To the Editor
Alopecia areata (AA) is a disfiguring human autoimmune skin disease with a complex genetic basis that targets hair follicles in the actively growing anagen phase of the hair cycle. AA is characterized by focal hair loss that waxes and wanes. Bald spots can become extensive involving the entire scalp (alopecia totalis) or body (alopecia universalis). Anagen stage hair follicles are disrupted from the bulb to just under the sebaceous gland by an infiltration of CD4+ and CD8+ T cells.
The C3H/HeJ inbred mouse strain spontaneously develops an AA-like disease as early as 3–6 mo. of age but often by 12 mo. of age (Sundberg et al., 1994). As with the human disease, there is a mixture of CD4+ and CD8+ T cells in and around hair follicles spanning from just above the bulb to just below the sebaceous gland. Full thickness skin grafts from affected AA mice transplanted onto young, clinically normal, histocompatible mice reproducibly regenerate the disease beginning at 10 weeks post engraftment (McElwee et al., 1998; McElwee et al., 2003).
Interferon gamma (IFNG) has previously been implicated in the pathogenesis of AA by inducing ectopic expression of MHC class I molecules in the anagen hair bulb (Ito et al., 2004). Since major histocompatibility complex (MHC) class I molecules are normally not expressed there (Paus et al., 1999), this expression is thought to contribute to the disruption of immune privilege maintenance of the hair bulb. Genetic deletion of Ifng from C3H/HeJ mice provided complete protection against the development of AA using the AA skin graft model (Freyschmidt-Paul et al., 2006a).
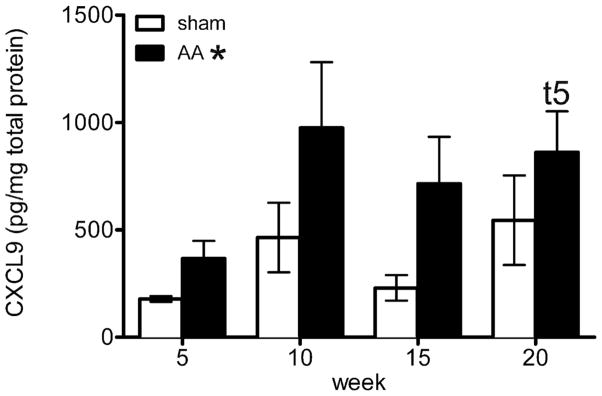
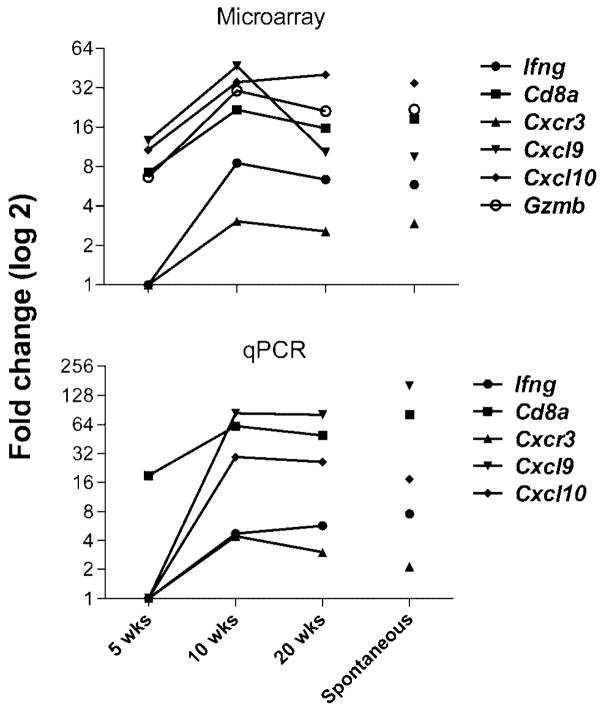
To identify potentially important genes in the pathogenesis of AA, a cross sectional transcriptome analysis was done using Affymetrix gene arrays and large scale quantitative real time RT PCR with further validation by ELISA of selected cytokines in protein extracts from mouse skin. Skin was collected at 5, 10, and 20 weeks after skin graft induction of AA or from spontaneous cases with appropriate controls (see Supplemental Data for Materials and Methods). Ifng mRNA was increased by 10 weeks, consistent with previous findings in mouse and human AA (Carroll et al., 2002; Freyschmidt-Paul et al., 2006a; Freyschmidt-Paul et al., 2006b; Ito et al., 2004). Also at 10 wks, there was a modestly significant increase of chemokine (C-X-C motif) receptor 3 (Cxcr3), and a marked increase of its ligands, chemokine (C-X-C motif) ligand 9 and 10 (Cxcl9, and Cxcl10) as early as 5 wks post transplant. This increased expression prior to the development of AA suggests that these chemokines are involved in the early pathogenesis of AA. Transcripts of the less well characterized ligand, Cxcl11, were only significantly increased in the spontaneous lesions (data not shown) and therefore may not be as important early in disease. In support of these findings, the concentration of CXCL9 protein in the skin of AA mice increased post engraftment compared with the sham control in an independent experiment (Fig. 1).
Figure 1. CXCL9 protein levels are significantly increased in mice with AA.
An ELISA against CXCL9 was performed on dorsolumbar skin from mice receiving a graft from an AA mouse or their own skin rotated 180° (sham) 5, 10, 15, or 20 weeks post grafting. These values were natural log transformed and analyzed by univariate analysis of variance using a full factorial model in SPSS, v19. Bars indicate means +/− SE, n=8–10. * = Mice receiving the AA graft had significantly more CXCL9 (p < 0.001) than those mice receiving the sham graft at all time points shown. t5 = CXCL9 is significantly increased in week 20 AA mice compared to week 5 AA mice (p < 0.01).
These findings are consistent with human studies where CXCR3 is increased around the hair follicles of inflammatory AA patients and is associated with increased myxovirus protein A (MxA), an IFNG-related protein (Ghoreishi et al., 2010). Mx2, the mouse homolog of MxA, was mildly upregulated at all time points examined and did not increase with time (data not shown). The early up-regulation of the CXCR3 axis suggests that it is important in the earliest stages of the disease, potentially contributing to the loss of immune privilege of the hair follicle.
Cd8a transcripts, the alpha component of the CD8 costimulatory molecule on CD8+ T cells, are increased as early as 5 weeks post engraftment (Fig. 2), suggesting CD8+ T cell numbers in AA skin increase very early in disease. Granzyme B (GZMB), a cytotoxic molecule present in activated CD8+ T cells, was previously shown to be increased around the hair follicles in human inflammatory AA along with another cytotoxic protein, cytotoxic granule associated RNA binding protein I (TIA1) (Ghoreishi et al., 2010). Gzmb transcripts were also increased at the 5 wk time point (Fig. 2) and this elevation was seen in the absence of Tia1 dysregulation (data not shown), suggesting that GZMB secretion may be the preferred method of cellular targeting by CD8+ T cells in the C3H/HeJ model of AA.
Figure 2. Transcripts of the CXCR3 axis as well as MHC class I associated molecules are significantly increased in mice with AA.
(A) Gene expression analysis using Affymetrix microarrays of C3H/HeJ mice at 5, 10, and 20 weeks post engraftment compared with sham control as well as C3H/HeJ mice spontaneously developing AA. Values shown are significant by ANOVA analysis with q value < 0.05. Values are expressed as Log2. n = 3 for each group. (B) Gene expression analysis using quantitative RT PCR from mice shown in (A). Values shown are significant (p < 0.05) as determined by GPR analysis. Values are expressed as Log2. n = 3 for each group.
The CXCR3 axis is associated with numerous human autoimmune diseases including psoriasis, rheumatoid arthritis, type I diabetes, and systemic lupus erythematosus (Groom and Luster, 2011). It is composed of CXCR3 and its ligands CXCL9, CXCL10, and CXCL11, all of which are strongly induced by IFNG (Groom and Luster, 2011). Secretion of these chemokines results in enhanced Th1-mediated immune responses and a positive feedback loop is thought to exist because CXCR3-positive Th1 cells and NK cells produce IFNG which stimulates production of CXCL9, CXCL10, and CXCL11, resulting in the recruitment of more Th1 and NK cells (Groom and Luster, 2011; Rotondi et al., 2007). CXCR3 is expressed primarily on Th1 CD4+ T cells and CD8+ T cells as well as NK and NKT cells, while its ligands are secreted by many tissue resident cells including dendritic cells. In addition to CXCR3 found by Ghoreishi et al., a study of human AA revealed overexpression of the chemokines CX3CL1, CXCL1, CCL5, and CXCL10 (Subramanya et al., 2010). CX3CL1, like CXCR3, is induced by IFNG and can act as an amplification circuit of polarized Th1 responses (Fraticelli et al., 2001), consistent with a Th1 type of response in AA skin.
These results suggest that CXCL9 and CXCL10 are increased early in the skin of mice predisposed to developing AA and these ligands may assist in attracting CD8+ T cells and IFNG-secreting Th1 cells, which both express the CXCR3 receptor, to the hair follicles. Genetic deletion by crossing with CXCR3-deficient mice (Hancock et al., 2000) or blocking of the CXCR3 receptor in the C3H/HeJ skin graft model would be of interest in further delineating the role of IFNG and the CXCR3 axis inTh1 immune response up-regulation and CD8+ T cell activity in this mouse model of AA.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Alopecia Areata Foundation (to JPS, LEK, HBE, and DCR), National Institutes of Health (AR056635 to JPS), and The Ellison Medical Foundation (to JPS).
Abbreviations
- AA
alopecia areata
- IFNG
interferon gamma
- CD8A
CD8 antigen, alpha chain
- CXCR3
chemokine (C-X-C motif) receptor 3
- CXCL9
chemokine (C-X-C motif) ligand 9
- CXCL10
chemokine (C-X-C motif) ligand 10
- CXCL11
chemokine (C-X-C motif) ligand 11
- QTL
quantitative trait locus
- NK
natural killer cell
- Th1
immune response
- GZMB
granzyme B
- TIA1
cytotoxic granule associated RNA binding protein I
- MHC
major histocompatability complex
- MxA
myxovirus protein A
- Mx2
the mouse homolog of MxA
Footnotes
Conflicts of interest: DCR is a founder and director of Bar Harbor Biotechnology, which received reimbursement for the qPCR studies.
References
- Carroll JM, McElwee KJ, King ELJ, Byrne MC, Sundberg JP. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392–402. doi: 10.1046/j.1523-1747.2002.01811.x. [DOI] [PubMed] [Google Scholar]
- Fraticelli P, Sironi M, Bianchi G, D’Ambrosio D, Albanesi C, Stoppacciaro A, et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–81. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyschmidt-Paul P, McElwee KJ, Hoffmann R, Sundberg JP, Vitacolonna M, Kissling S, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515–21. doi: 10.1111/j.1365-2133.2006.07377.x. [DOI] [PubMed] [Google Scholar]
- Ghoreishi M, Martinka M, Dutz JP. Type 1 interferon signature in the scalp lesions of alopecia areata. Br J Dermatol. 2010;163:57–62. doi: 10.1111/j.1365-2133.2010.09775.x. [DOI] [PubMed] [Google Scholar]
- Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–15. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–20. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Ito N, Bettermann A, Tokura Y, Takigawa M, Paus R. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am J Pathol. 2004;164:623–34. doi: 10.1016/S0002-9440(10)63151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee KJ, Boggess D, King LE, Jr, Sundberg JP. Experimental induction of alopecia areata-like hair loss in C3H/HeJ mice using full-thickness skin grafts. J Invest Dermatol. 1998;111:797–803. doi: 10.1046/j.1523-1747.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Freyschmidt-Paul P, Sundberg JP, Hoffmann R. The pathogenesis of alopecia areata in rodent models. J Investig Dermatol Symp Proc. 2003;8:6–11. doi: 10.1046/j.1523-1747.2003.12164.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Christoph T, Muller-Rover S. Immunology of the hair follicle: a short journey into terra incognita. J Investig Dermatol Symp Proc. 1999;4:226–34. doi: 10.1038/sj.jidsp.5640217. [DOI] [PubMed] [Google Scholar]
- Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune disease. Endocr Rev. 2007;28:492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- Subramanya RD, Coda AB, Sinha AA. Transcriptional profiling in alopecia areata defines immune and cell cycle control related genes within disease-specific signatures. Genomics. 2010;96:146–53. doi: 10.1016/j.ygeno.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, Cordy WR, King LE. Alopecia areata in aging C3H/HeJ mice. J Invest Dermatol. 1994;102:847–56. doi: 10.1111/1523-1747.ep12382416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.