Abstract
Although the pathogenic mechanisms that underlie autism are not well understood, there is evidence showing that metabotropic and ionotropic glutamate receptors are hyper-stimulated and the GABAergic system is hypo-stimulated in autism. Memantine is an uncompetitive antagonist of NMDA receptors and is widely prescribed for treatment of Alzheimer's disease treatment. Recently, it has been shown to improve language function, social behavior, and self-stimulatory behaviors of some autistic subjects. However the mechanism by which memantine exerts its effect remains to be elucidated. In this study, we used cultured cerebellar granule cells (CGCs) from Fmr1 knockout (KO) mice, a mouse model for fragile X syndrome (FXS) and syndromic autism, to examine the effects of memantine on dendritic spine development and synapse formation. Our results show that the maturation of dendritic spines is delayed in Fmr1-KO CGCs. We also detected reduced excitatory synapse formation in Fmr1-KO CGCs. Memantine treatment of Fmr1-KO CGCs promoted cell adhesion properties. Memantine also stimulated the development of mushroom-shaped mature dendritic spines and restored dendritic spine to normal levels in Fmr1-KO CGCs. Furthermore, we demonstrated that memantine treatment promoted synapse formation and restored the excitatory synapses to a normal range in Fmr1-KO CGCs. These findings suggest that memantine may exert its therapeutic capacity through a stimulatory effect on dendritic spine maturation and excitatory synapse formation, as well as promoting adhesion of CGCs.
Introduction
Autism is the most severe of a group of neurodevelopmental disorders referred to autism spectrum disorders (ASDs), and is characterized by problems in communication, social skills, and repetitive behavior. Susceptibility to autism is attributable to genetic factors [1], [2], [3], [4], but the exact cause of this disorder is not yet known. There is some evidence showing that metabotropic and ionotropic glutamate receptors are affected in autism. Purcell et al [5] demonstrated that there is excessive glutaminergic activity in autistic brain. A normal level of glutamate is important for neurotransmitters that play a key role in long-term potentiation, learning and memory [6], [7], [8], [9]. Hypo-function of the inhibitory GABAergic system and hyperactivity of the excitatory glutamate system has been theorized to have a causal role in autism [10].
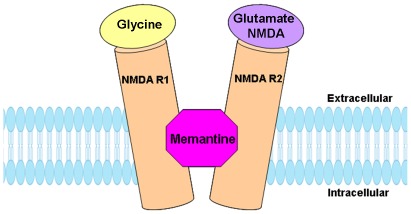
Memantine is a low-affinity voltage-dependant uncompetitive antagonist at NMDA receptors [11], [12], as shown in Figure 1. It is widely prescribed for the treatment of Alzheimer's disease (AD). Studies have shown that memantine can be rapidly displaced from the NMDA receptor, which may avoid prolonged receptor blockade and the associated detrimental effects on learning and memory [13]. In addition, memantine has been demonstrated to act as an antagonist at nicotinic acetylcholine receptors and at 5-HT receptors [14], [15]. Both in vitro and in vivo studies indicate that memantine offers protective effects from neurotoxicity and can improve learning and memory in several preclinical models of AD [16], [17], [18], [19]. Perry et al [20] reported decreased nicotinic receptors and decreased cholinergic receptors in the cortices of autistic brain. Several studies have reported beneficial results with the utilization of cholinesterase inhibitors in AD to enhance frontal lobe function and promote executive language and social response in autism [21], [22], [23]. Most recently, two small open-label studies reported the effect of memantine on improving language and behavior of autistic subjects [24], [25]. In a large scale clinical trial study involving 151 subjects, Chez et al [26] further observed that open-label use of memantine significantly improved language function, social behavior, and self-stimulatory behaviors of autistic subjects. Studies are underway with memantine and larger numbers of autistic subjects (Clinicaltrials.gov). In addition, Erickson et al [27] reported that 4 of 6 patients with Fragile X syndrome (FXS) and a co-morbid diagnosis of pervasive developmental disorder (PDD) with memantine treatment showed global clinical benefit on ratings with the Clinical Global Impressions-Improvement subscale (CGI-I). However the cellular mechanism by which memantine improves language and social behaviors in autism remains to be elucidated.
Figure 1. Schematic representation of memantine and NMDA receptor.
In this study, we examined the effects of memantine on the cultured cerebellar granule cells (CGCs) from Fmr1 knockout (Fmr1-KO) mice, which is a mouse model for FXS and currently also used as a mouse model for autism studies [28], [29], [30]. Our results show that the maturation of dendritic spines is delayed in Fmr1-KO CGCs. We also detected reduced excitatory synapse formation in Fmr1-KO CGCs. Memantine treatment of Fmr1-KO CGCs promoted cell adhesion properties. Memantine also stimulated the development of mushroom-shaped dendritic spines and restored dendritic spine to normal levels seen in Fmr1-KO CGCs. Furthermore, we demonstrate that memantine treatment promoted synapse formation and restored the excitatory synapses to a normal range in Fmr1-KO CGCs.
Methods
Fmr1 knockout mice
Mice were from a colony of congenic C57BL/6 fragile X mice derived from C57BL/6-129 hybrid mice carrying the Fmr1 knockout mutation originally provided through the generosity of B. Oostra, P.Willems and S.Warren. Offspring of mice that carried the knockout mutation were distinguished from their normal siblings by polymerase chain reaction (PCR) analysis with 1.5 mM MgCl2 and using S1, S2 primers for the normal allele and M2, N2 primers for the knockout allele essentially as originally described [28]. All experiments with live animals conformed to the guidelines for the ethical use of animals of the NYS Institute for Basic Research in Developmental Disabilities Institutional Animal Care and Use Committee (IACUC).
Cell culture and memantine treatment
CGCs were prepared from wild type (WT) and Fmr1 KO 5–6 day postnatal pups as described previously [31]. Briefly, the entire cerebellum was dissected out, and single cell suspensions were prepared by trypsinization and trituration in 1% trypsin in Ca2+-free isotonic phosphate buffer (CF-PBS). Cells were washed in CF-PBS and resuspended in culture medium (MEM), supplemented with 0.25% glucose, 2 mM glutamine, 10% HS, 5% FCS, and both penicillin and streptomycin. Cells were seeded into poly-D-lysine-coated dishes and incubated at 37°C in a moist chamber under 5% CO2. After 24 hr in vitro the medium was replaced with serum-free medium containing 15% N-2 supplement and 15 mM KCl in the medium. After 5–7 days in vitro (DIV) CGCs were used for the experiments reported in the present study. When required, the CGCs were treated for 72 h with memantine (Sun Pharma) at a concentration of 100 µM, referred from the literature [32], [33].
Cell adhesion assay
Approximately 10,000 CGCs were plated per well in 96 well tissue culture plates coated with poly-D-lysine (Sigma-Aldrich) at a final concentration of 10 µg/ml. After 1.5 h of attachment, unattached cells were removed by aspiration and adherent cells were quantified by the colorimetric aqueous MTS assay (CellTiter 96 AQueous One Solution kit, Promega).
Cell migration assay
CGCs were labeled with fluorescent Calcein AM (BD Biosciences) at a final concentration of 2.5 µM. Approximately 10,000 labeled cells were plated in 0.8 ml DMEM +1% FBS in each well of a 24 well chamber plate adapted for the HTS Fluoroblock (BD Falcon) apparatus. After 3 h, 5% FBS was added to the lower chamber medium to establish a 1–5% serum gradient and migration of cells from the upper to lower chamber were quantified at 2 h using a microfluorimetric plate reader (CytoFluor 4000, MTX Lab Systems) [34].
DiI labeling
The culture was labeled using a protocol adapted from Hering et al [35] Briefly, CGCs were fixed in 4% formaldehyde for 15 min and incubated with Vybrant-DiI cell-labeling solution (1∶200, Invitrogen) for 25 min at 37°C. Cultures were washed in warmed PBS, incubated in PBS at 4°C for 24–48 hr to allow dye diffusion within membranes, mounted on glass slides with ProLong Gold antifade reagent (Invitrogen), and then imaged using a Nikon Eclipse E800 microscope. Spines were classified according to previously described criteria [36], [37]: the mushroom type has a large head with short neck; the stubby type has a short protrusion with no clear neck; the thin type has a long neck and a small head. Spine length was measured from shaft to tip using a bent-line tool [38]. The spines were counted in 12–18 neurons/group from the independent experiments. For each neuron, 1–4 dendrites were analyzed. The spine value was averaged in each independent experiment for the statistical test. The n value refers to the number of independent experiments analyzed.
Immunofluorescence
CGCs were fixed in 4% formaldehyde for 15 min and blocked with 3% goat serum/0.3% Triton X-100 in PBS and incubated with an anti-Syp polyclonal antibody (anti-synaptophysin, 1∶200, Cell Signaling Technology), anti-VGLUT1 monoclonal antibody (1∶500, Millipore), and anti-VGAT polyclonal antibody (1∶500, Millipore) overnight at 4°C, followed by incubation with Alexa Fluor 488 anti-rabbit IgG and Alexa Fluor 555 anti-mouse IgG (1∶1000, Invitrogen) for 1.5 h at room temperature. Cell number and distribution were analyzed following staining with nuclear marker Hoechst 33258 (Sigma-Aldrich) for 5 min at room temperature. Cultures were mounted on glass slides with ProLong Gold antifade reagent (Invitrogen), and imaged using a Nikon Eclipse E800 microscope.
The analysis of immunofluorescent images was done as described previously [39]. Images were acquired in the linear range with constant settings and analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA). All analyses were performed blind as to the treatment of the culture. Immunoreactive puncta were defined as discrete regions along the dendrite with fluorescence intensity twice the background and average size of the puncta were normalized with data from the WT CGCs group, respectively. For quantification, 20–30 neurons from two to three different batches of cultures and experiments for each condition were randomly chosen on the basis of having a healthy morphology. Negative controls, in which the primary antibodies were omitted and treated only with the secondary antibodies, were run for each condition to exclude false positive secondary antibody binding. The n value refers to the number of cells analyzed.
Statistics
The statistical analyses were carried out by one-way analysis of variance (ANOVA) with Fisher's PLSD post hoc test using the StatView 5.0 software (SAS Institute, Inc.). The Kolmogorov-Smirnov test was used to compare the two distributions. All data is presented as means ± SE. Significance was accepted at p<0.05 or better.
Results
Memantine promotes CGCs adhesion
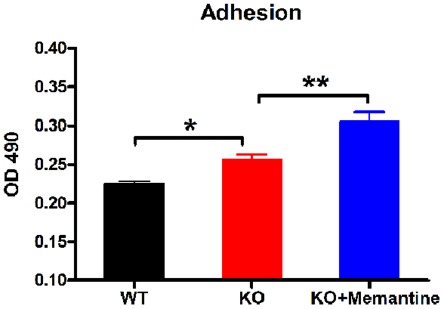
To test the effect of memantine on adhesion of CGCs, Fmr1-KO and WT CGCs were seeded on the PDL-coated surface. Cell adhesion was evaluated by a modified MTS assay based on dehydrogenase conversion of MTS to colored tetrazolium salt, a reaction that is mediated only by viable cells. The amount of colored product formed at 490 nm (OD 490) was proportional to the number of attached cells. As shown in Figure 2, there was an increase in cell adhesion in Fmr1-KO CGCs compared to WT CGCs (one-way ANOVA, F = 22.42, p<0.0001; Fisher's PLSD, p = 0.03), and after treatment with memantine, Fmr1-KO CGCs exhibited a 19% increase in adhesion (Fisher's PLSD, p = 0.002).
Figure 2. Memantine increased the adhesion property in Fmr1-KO CGCs.
Comparison of CGCs adhesion. The values are mean ±SE from three independent experiments with five replicates in each experiment, *p<0.05, **p<0.01. OD: optical density.
Memantine has little effect on CGCs migration
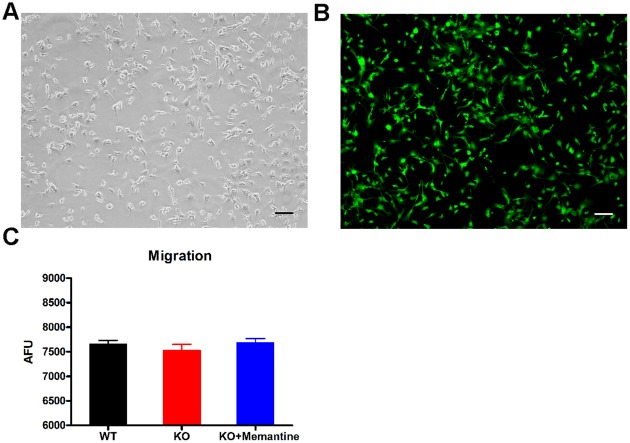
Cell migration is modulated by a complex of adhesion molecules interacting between migrating cells and the surrounding extracellular matrix proteins, and is critically affected by cell adhesion [40]. Since we observed that memantine promotes CGC adhesion, we further investigated whether memantine affects CGC migration with a modified Boyden chamber assay [34]. This assay enabled us to count the cells migrating through the insert into lower chamber. Our results indicated that memantine has little effect on CGCs migration as we observed no significant difference in cell migration in comparing WT CGCs, Fmr1-KO CGCs and Fmr1-KO CGCs treated with memantine (one-way ANOVA, F = 0.70, p = 0.52, Figure 3).
Figure 3. Memantine had little effect on CGCs migration.
A. Cultured CGCs. Scale bar, 100 µm. B. CGCs stained with Calcein AM dye in modified Boyden chamber migration assay. Scale bar, 100 µm. C. Comparison of CGCs migration. The values are mean ±SE from three independent experiments with five replicates in each experiment. AFU: arbitrary fluorescence units.
Memantine treatment restores dendritic spine to normal levels in Fmr1-KO CGCs
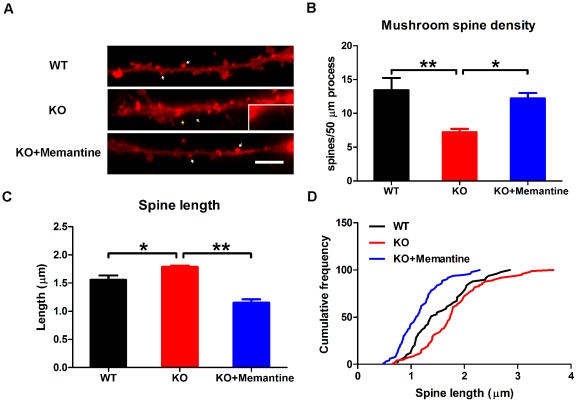
Dendritic spine dynamics plays an important role in mediating learning and memory, and is of essential importance to synaptic function [41], [42]. To test whether memantine treatment affected the development of dendritic spines, DiI labeling was employed to outline the shape of dendritic spines (Figure 4A). Our results showed that the density of mushroom-shaped mature dendritic spines was significantly decreased in Fmr1-KO CGCs as compared with WT CGCs (one-way ANOVA, F = 7.86, p = 0.02; Fisher's PLSD, p<0.01, Figure 4B). However, treatment with memantine stimulated the development of dendritic spines and restored the density of mushroom-shaped spines in Fmr1-KO CGCs to the normal level (Fisher's PLSD, p = 0.02, Figure 4B). We examined the effects of memantine on spine length and found that dendritic spines were longer in Fmr1-KO CGCs than in WT CGCs (one-way ANOVA, F = 31.43, p<0.0001; Fisher's PLSD, p = 0.03). Memantine decreased the spine length in Fmr1-KO CGCs (Fisher's PLSD, p<0.0001, Figure 4C). In addition, the distribution of different length of spines was altered after memantine treatment (Figure 4D). Fmr1-KO CGCs exhibited fewer short spines and more medium to long spines (Kolmogorov-Smirnov Comparison, p = 0.01), while more short spines were present in memantine-treated Fmr1-KO CGCs as compared with Fmr1-KO CGCs without treatment (Kolmogorov-Smirnov Comparison, p<0.0001).
Figure 4. Memantine restored dendritic spine to normal levels in Fmr1-KO CGCs.
A. Cultured CGCs were stained with Vybrant-DiI to outline the shape of dendritic spines. Arrows colored in white show the mushroom-shaped spines, while arrows colored in yellow show the thin-shaped spines, Scale bars, 10 µm. Histogram showed the density of mushroom-shaped spines (B), spine length (C) and cumulative frequency distribution of spine lengths (D) in WT CGCs, Fmr1-KO CGCs and Fmr1-KO CGCs treated with memantine. The data are the mean ± SE, *p<0.05, **p<0.01. n = 3.
Memantine stimulates synapse formation and restores the excitatory synapses to a normal range in KO CGCs
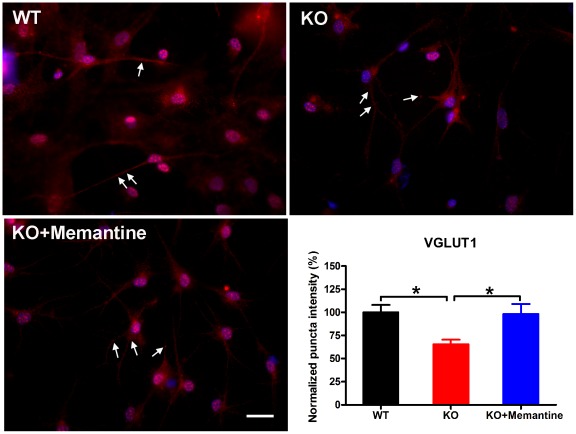
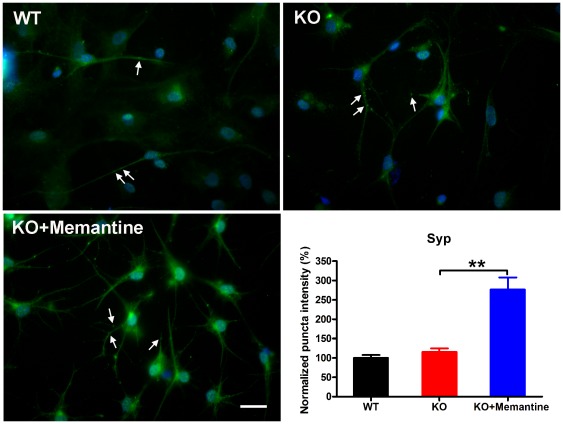
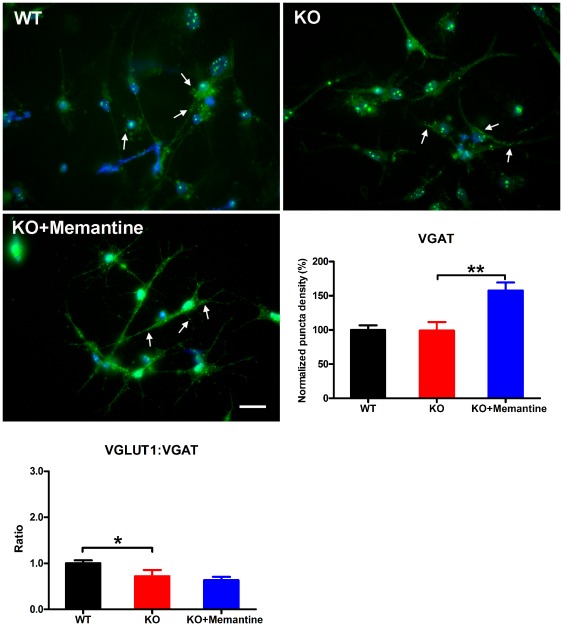
To further investigate the effects of memantine on synapse formation, using antibodies to synaptic vesicle proteins we examined synapse formation comparing WT CGCs, Fmr1-KO CGCs and Fmr1-KO CGCs treated with memantine. We stained the CGCs with antibodies to synaptophysin (a general marker of all synapses) and to the vesicular glutamate transporter VGLUT1 and the vesicular GABA transporter VGAT (markers of excitatory and inhibitory synapses respectively). We observed a dramatic decrease in the immunoreactivity of excitatory synapses (VGLUT1 staining) in Fmr1-KO CGCs as compared with that in WT CGCs (one-way ANOVA, F = 5.44, p = 0.01; Fisher's PLSD, p = 0.01, Figure 5). Memantine treatment significantly stimulated excitatory synapse formation in Fmr1-KO CGCs (Figure 5, Fisher's PLSD, p = 0.01) and fully restored the intensity of excitatory synapse to the level observed in WT CGCs (Figure 5). In addition, we found that the intensities of total synapses (Syp staining) and inhibitory synapses (VGAT staining) were not altered in Fmr1-KO CGCs as compared with that in WT CGCs (one-way ANOVA, F = 25.52, p<0.0001; Fisher's PLSD, p = 0.58, Figure 6 and one-way ANOVA, F = 10.53, p = 0.001; Fisher's PLSD, p = 0.95, Figure 7, respectively). Memantine treatment also stimulated the formation of total synapses and inhibitory synapses in Fmr1-KO CGCs (Fisher's PLSD, p<0.0001, Figure 6 and p = 0.001, Figure 7, respectively). The ratio of excitatory to inhibitory synapses in Fmr1-KO CGCs was lower than in WT CGCs (one-way ANOVA, F = 4.70, p = 0.02; Fisher's PLSD, p = 0.04). However, memantine treatment did not change the ratio in Fmr1-KO CGCs (Fisher's PLSD, p = 0.52, Figure 7).
Figure 5. Memantine fully restored the intensity of excitatory synapses to a normal range in Fmr1-KO CGCs.
Representative photomicrographs of labeling of VGLUT1 (arrow) in WT CGCs, Fmr1-KO CGCs and Fmr1-KO CGCs treated with memantine. Red, VGLUT1; Blue, Hoechst, scale bars, 25 µm. The histogram shows quantification of puncta size using image analysis. WT, n = 23; Fmr1-KO, n = 25; Fmr1-KO+Memantine, n = 26. *p<0.05.
Figure 6. Memantine stimulated the formation of total synapses in Fmr1-KO CGCs.
Representative photomicrographs of labeling of Syp (arrow) in WT CGCs, Fmr1-KO CGCs and Fmr1-KO CGCs treated with memantine. Green, Syp; Blue, Hoechst, scale bars, 25 µm. The histogram shows quantification of puncta size using image analysis. WT, n = 23; Fmr1-KO, n = 25; Fmr1-KO+Memantine, n = 26. **p<0.01.
Figure 7. Memantine stimulated the formation of inhibitory synapses in Fmr1-KO CGCs.
Representative photomicrographs of labeling of VGAT (arrow) in WT CGCs, KO CGCs and KO CGCs treated with memantine. Green, VGAT; Blue, Hoechst, scale bars, 25 µm. The histogram shows quantification of puncta size using image analysis and ratio of excitatory synapses to inhibitory synapse. WT, n = 23; Fmr1-KO, n = 25; Fmr1-KO+Memantine, n = 25. *p<0.05, **p<0.01.
Discussion
The hypo-function of the GABAergic system and the glutamate toxicity found in autism have been implicated as possibly having a causal role for autism [5], [20]. Memantine is an uncompetitive antagonist at NMDA receptors [11], [12] and is widely used for the treatment of AD. Memantine has been demonstrated to have neuroprotective effects and promote neurogenesis [43], [44]. Most recently, several clinical trials have reported that memantine can significantly improve language function and social behavior of patients with autism and fragile X syndrome (FXS) [24], [25], [26], [27]. Neuroanatomical studies on autistic individuals have revealed loss of purkinje and granule cells of the cerebellum [45], [46] and cerebellar dysfunction in animal models can induce autism-like behavioral deficits [47], [48]. Granule cells of the cerebellum constitute the largest homogeneous neuronal population of mammalian brain. Due to their postnatal generation and the feasibility of well characterized primary in vitro cultures, cerebellar granule cells (CGCs) are a model of choice for the study of neural development, function and pathology [49]. In this study, using cultured CGCs from Fmr1-KO, we first examined how memantine affects the cell properties. We found that memantine significantly promoted the adhesion of CGCs, while having little effect on the cell migration.
Integrin-mediated cell adhesion has been shown to be essential in the regulation of neuronal migration, which is a critical process that determines the final location of neurons, and thus establishes the basis for neural circuits [40], [50], [51], [52]. In addition, the complexity and specificity of synaptogenesis relies upon regulation of cell adhesion molecules that mediate contact initiation, synapse formation and plasticity. Disruption of adhesion could result in an imbalance in the structure and function of synapses [53], [54]. Recently, several lines of studies have shown that mutations in the synaptic adhesion molecules Neurexin 1 and Neuroligins 3 and 4 are associated with autism [54], [55]. Mutations in scaffolding molecule Shank3 that interacts with Neuroligins have also been detected in some autistic subjects [56], [57]. These observations imply that a defect in synaptic cell adhesion may alter synapse formation, maturation and plasticity in autistic subjects. Since memantine promotes CGC adhesion, we reason that it could play a role in the regulation of neural synaptogenesis. Thus, memantine might counteract some of the defects in synaptic cell adhesion molecules found in autism. In our study, we found that the adhesion of Fmr1-KO CGCs was increased as compared with controls. Whether there is a defect in adhesion-associated molecules in Fmr1-KO mice or FXS patients is unknown and our results suggest that it is of important significance to further investigate synaptic cell adhesion and migration properties in FXS in the future.
Our study further demonstrated that memantine stimulated synapse formation. In particular, memantine restored the number of excitatory synapses found in Fmr1-KO CGCs to the level of that in the WT CGCs. Synapses critically mediate neuronal communication. The number, type, and connectivity patterns of synapses determine the formation, maintenance, and function of neural circuitries [58]. Recently, Yun and Trommer [59] reported that that the peak amplitude of NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) was smaller in Fmr1-KO mice as compared with controls, while AMPA receptor-mediated EPSCs were comparable in the two groups. In addition, they found diminished medial perforant path-granule cell long-term potentiation (LTP), complementing previous findings that demonstrated impaired LTP in CA1, neocortex, and amygdala and exaggerated long-term depression in CA1 of Fmr1-KO mice [60]. We suggest that the stimulatory effect of memantine on excitatory synapse formation could counter the smaller EPSCs found in Fmr1-KO CGCs and exert a therapeutic role. However whether memantine can actually promote excitatory synaptic transmission remains to be further studied.
In addition, we found that memantine also stimulated the formation of inhibitory synapses. Thus memantine treatment did not change the ratio of excitatory to inhibitory synapses. This leaves open the question of whether memantine affects the neural circuit balance. Future studies will be needed to answer this question. The stimulatory effect of memantine on both excitatory and inhibitory synapse formation suggests that memantine may have a general role in promoting neurogenesis. Recently, several studies have shown that memantine promotes cell proliferation and production of mature granule neurons in the adult hippocampus [43], [61]. Memantine has also been demonstrated to stimulate the proliferation of hippocampal progenitor cells [62]. All these findings imply that memantine can play a role in neurogenesis.
To further elucidate the role of memantine on neural development and function, we examined dendritic spines. We showed that memantine treatment promotes the development of mature (mushroom-shaped) dendritic spines and can restore the dendritic spine to normal levels in Fmr1-KO CGCs. Several categories of spines have been identified based on their shape and size, including thin, stubby, cup, and mushroom shaped [63]. Spine morphology is linked to synapse function and the mushroom-shaped spines considered to represent the most mature and stable spine morphology [36], [64]. Recent studies suggest that excitatory synapses mainly connect to mushroom-shaped dendritic spines [65]. Thus, the increase in mushroom-shaped spines in response to memantine treatment is consistent with our findings that memantine stimulates the formation of excitatory synapses. The effect of memantine on spines in Fmr1-KO CGCs also suggests a role of memantine in restoring synaptic transmission.
A number of studies have reported defects of dendritic spines in Fmr1-KO mice [38], [66]. It has been reported that the length of dendritic spines, a measure of immaturity, is increased in Fmr1-KO mice, suggesting a delay in the development of dendritic spines. In our study, we found that the length of dendritic spines was significantly impaired in Fmr1-KO CGCs, which is supportive of the findings from Nimchinsky et al [66]. The effect of memantine in enhancing dendritic spine maturation could contribute to its observed therapeutic effects in autistic and FXS patients. We reckon there could be two ways memantine exert its restoring role in FMRP deficient neurons. It could specifically counteract the loss of FMRP and therefore only acting on those dendritic spines that are immature as a result of FMRP loss. Alternatively memantine could have a more general effect on the development and maturation of dendritic spines and not directly target those neurons altered by the loss of FMRP. Studies will be carried out on wild type mice to further elucidate the mechanism through which memantine affects the neuronal properties, synapse formation and dendritic spine maturation.
In summary, we have shown that Fmr1-KO CGCs exhibit delayed development of dendritic spines and reduced excitatory synapse formation. Memantine treatment of Fmr1-KO CGCs promoted cell adhesion. Memantine also stimulated the development of mushroom-shaped dendritic spines and restored the dendritic spine to normal levels in Fmr1-KO CGCs. Furthermore, we demonstrated that memantine treatment can enhance synapse formation, particularly excitatory synapses and it restored the excitatory synapses to a normal range in Fmr1-KO CGCs. These findings suggest that the stimulatory effect of memantine on dendritic spines development and excitatory synapse formation, as well as the adhesion promoting effect could be mechanisms by which memantine exerts its therapeutic effect in autism and FXS.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the NYS Office for People with Developmental Disabilities, the Rural India Charitable Trust, and Northfield Bank Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buxbaum JD, Baron-Cohen S, Devlin B. Genetics in psychiatry: common variant association studies. Mol Autism. 2010;1:6. doi: 10.1186/2040-2392-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devlin B, Melhem N, Roeder K. Do common variants play a role in risk for autism? Evidence and theoretical musings. Brain Res. 2011;1380:78–84. doi: 10.1016/j.brainres.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss LA. Autism genetics: emerging data from genome-wide copy-number and single nucleotide polymorphism scans. Expert Rev Mol Diagn. 2009;9:795–803. doi: 10.1586/erm.09.59. [DOI] [PubMed] [Google Scholar]
- 5.Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- 6.Kim MH, Choi J, Yang J, Chung W, Kim JH, et al. Enhanced NMDA receptor-mediated synaptic transmission, enhanced long-term potentiation, and impaired learning and memory in mice lacking IRSp53. J Neurosci. 2009;29:1586–1595. doi: 10.1523/JNEUROSCI.4306-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEntee WJ, Crook TH. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology (Berl) 1993;111:391–401. doi: 10.1007/BF02253527. [DOI] [PubMed] [Google Scholar]
- 8.Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, et al. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornhuber J, Bormann J, Retz W, Hubers M, Riederer P. Memantine displaces [3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur J Pharmacol. 1989;166:589–590. doi: 10.1016/0014-2999(89)90384-1. [DOI] [PubMed] [Google Scholar]
- 13.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 14.Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther. 2005;312:1195–1205. doi: 10.1124/jpet.104.077172. [DOI] [PubMed] [Google Scholar]
- 15.Buisson B, Bertrand D. Open-channel blockers at the human alpha4beta2 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:555–563. doi: 10.1124/mol.53.3.555. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, et al. Memantine improves cognition and reduces Alzheimer's-like neuropathology in transgenic mice. Am J Pathol. 2010;176:870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minkeviciene R, Banerjee P, Tanila H. Memantine improves spatial learning in a transgenic mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2004;311:677–682. doi: 10.1124/jpet.104.071027. [DOI] [PubMed] [Google Scholar]
- 18.Van Dam D, Abramowski D, Staufenbiel M, De Deyn PP. Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology (Berl) 2005;180:177–190. doi: 10.1007/s00213-004-2132-z. [DOI] [PubMed] [Google Scholar]
- 19.Van Dam D, De Deyn PP. Cognitive evaluation of disease-modifying efficacy of galantamine and memantine in the APP23 model. Eur Neuropsychopharmacol. 2006;16:59–69. doi: 10.1016/j.euroneuro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, et al. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry. 2001;158:1058–1066. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- 21.Chez MG, Aimonovitch M, Buchanan T, Mrazek S, Tremb RJ. Treating autistic spectrum disorders in children: utility of the cholinesterase inhibitor rivastigmine tartrate. J Child Neurol. 2004;19:165–169. [PubMed] [Google Scholar]
- 22.Chez MG, Buchanan TM, Becker M, Kessler J, Aimonovitch MC, et al. Donezepil hydrochloride: a double-blind study in autistic children. J Pediatr Neurol. 2003;1:83–88. [Google Scholar]
- 23.Hardan AY, Handen BL. A retrospective open trial of adjunctive donepezil in children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol. 2002;12:237–241. doi: 10.1089/104454602760386923. [DOI] [PubMed] [Google Scholar]
- 24.Chez MG, Chin K, Memon S, Hung P. Memantine as add-on therapy in pediatric epileptic patients: Effects on cognitive development and seizure frequency. Epilepsia. 2004;45(Suppl. 7):152. [Google Scholar]
- 25.Chez MG, Hung PC, Chin K, Memon S, Kirschner S. Memantine experience in children and adolescents with autistic spectrum disorders. Ann Neurol. 2004;56(Suppl. 8):109. [Google Scholar]
- 26.Chez MG, Burton Q, Dowling T, Chang M, Khanna P, et al. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. J Child Neurol. 2007;22:574–579. doi: 10.1177/0883073807302611. [DOI] [PubMed] [Google Scholar]
- 27.Erickson CA, Mullett JE, McDougle CJ. Open-Label Memantine in Fragile X Syndrome. J Autism Dev Disord. 2009;39:1629–1635. doi: 10.1007/s10803-009-0807-3. [DOI] [PubMed] [Google Scholar]
- 28.Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, et al. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- 29.Bernardet M, Crusio WE. Fmr1 KO mice as a possible model of autistic features. ScientificWorldJournal. 2006;6:1164–1176. doi: 10.1100/tsw.2006.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietropaolo S, Guilleminot A, Martin B, D'Amato FR, Crusio WE. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS One. 2011;6:e17073. doi: 10.1371/journal.pone.0017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci. 1999;19:9459–9468. doi: 10.1523/JNEUROSCI.19-21-09459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanchard AP, Guillemette G, Boulay G. Memantine potentiates agonist-induced Ca2+ responses in HEK 293 cells. Cell Physiol Biochem. 2008;22:205–214. doi: 10.1159/000149798. [DOI] [PubMed] [Google Scholar]
- 33.Erdo SL, Schafer M. Memantine is highly potent in protecting cortical cultures against excitotoxic cell death evoked by glutamate and N-methyl-D-aspartate. Eur J Pharmacol. 1991;198:215–217. doi: 10.1016/0014-2999(91)90625-z. [DOI] [PubMed] [Google Scholar]
- 34.Lamszus K, Schmidt NO, Jin L, Laterra J, Zagzag D, et al. Scatter factor promotes motility of human glioma and neuromicrovascular endothelial cells. Int J Cancer. 1998;75:19–28. doi: 10.1002/(sici)1097-0215(19980105)75:1<19::aid-ijc4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, et al. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng J, Dunaevsky A. Delayed Development of Dendritic Spines in Fxr2 Knockout Mouse. The Open Neuroscience Journal. 2009;3:148–153. [Google Scholar]
- 39.Wei H, Zou H, Sheikh A, Malik M, Dobkin C, et al. IL-6 is increased in the cerebellum of the autistic brain and alters neural cell adhesion, migration and synapse formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valiente M, Marin O. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 2010;20:68–78. doi: 10.1016/j.conb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Maekawa M, Namba T, Suzuki E, Yuasa S, Kohsaka S, et al. NMDA receptor antagonist memantine promotes cell proliferation and production of mature granule neurons in the adult hippocampus. Neurosci Res. 2009;63:259–266. doi: 10.1016/j.neures.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006;23:2611–2622. doi: 10.1111/j.1460-9568.2006.04787.x. [DOI] [PubMed] [Google Scholar]
- 45.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- 47.Bobee S, Mariette E, Tremblay-Leveau H, Caston J. Effects of early midline cerebellar lesion on cognitive and emotional functions in the rat. Behav Brain Res. 2000;112:107–117. doi: 10.1016/s0166-4328(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 48.Caston J, Chianale C, Delhaye-Bouchaud N, Mariani J. Role of the cerebellum in exploration behavior. Brain Res. 1998;808:232–237. doi: 10.1016/s0006-8993(98)00847-6. [DOI] [PubMed] [Google Scholar]
- 49.Contestabile A. Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum. 2002;1:41–55. doi: 10.1080/147342202753203087. [DOI] [PubMed] [Google Scholar]
- 50.Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- 51.Schmid RS, Shelton S, Stanco A, Yokota Y, Kreidberg JA, et al. alpha3beta1 integrin modulates neuronal migration and placement during early stages of cerebral cortical development. Development. 2004;131:6023–6031. doi: 10.1242/dev.01532. [DOI] [PubMed] [Google Scholar]
- 52.Stanco A, Szekeres C, Patel N, Rao S, Campbell K, et al. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci U S A. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giagtzoglou N, Ly CV, Bellen HJ. Cell adhesion, the backbone of the synapse: “vertebrate” and “invertebrate” perspectives. Cold Spring Harb Perspect Biol. 2009;1:a003079. doi: 10.1101/cshperspect.a003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yun SH, Trommer BL. Fragile X mice: reduced long-term potentiation and N-Methyl-D-Aspartate receptor-mediated neurotransmission in dentate gyrus. J Neurosci Res. 2011;89:176–182. doi: 10.1002/jnr.22546. [DOI] [PubMed] [Google Scholar]
- 60.Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol. 2006;96:1734–1745. doi: 10.1152/jn.00221.2006. [DOI] [PubMed] [Google Scholar]
- 61.Jin K, Xie L, Mao XO, Greenberg DA. Alzheimer's disease drugs promote neurogenesis. Brain Res. 2006;1085:183–188. doi: 10.1016/j.brainres.2006.02.081. [DOI] [PubMed] [Google Scholar]
- 62.Namba T, Yabe T, Gonda Y, Ichikawa N, Sanagi T, et al. Pigment epithelium-derived factor up-regulation induced by memantine, an N-methyl-D-aspartate receptor antagonist, is involved in increased proliferation of hippocampal progenitor cells. Neuroscience. 2010;167:372–383. doi: 10.1016/j.neuroscience.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 63.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 65.van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10:207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]