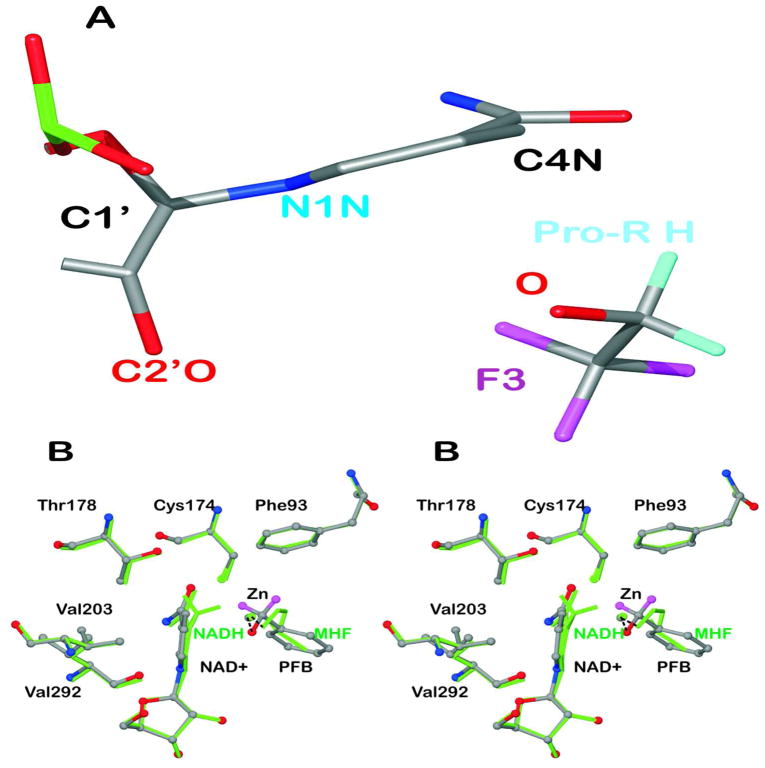
Figure 4.
Puckering of the nicotinamide rings. (A) The structure of the complex with NAD+ and TFE (subunit A) is shown with atoms C2N and C3N aligned on top of atoms C5N and C6N so that the displacements of N1N and C4N from the plane are visible. (B) Comparison of the structure of the complex with NAD+ and pentafluorobenzyl alcohol to that with NADH and methylhexylformamide. The “A” subunits of the structures (amino acid residues A1 to A374) were superimposed, with an rmsd of 0.25 Å. The complex with NAD+ and pentafluorobenzyl alcohol (with H atoms in magenta but F atoms removed for clarity) are shown in atom coloring with ball and stick representation. The complex with NADH (with H atoms on C4N) and methylhexylformamide (“MHF”, with atoms C3-C6 removed for clarity) is shown in green. The residues making close contacts are labeled. The distances are given in Table 5.