020009e. [DOI] [PubMed] [Google Scholar]
- 17.Clark DA, Clark JR, Diver ST. Org Lett. 2008;10:2055–2058. doi: 10.1021/ol800523j. [DOI] [PubMed] [Google Scholar]
- 18.Donohoe TJ, Bower JF. Proc Natl Acad Sci USA. 2010;107:3373–3376. doi: 10.1073/pnas.0913466107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai CX, Lu XB, He R, Zhang WZ, Feng XJ. Org Biomol Chem. 2005;3:4139–4142. doi: 10.1039/b510776h. [DOI] [PubMed] [Google Scholar]
- 20.Coquerel Y, Rodriguez J. Eur J Org Chem. 2008:1125–1132. [Google Scholar]
- 21.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto T, Kawasaki T, Hino O. Biochem Pharmacol. 2003;65:677–681. doi: 10.1016/s0006-2952(02)01606-4. [DOI] [PubMed] [Google Scholar]
- 23.TPGS-750-M will be offered in February, 2011, as a 2 wt % solution in water by Sigma-Aldrich This product will be listed under catalog #733857.
- 24.Lipshutz BH, Ghorai S, Abela AR, Moser R, Nishikata T, Duplais C, Krasovskiy A, Gatson RD, Gadwood RC. J Org Chem. 2011 doi: 10.1021/jo101974u. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Dwars T, Paetzold E, Oehme G. Angew Chem, Int Ed. 2005;44:7174–7199. doi: 10.1002/anie.200501365. [DOI] [PubMed] [Google Scholar]; (b) Khan MN. Micellar Catalysis. CRC Press; Boca Raton, FL: 2006. [Google Scholar]
- 26.Sheldon RA, Arends IWCE, Hanefeld U. Green Chemistry and Catalysis. Wiley-VCH; Weinheim, Germany: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.