Abstract
Delayed engraftment remains a major hurdle following cord blood (CB) transplantation. It may be due, at least in part, to low fucosylation of cell surface molecules important for homing to the BM microenvironment. Since fucosylation of specific cell surface ligands is required before effective interaction with selectins expressed by the BM microvasculature can occur, a simple 30 minute ex vivo incubation of CB HPC with fucosyltransferase (FT) - VI and its substrate (GDP-fucose) was performed to increase levels of fucosylation. The physiologic impact of CB HPC hypo-fucosylation was investigated in vivo in NOD-SCID IL-2Rγnull (NSG) mice. By isolating fucosylated and non-fucosylated CD34+ cells from CB we show that only fucosylated CD34+ cells are responsible for engraftment in NSG mice. Further, since the proportion of CD34+ cells that are fucosylated in CB is significantly less than in BM and PB, we hypothesize that these combined observations might explain, at least in part, the delayed engraftment observed following CB transplantation. Since engraftment appears to be correlated with the fucosylation of CD34+ cells, we hypothesized that increasing the proportion of CD34+ cells that are fucosylated would improve CB engraftment. Ex vivo treatment with fucosyltransferase (FT)-VI significantly increases the levels of CD34+ fucosylation and, as hypothesized, this was associated with improved engraftment. Ex vivo fucosylation did not alter the biodistribution of engrafting cells, or pattern of long-term, multi-lineage, multi-tissue engraftment. We propose that ex vivo fucosylation will similarly improve the rate and magnitude of engraftment for CB transplant recipients in a clinical setting.
Keywords: Hematopoiesis, cord blood, transplantation, engraftment, fucosylation
Introduction
Cord blood (CB) has become an important source of hematopoietic support for cancer patients lacking human leukocyte antigen (HLA) matched donors. The ethnic diversity, relative ease of collection, ready availability, reduced incidence and severity of graft versus host disease (GvHD) and tolerance of higher degrees of HLA disparity between donor and recipient, are positive attributes when compared to unrelated donor bone marrow (BM) [1-4] or cytokine-mobilized peripheral blood (PB) [5-7]. However, significantly delayed neutrophil and platelet engraftment and elevated risk of graft failure are also associated with CB transplantation when compared to BM or PB transplantation [8-13].
A critical part of engraftment is the recruitment of primitive hematopoietic progenitor cells (HPC) to the BM. This process is likely governed by a cascade of molecular interactions between members of the selectin, integrin and CD44 superfamilies of adhesion molecules expressed by HPC and their various counter-receptors expressed by the microvasculature of the BM [14-21]. The initial step associated with homing is the rolling of HPC on the vascular endothelium of the hematopoietic microenvironment [14-24], an interaction mediated, at least in part, by P- and E-selectins constitutively expressed by the BM endothelium and selectin ligands expressed by the HPC including P-selectin glycoprotein ligand (PSGL)-1 (CD162) [25].
The delayed engraftment and elevated risk of graft failure observed for CB recipients are, at least in part, due to low total nucleated cell (TNC) [26,27] and CD34+ cell [28] dose transplanted. However, there is also evidence that CB CD34+ cells possess a defect in homing to BM [29,30]. Appropriate carbohydrate modification (fucosylation) of selectin ligands appears critical for the rolling of primitive HPC on P- and E-selectins expressed by the hematopoietic microvasculature [23,29,30]. CB CD34+ cells interact poorly with selectins when compared to CD34+ cells from PB or BM. A correlation between the level of CB CD34+ fucosylation and binding to P- and E-selectins exists with more heavily fucosylated CD34+ cells exhibiting a greater affinity for P- and E-selectins [29]. Consistent with this observation, we demonstrate that fucosylated rather than non-fucosylated CD34+ cells are responsible for engraftment in the NOD-SCID IL-2Rγnull (NSG) mouse model [33]. These observations provide a rationale for increasing the level of CD34+ cell surface fucosylation with the goal of improving CB engraftment. Levels of cell surface fucosylation can be increased by ex vivo treatment with α1-3 fucosyltransferase (FT)-VI [29,30,32]. Increased levels of fucosylation augment the level of P- and E-selectin binding of CD34+ cells and increase the levels of rolling on P- or E-selectin in vitro [29] and BM microvasculature in vivo [30]. However the impact of ex vivo fucosylation on hematopoietic engraftment of CB remains unclear with different groups reporting opposing findings [29,30].
A correlation between the level of fucosylation of engrafting hematopoietic cells and their interaction with selectins expressed by the hematopoietic microvasculature is consistent with a potentially key role for fucosylation in recruitment to the BM. Ultimately, a better understanding of the deficiencies in homing of CB CD34+ cells will be necessary to enable the development of more effective CB transplant techniques. Herein, we used the NSG mouse model to further explore the impact of fucosylation on the rate and magnitude of CB engraftment with the goal of improving engraftment following CB transplantation in the clinic.
Materials and methods
Hematopoietic Cells
Samples were provided under University of Texas M. D. Anderson Cancer Center IRB-approved protocols. All animal work was performed under University of Texas M. D. Anderson Cancer Center IACUC-approved protocols. Unless otherwise stated, CB CD34+ cells were selected by magnetic activated cell sorting (MACS) according to manufacturer’s instructions (CD34 Reagent, Miltenyi Biotec, Auburn, CA.).
Ex vivo Fucosylation using FT-VI. CB CD34+ cells were incubated at 106 cells/ml for 30 minutes at room temperature with 1 mM GDP β-fucose (EMD Biosciences, San Diego, CA.), 1 mM MnCl2 in 1 ml Hanks Buffered Saline Solution (HBSS) containing 1% human serum albumin (HSA, Baxter Healthcare Corp., Westlake Village, CA.) and 94 mU/ml FT-VI (America Stem Cell, Inc., Carlsbad, CA.). Fucosylation reactions were initially performed using 10 mM Mn2+ (Fig 5A), however, this dose was associated with significant hematotoxicity (Fig 6A). A significantly less hematotoxic dose of 1mM Mn2+ was subsequently used without any reduction in enzyme activity (Fig 6C). Further, while preliminary reactions were performed at 37°C, subsequent studies revealed that reactions could be performed at room temperature without any reduction in enzyme efficacy. After incubation, cells were washed in HBSS containing 1% HSA. Levels of fucosylation were determined by flow cytometry. Fucosylation is characterized by the presence of sialyl Lewis X (sLeX) residues which are revealed by flow cytometry using antibody HECA-452 (BD Biosciences, San Jose, CA.) against cutaneous lymphocyte antigen (CLA). CLA shares a carbohydrate domain with the sLeX antigen [23,29,31]. Briefly, cells were stained with combinations of fluorochrome-conjugated anti-CD34, anti-CD45 and anti-CLA (HECA 452) antibodies (all from BD Biosciences) or with isotype controls. Antibody staining was revealed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA.) and analysis performed using CELLQuest Pro software (Becton Dickinson).
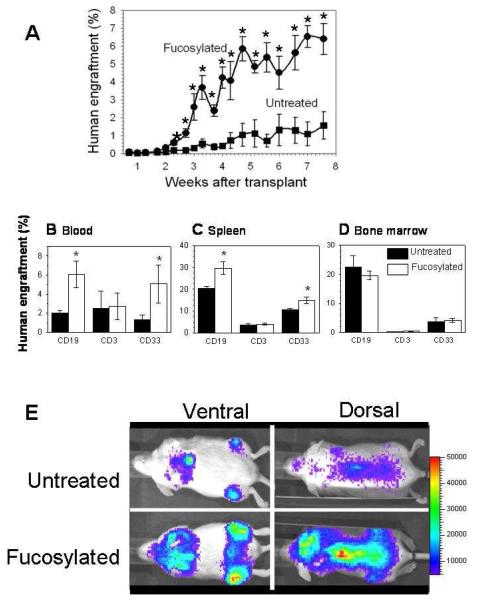
Figure 5.
A. Fucosylated CD34+ cells engraft more rapidly and with greater magnitude. B-D. Increased CD19+ and CD33+ cell engraftment is observed in PB and spleen in recipients of fucosylated CB. No differences observed in BM. E. FT-VI treatment (fucosylation) improved the rate and magnitude of engraftment without altering biodistribution.
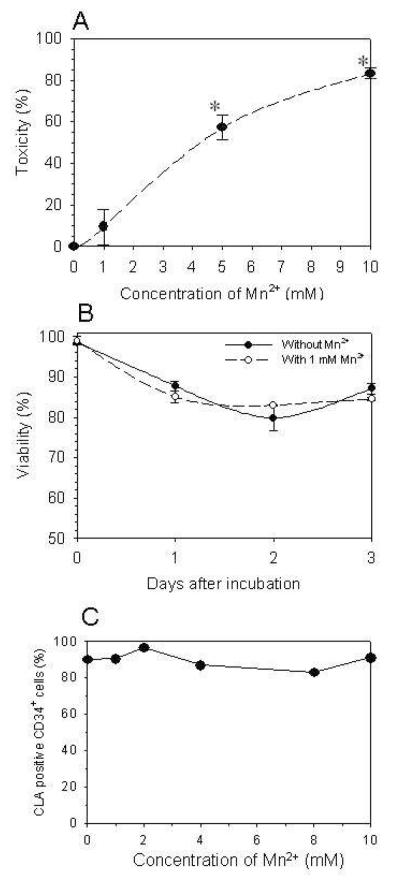
Figure 6.
A-B. Colony-forming unit (CFU) and viability assays provide evidence of manganese (Mn2+) toxicity when used in the reaction buffer at >1mM. C. Use of Mn2+ at <1mM does not compromise the activity of FT-VI. The enzyme is active in the absence of Mn2+.
NSG mouse model to assess the impact of endogenous fucosylation on human engraftment
MACS-isolated CD34+ cells from multiple CB units were pooled and stained with HECA-452 to reveal sialyl Lewis X (sLeX) residues indicative of fucosylation. Cells were resuspended in buffer containing 0.6 μg/ml DAPI (Invitrogen) to allow exclusion of dead cells and separated, by means of fluorescence-activated cell sorting (FACS), using a FACS Aria II (BD Bioscience, San Jose, CA) fitted with a 100μm nozzle and Blue Diode 488, HeNe 633 and UV 355 lasers, into three fractions: (1) “Total CD34+”, containing both fucosylated (HECA+) and unfucosylated (HECA−) CD34+ cells; (2) “CD34+HECA+”, containing only fucosylated CD34+ cells and (3) “CD34+HECA−”, containing only unfucosylated CD34+ cells. Recipient NSG mice were sublethally-irradiated (320 cGy using a 137Cs source delivered at approximately 300 cGy/minute, J. L. Shepherd and Associates Mark I-25 Irradiator, San Fernando, CA.) and sufficient cells collected to provide an intravenous dose of >104 Total CD34+, CD34+HECA+ or CD34+HECA− cells/mouse (5 mice/group). Human engraftment was evaluated by flow cytometric analysis of BM and spleens of mice >6 weeks after transplant using human CD45 and mouse CD45 antibodies (BD Biosciences), a FACSCalibur flow cytometer (Becton Dickinson) and CELLQuest Pro software (Becton Dickinson).
Fucosylation, PSGL-1 expression and homing
The human chronic myelogenous leukemia cell line K562 produces solid tumor masses in the liver, kidney and other organs, rather than engrafting to BM [45]. While CB CD34+ cells express PSGL-1 [16,34,35], K562 cells do not (Fig. 2A - upper panel). K562 cells expressing PSGL-1 (K562/PSGL-1) were generated by electroporation (Cell-Porator, Gibco BRL, Carlsbad, CA.) with pcDNA3.1 (Invitrogen, Carlsbad, CA.) containing the full cDNA for human PSGL-1 and a neomycin resistance cassette. Cells were then selected at low density in medium containing G418 (1 mg/ml), stained with anti-human PSGL-1 antibody KPL-1 (BD Biosciences) and isolated by FACS. To measure BM homing, K562/PSGL-1 and parental K562 lines, were labeled with carboxyfluorescein succinimidyl ester (CFSE). Cells (106 /ml) were incubated for 10 minutes at 37°C in 10 μM CFSE (Molecular Probes Invitrogen, Eugene, OR.), washed, incubated in medium for 1 hour at 37°C to allow efflux of unboun d stain and cells treated ± FT-VI. Four treatment groups were generated: (i) untreated K562, (ii) FT-VI treated K562, (iii) untreated K562/PSGL-1 and (iv) FT-VI treated K562/PSGL-1. Cells were injected intravenously into sublethally-irradiated NSG recipients at 5×106 cells/mouse (3 mice/gp). Mice were euthanized approximately 16 hours after injection, femora removed and marrow suspensions prepared for flow cytometric assessment of the levels of CFSE-positive (human K562) cells present, relative to marrow suspensions from control (uninjected) mice. DAPI (Invitrogen; 0.6 μg/ml) was added to identify non viable cells and >500,000 viable events acquired (LSRII flow cytometer, Becton Dickinson) and analyzed (CELLQuest Pro software, Becton Dickinson).
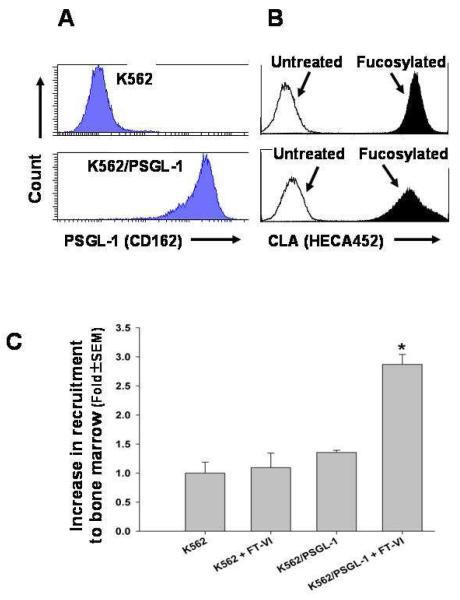
Figure 2.
A. K562 were engineered to express PSGL-1 (K562/PSGL-1). B. K562 and K562/PSGL-1 have low endogenous levels of fucosylation and become heavily fucosylated following treatment with FT-VI. C. Only FT-VI-treated (fucosylated) K562/PSGL-1 cells showed improved homing to femoral marrow.
Impact of fucosylation on homing of primary CB CD34+ cells
CD34+ cells were selected from a pool of fresh CB units using the Isolex Magnetic Cell Selection System (Baxter Healthcare Corp.) according to the manufacturer’s instructions. After labeling with CFSE and allowing efflux of unbound stain, cells were divided across five treatment groups. Groups 2-4 were not treated with FT-VI, but assessed the impact of different buffer components: Grp2: HBSS containing 0.5% HSA (HBSS/HSA); Grp3: HBSS/HSA with 1 mM βDFucose and Grp4: HBSS/HSA with 1 mM βDFucose + 1 mM Mn2+. Groups 5 and 6 were treated with FT-VI ± Mn2+: Grp5: HBSS/HSA with 1 mM βDFucose + FT-VI and Grp6: HBSS/HSA with 1 mM βDFucose + 1 mM Mn2+ + FT-VI. After incubation for 30 minutes at room temperature, cells were washed and numbers determined. Ex vivo fucosylation reaction was confirmed by flow cytometry using the HECA-452 antibody. Sublethally-irradiated NSG recipients (n=3/gp) received 3×105 CFSE-labeled CB CD34+ cells intravenously. Grp1 received no cells and provided negative background data. Mice were euthanized approximately 18 - 20 hours after injection, femora removed and BM cell suspensions stained with APC-conjugated anti-huCD34 and PE-Cy7-conjugated anti-human CD38 or isotype controls (BD Bioscience) to determine whether CFSE+ cells homing to the BM were skewed towards more primitive (CD34+38−) or more mature (CD34+38+) HPC. DAPI was used to exclude dead cells and data acquired using an LSRII flow cytometer (Becton Dickinson). An average of >106 viable events were acquired for each sample and analyzed using Diva Software (Becton Dickinson).
Colony-forming unit (CFU) assays to assess impact of fucosylation on homing
Primitive HPC with the capacity to engraft immunodeficient mice represent a small proportion of total CD34+ cells. While as many CB CD34+ cells were infused per mouse as was practically possible in the CB CD34+ ± FT-VI experiment described above and large data files acquired during flow cytometry, concerns were raised that the rarity of human events might not provide sufficient power to accurately detect differences in BM homing as a result of treatment with FT-VI. To address this concern, a parallel experimental approach was adopted. The content of human myeloid (CFU-GM), erythroid (BFU-E) and multipotent (CFU-GEMM) clonogenic progenitors in the BM of NSG mice was measured at early timepoints (7, 14 and 21 days) following transplantation of purified CD34+ cells ± FT-VI. We reasoned that treatments resulting in enhanced numbers of human clonogenic HPC at these early times following CD34+ cell infusion would reflect increased recruitment of primitive HPC to the BM. The ability of these CFU assays to detect only human HPC would be confirmed by the absence of colonies in cultures performed with BM from sublethally-irradiated, non-transplanted NSG mice.
Marrow cells were plated in triplicate 1ml cultures in 35-mm plates in medium comprising 0.9% methyl cellulose in IMDM supplemented with 30% FBS and 3 mM L-glutamine. Growth of human CFU was stimulated by the addition of recombinant human interleukin 3 (IL-3; 10 ng/ml), human granulocyte-macrophage colony stimulating factor (GM-CSF; 10 ng/ml), human stem cell factor (SCF; 50 ng/ml) (all from R&D Systems, Minneapolis, MN.) and human erythropoietin (4U/ml)(Epoetin alfa, Amgen, Inc., Thousand Oaks, CA.). After 14 days of incubation at 37°C in a 5% CO2, fully humidified atmosphere, colonies were scored using an inverted microscope.
Impact of fucosylation on hematopoietic engraftment in NSG mice
CB CD34+ cells were divided into 2 fractions, treated ± FT-VI, washed and equivalent numbers of fucosylated or untreated cells injected into groups of sublethally-irradiated NSG mice (n=5 mice/gp). Human engraftment was followed by analysis of serial, retro-orbital blood samples. Blood samples were treated with Lysing Buffer (BD Biosciences) to remove red blood cells before antibody staining and flow cytometric analysis performed. At specific timepoints, multi-lineage human engraftment was assessed (human T-lymphocytes: huCD3, huCD4, huCD8; human B-lymphocytes: huCD19; human myeloid cells: huCD33; human platelets: huCD41a, huCD61 and human erythrocytes: huCD235a (Glycophorin A), all BD Biosciences). For T- and B-lymphocytes and myeloid cells, multi-lineage composition of the human engraftment was investigated by gating on the huCD45+ cell population. The proportion of human-derived erythrocytes or platelets was determined from the proportion of human-stained events (huCD235a for erythrocytes and huCD41a and/or huCD61 for platelets) in the total erythrocyte or platelet population (mouse and human) as defined by their distinct forward and side scatter. Mice were euthanized after 7 weeks and human multi-lineage (huCD34+, myeloid and lymphoid) and multi-tissue (PB, BM, spleen and thymus) engraftment was assessed by flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson) and CELLQuest Pro software (Becton Dickinson).
Non-invasive bioluminescent imaging
CB CD34+ cells were transduced with a lentivirus vector coding for Firefly luciferase (FFLuc) as previously described [37-39]. Supernatant containing the lentivirus was adsorbed to a plate-bound recombinant fibronectin fragment (FN CH-296; Retronectin; Takara Shuzo, Otsu, Japan.) and CB CD34+ cells added. After lentiviral transduction, the cells were incubated at 37°C for 72 hours in medium containing 100 ng/ml each of SCF, Flt-3 Ligand and Thrombopoietin (TPO) (all CellGenix, Antioch, IL.) and granulocyte colony-stimulating factor (G-CSF) (Neupogen, Amgen, Inc.). Transfection efficiencies ranged from 10-20%. Half of the cells were then treated with FT-VI. CD34+ ± FT-VI cells were then transplanted into sublethally-irradiated NSG mice. To reveal the presence of FFLuc-labeled cells, anesthetized mice were injected intraperitoneally with 150 mg/kg D-luciferin (Gold Biotechnology, St. Louis, MO.) and visualized after 10 minutes using a Xenogen-IVIS Imaging System (Caliper Life Sciences, Hopkinton, MA.).
Assessment of Mn2+ toxicity
A series of experiments were undertaken to investigate the potential toxicity of Mn2+ present in the reaction mixture used for fucosylation. (i) CB CD34+ cells were incubated in HBSS/HSA containing 0, 1, 5 or 10 mM Mn2+ for 30 minutes at room temperature, washed and plated at 1000 cells per 30 mm culture dish (1 ml) in Methocult GF+ H4435 growth medium (StemCell Technologies, Vancouver, BC, Canada.) to determine the number of CFC present and cultured for 14-21 days at 37°C in a 5%CO2-in-air fully humidified atmosphere. Colonies (defined as aggregates of >40 cells) were enumerated using a dissecting microscope and dark field illumination. (ii) CB CD34+ cells were incubated in HBSS/HSA containing 0 or 1 mM Mn2+ for 30 minutes at room temperature, washed and plated at approximately 106cells/ml in αMEM medium (HyClone, Logan, UT.) containing 10% fetal bovine serum.(HyClone). Cell viabilities were assessed using trypan blue dye exclusion immediately before and 1, 2 and 3 days after culture. (iii) CB CD34+ cells were incubated in HBSS/HSA containing FT-VI, 1 mM GDP β-fucose and 0, 1, 2, 4, 8, or 10 mM Mn2+ for 30 minutes at room temperature. After incubation, cells were washed and levels of fucosylation assessed by flow cytometry using the HECA-452 antibody (BD Bioscience).
Statistical analysis
Data were compared using the Student’s 2-sample t-test to test for differences between treatment groups using a 2-sided significance level of 0.05.
Results
CB CD34+ cells have lower levels of endogenous fucosylation than BM or PB CD34+ cells
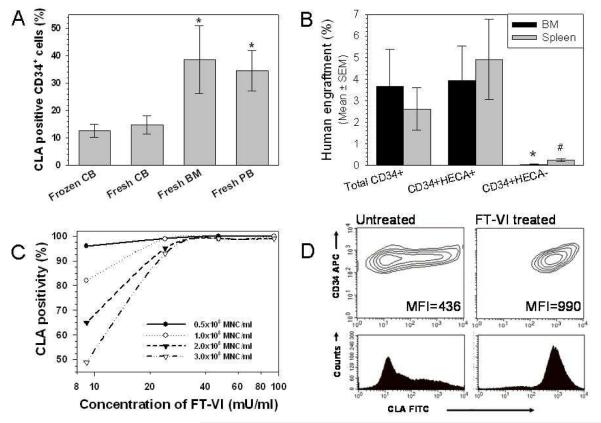
Flow cytometric analysis revealed that 15±3% (n=18, mean±SEM) of fresh CB CD34+ cells and 13±2% (n=9) of thawed CB CD34+ cells were fucosylated as defined by the binding of anti-CLA antibody HECA-452. By comparison, the fucosylation of BM and PB CD34+ cells was significantly greater (>2-fold, P≤0.05) than that found on CB CD34+ cells at 39±12% (n=6) and 35±7% (n=27), respectively (Fig. 1A).
Figure 1.
A. Endogenous fucosylation of BM and PB CD34+ cells is significantly greater (*P≤0.05) than fresh or frozen CB CD34+ cells. B. Fucosylated (HECA+) CD34+ cells are responsible for engraftment. C. FT-VI activity is limiting at higher cell and lower enzyme concentrations. D. Representative CLA antibody staining of untreated and FT-VI-treated CB CD34+ cells.
Engraftment associated with CB CD34+ cells is contained within the endogenously fucosylated (HECA+) fraction
Three replicate experiments were performed and revealed that the human engraftment observed in mice >6 weeks after receiving >104 Total CD34+ cells (containing fucosylated and unfucosylated CD34+ cells) was similar to that observed in mice >6 weeks after receiving >104 CD34+HECA+ cells (containing only fucosylated CD34+ cells). Mice receiving >104 CD34+HECA− cells (containing only unfucosylated CD34+ cells) failed to show significant levels of human engraftment. Representative data from one experiment is shown (Fig. 1B). After receipt of >104 Total CD34+ cells (containing fucosylated and non-fucosylated CD34+ cells) the levels of human engraftment in the BM and spleen were 3.67±1.71% and 2.62±0.98%, respectively. By comparison, the levels of human engraftment in the BM and spleen of mice receiving >104 CD34+HECA+ cells (containing only fucosylated CD34+ cells) were not significantly different (P>0.05) at 3.94±1.59% and 4.92±1.85%, respectively. However, levels of human engraftment in the BM and spleen of mice receiving >104 CD34+HECA− cells (containing only unfucosylated CD34+ cells) were significantly less (P≤0.05) than those observed in mice receiving Total CD34+ cells at 0.05±0.02% and 0.25±0.06%, respectively.
Ex vivo FT-VI treatment increases fucosylation of CB CD34+ cells
Optimization of the fucosylation reaction involved the assay of enzyme concentration, duration of reaction, Mn2+ concentration, temperature and the presence of GDP β-fucose. With a fixed number of CB mononuclear cells (MNC), fucosylation levels increased in proportion to enzyme concentration and duration of incubation until a plateau of >95% fucosylation was achieved (Fig. 1C). Incubation at 37°C did not improve levels of fucosylation over incubation at room temperature (data not shown). While the enzymatic reaction was absolutely dependent on the addition of the GDP β-fucose substrate, it was not absolutely dependent on the presence of Mn2+ (see Fig. 6C). The reaction conditions selected for these studies were treatment of CD34+ cells/ml for 30 minutes at room temperature with 94 mU/ml recombinant FT-VI in a HBSS/HSA reaction mixture containing 1 mM GDP β-fucose and 1 mM Mn2+. These conditions reproducibly increased the proportion of fucosylated (HECA+) CB CD34+ cells to >90%. Use of mean fluorescence intensity (MFI) allowed a more quantitative measure of fucosylation. In a representative example (Fig. 1D), MFI of untreated CB CD34+ cells was increased >2-fold from 436 to 990 arbitrary units when treated with FT-VI. The increased level of cell surface fucosylation after incubation remained stable for at least 2 hours at room temperature (data not shown).
Evidence for a fucosylation-dependent, PSGL-1-mediated mechanism for BM homing
Flow cytometric analysis confirmed high level expression of PSGL-1 by K562 following transfection (K562/PSGL-1) (Fig. 2A). Following FTVI-mediated fucosylation, both parental (upper panel) and K562/PSGL lines (lower panel) demonstrated high levels of binding of HECA-452 indicating efficient fucosylation (Fig. 2B). Sublethally-irradiated mice were then injected intravenously with equal numbers of parental K562 ± FT-VI, or K562/PSGL-1 ± FT-VI and euthanized after 16 hours. Flow cytometry of BM cells revealed the number of CFSE-positive K562 cells. Analysis revealed: (i) Consistent with previous reports [45] parental K562 cells did not home aggressively to the BM; (ii) FT-VI treatment of K562 cells did not improve BM homing observed with parental K562 (Fig. 2C); (iii) homing of K562/PSGL1 cells was not significantly different from that of K562 parental, or FT-VI treated K562 cells; (iv) treatment of K562/PSGL-1 cells with FT-VI (K562/PSGL-1 + FT-VI) markedly increased the level of fucosylation (Fig. 2B - lower panel, black fill) and significantly (P≤0.05) improved BM homing (2.8-fold) over that observed with K562 cells alone (Fig. 2C). Collectively, these data provide evidence for a fucosylation-dependent, PSGL-1-mediated mechanism for BM homing.
Flow cytometric analysis fails to reveal any improvement in CB CD34+ cell recruitment to BM following FT-VI treatment
Analysis of untreated cells (Grps 2-4) revealed that the endogenous level of CB CD34+ fucosylation was 12.6±0.7%. These data are consistent with those reported in Fig. 1A. Analysis of FT-VI treated cells (Grps 5-6) revealed that the level of CB CD34+ fucosylation after treatment with FT-VI was significantly (P=0.00003) increased to 74.2±1.5%.
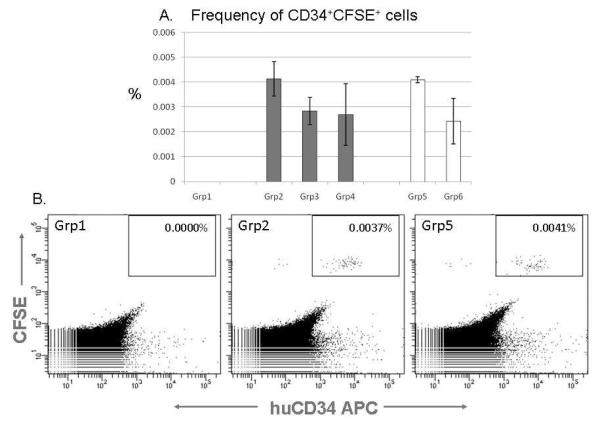
Approximately 105 CFSE-labeled CB CD34+ cells were injected per mouse in triplicate for each group. Flow cytometric analysis of femoral marrow was performed 18-20 hours after injection. BM from sublethally-irradiated NSG mice receiving no cells provided a negative control (Grp 1) (Fig. 3A). No significant differences were detected between the levels of homing of CD34+ cells under any of the conditions used, irrespective of buffer composition, or treatment with FT-VI (Grps 2-6). Representative flow cytometric analyses of CFSE positive CD34+ cells from femoral BM of mice receiving no CD34+ cells (Grp 1), untreated (Grp 2) and FT-VI-treated CD34+ cells (Grp 5) is shown (Fig. 3B). Flow cytometric analysis also revealed that 89.5±1.1% (n=15) of the CFSE-positive cells injected were CD34+ and 5.5±0.8% (n=15) were CD34+38lo/-. This was not changed when human cells from the NSG recipient BM were assayed.
Figure 3.
A. No evidence of an FT-VI-associated improvement in the homing of CB CD34+ cells to BM of NSG mice detected. (Solid bar - Untreated. Open bar - FT-VI treated). B. Representative flow cytometric analyses of BM from mice receiving: no cells (Grp1) or CFSE-stained, untreated (Grp2) or CFSE-stained, FT-V-treated CB CD34+ cells (Grp5).
CFC assays reveal an FT-VI-mediated improvement in CB CD34+ cell recruitment to BM
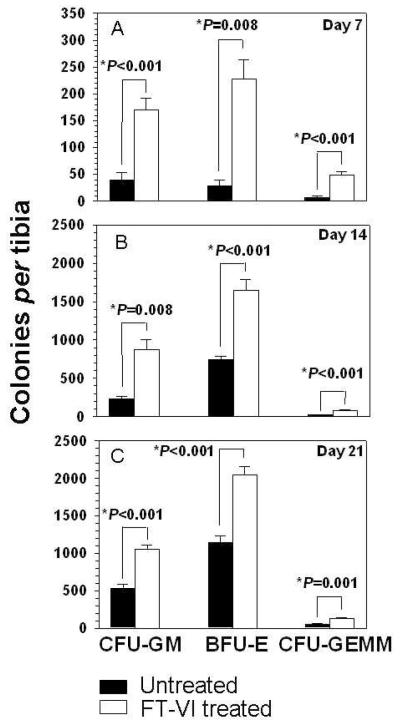
As shown in Fig. 4A-C, FT-VI-mediated fucosylation of transplanted CD34+ cells resulted in a significant 4.4-fold increase (P<0.001) in the numbers of human CFU-GM (myeloid), an 8.1 fold increase (P=0.008) in the numbers of human BFU-E (erythroid) and a 6.5-fold increase (P<0.001) in the numbers of human CFU-GEMM (multipotent) in the BM of recipient mice 7 days after transplant over the corresponding levels of human progenitors in mice receiving untreated CD34+ cells. This trend was similarly maintained for all of these measures of HPC (CFU-GM, BFU-E and CFU-GEMM) at 14 and 21 days after transplant. In recipients of FT-VI-treated CD34+ cells, CFU-GM numbers were increased 3.8 and 2-fold (P=0.008 and P<0.001, respectively) over numbers found in marrow of recipient mice receiving untreated CD34+ cells at 14 and 21 days. BFU-E were similarly increased by 2.2 and 1.8-fold (P<0.001 for both 14 and 21 days) and CFU-GEMM were similarly increased by 3.5 and 2.4-fold at 14 and 21 days (P<0.001 and P=0.001, respectively) (Fig 4B and Fig 4C).
Figure 4.
A-C. Evidence that FT-VI treatment (fucosylation) improves the homing of CB CD34+ cells to BM of NSG mice. Significantly greater numbers of human CFU-GM, BFU-E and CFU-GEMM were found in BM of NSG mice receiving CB CD34+ cells treated with FT-VI when assessed 7, 14 and 21 days after transplant.
Rapid and sustained engraftment following transplantation of FT-VI-treated CB CD34+ cells
We investigated the effects of fucosylation on the kinetics and duration of multilineage engraftment. In NSG mice receiving 4.5×104 fucosylated CB CD34+ cells, flow cytometry revealed a rapid increase of huCD45+ cells in the PB approximately 3 weeks after transplantation (Fig. 5A), more rapid than in mice receiving the same number of untreated huCD34+ cells. Long-term human engraftment was measured by the magnitude of human engraftment in PB at 8 weeks after transplant. In recipients of fucosylated CB CD34+ cells, the huCD45+ fraction was approximately 6%, as compared to approximately 2% in recipients of untreated CB CD34+ cells (P≤0.05).
Fucosylation enhances multilineage reconstitution in the BM and spleen
Eight weeks after transplantation, analysis of PB revealed that transplant of CB CD34+ + FT-VI cells increased the level of huCD33+ (myeloid) and huCD19+ (B-cell) cell engraftment approximately 3-fold, over that observed in mice receiving untreated CB CD34+ cells.(Fig. 5B) Other lineages, including red blood cells and platelets, were statistically indistinguishable between FT-VI-treated and untreated groups. In the spleen, transplant of CB CD34+ + FT-VI cells enhanced the level of huCD33+ (P=0.04) and huCD19+ cell (P=0.03) engraftment after 8 weeks (Fig. 5C), a similar pattern to that observed in PB. A comparison of the multi-lineage contribution to human engraftment in BM revealed no statistical difference between lineages for FT-VI-treated and untreated recipients (Fig. 5D). Consistent with the absence of any difference in huCD3+ T-cells in the PB, spleen and BM, no differences in huCD3+ T-cells, or in the huCD4:huCD8 cell ratio, were seen in the thymuses of either group (data not shown).
Fucosylation does not disrupt the spatial distribution of engrafting CD34+ CB in vivo
A representative image taken 11 days after transplant of luciferase-labeled CB CD34+ cells revealed 2.5-fold greater levels of bioluminescence (2.5 times the mean signal intensity) in mice receiving the FT-VI-treated CB CD34+ cells when compared to mice receiving untreated CB CD34+ cells.(Fig. 5E) Ventral views suggest engraftment in the long bones of the hind limb, the sternum and rib cage. Dorsal views suggest engraftment in the vertebral column and cranium. These data confirm that FT-VI treatment enhanced early engraftment (rate and magnitude), but did not alter the spatial distribution of transplanted cells.
Mn2+ in the reaction buffer
A 30 minute incubation of CB CD34+ cells with 5 and 10 mM Mn2+ significantly reduced (P<0.05) the number of CFC by 57.2±6.1% and 83.2±2.5%, respectively, when compared to CB CD34+ cells incubated without Mn2+. These data demonstrate toxicity associated with the use of Mn2+ at these concentrations.(Fig. 6A) The use of 1 mM Mn2+ did not significantly reduce (P=0.31) the number of CFC when compared to CB CD34+ cells incubated without Mn2+. These data suggest that Mn2+ at a concentration of 1 mM is not toxic by this measure. Further, measurement of cell viability immediately after and 1, 2 or 3 days after a 30 minute incubation of CB CD34+ cells with 1 mM Mn2+ revealed no evidence of toxicity when compared with CB CD34+ cells incubated without Mn2+.(Fig. 6B) These data suggest that Mn2+ can be used at 1 mM without toxicity. When CB CD34+ cells were incubated in reaction mixture containing FT-VI, βDFucose and reducing concentrations of Mn2+ (10, 8, 4, 2, 1 and 0 mM) for 30 minutes at room temperature, there was no reduction in the proportion of CD34+ cells that became fucosylated (Fig. 6C). These data suggest that fucosylation driven by FT-VI can occur in reduced concentrations of Mn2+ (<10mM) and even in the absence of Mn2+.
Discussion
Delayed neutrophil and platelet engraftment remains a major barrier to the successful use of CB transplantation in the clinic when compared to BM and PB sources. This may be due, at least in part, to a deficiency in the homing of CB HPC and is likely a consequence of low levels of endogenous fucosylation [29,30] (Fig. 1A). We show that the engraftment observed in NSG mice when transplanted with total CD34+ cells (containing both fucosylated and unfucosylated CD34+ cells) is the result of the fucosylated (HECA+) CD34+ population (Fig. 1B) contained therein. These data might therefore predict that engraftment would be delayed following CB transplantation when compared to the BM or PB transplantation. Such observations provide the rationale for increasing the levels of fucosylation of CB CD34+ cells by the ex vivo use of FT-VI (Fig. 1D) with the goal of improving engraftment. We hypothesize that increasing the levels of fucosylation of CB HPC will increase the affinity with which the cells bind to P- and E-selectins constitutively expressed by BM endothelium thereby improving homing and subsequently improving CB HPC engraftment in the clinic.
To investigate the potential role of specific cell surface molecules in the fucosylation process, we developed a model system using the NSG mouse model and human K562 cells engineered to express PSGL-1 (K562/PSGL-1) [21]. In a series of short-term homing experiments in sublethally-irradiated NSG mice, the infusion of CFSE-labeled K562 ± PSGL-1 ± FT-VI cells allowed homing to femoral BM to be followed by flow cytometry. Consistent with previous observations [45], K562 cells did not home efficiently to the BM. However, neither did K562 cells treated with FT-VI, despite being heavily fucosylated (Fig. 2B), indicating that fucosylation alone does not confer BM homing. K562/PSGL-1 cells did not appear to home to the BM any more efficiently than parental K562 cells, indicating that the expression of PSGL-1 did not intrinsically confer improved BM homing. Only the treatment of the K562/PSGL-1 cells with FT-VI markedly improved BM homing. This provided evidence for a fucosylation-dependent, PSGL-1-mediated mechanism for BM homing (Fig. 2C). A similar fucosylation-dependent mechanism for CB CD34+ cells was sought in a similar short-term homing experiment.
However, when CFSE-labeled CB CD34+ cells were treated ± FT-VI and transplanted into recipient NSG mice, no evidence for a fucosylation-dependent improvement in CB CD34+ cell homing was found (Fig. 3). These data are consistent with observations reported by Hidalgo and Frenette [30], who similarly used flow cytometry and failed to find evidence for a fucosylation-dependent improvement in CB CD34+ cell homing. However, despite this failure to demonstrate a fucosylation-dependent improvement in the shorter-term process of homing, evidence for a fucosylation-dependent improvement in the longer-term process of engraftment was found. This included: (i) an increase in the rate and magnitude of human engraftment in the PB of mice receiving CB CD34+ treated with FT-VI over those receiving untreated CB CD34+ cells (Fig. 5A), (ii) an increase in the presence of human hematopoietic progenitors (Fig. 4A-C) in the marrow of mice receiving CD34+ treated with FT-VI over those receiving untreated CB CD34+ cells and (iii) an increase in the magnitude of bioluminescence in mice subjected to non-invasive imaging after transplant of luciferase-labeled CD34+ treated with FT-VI over those receiving untreated CB CD34+ cells (Fig. 5E). The authors suggest that the failure to demonstrate improved homing in the short-term model might, at least in part, have been a consequence of the relatively low numbers of CD34+ cells transplanted. Despite as many CB CD34+ cells being infused per mouse as was practically possible, the limitations associated with the acquisition of rare events, despite the acquisition of large data files, might have obscured any differences in the homing of CD34+ cells as a result of FT-VI treatment.
Although the treatment of CB HPC with FT-VI improved the rate and magnitude of engraftment (Fig. 5A), it did not alter the spatial biodistribution pattern of the engrafting human cells (Fig. 5E). Further, multi-lineage flow cytometric analysis of PB revealed that the improved human engraftment observed was a consequence of improved myeloid and B-cell cell numbers (Fig. 5B). Although the generation of human lineage-committed cells in the NSG mouse is likely limited by the species-specific nature of some hematopoietic growth factors [40-43], the observation that FT-VI-treatment significantly improved neutrophil engraftment is encouraging and consistent with the finding of increased numbers of CFU-GM and CFU-GEMM observed in the BM of recipients of CB CD34+ cells treated with FT-VI (Fig. 4A-C). Further, the treatment of CB CD34+ cells with FT-VI does not appear to compromise long-term engraftment and, consistent with previous reports [44], we show that the fucosylation reaction has no absolute requirement for Mn2+, otherwise a potentially hematotoxic material (Fig. 6A-C).
Summary
We demonstrate that ex vivo fucosylation of CB CD34+ cells enhances both the rate and magnitude of human engraftment in NSG mice without changing the biodistribution of the engrafting cells. These studies provide evidence that ex vivo treatment of CB CD34+ cells with FT-VI (fucosylation) prior to transplant may be an effective method by which to enhance engraftment in CB recipients. These observations provide the rationale and impetus to test the effect of ex vivo fucosylation in a clinical setting with the goal of improving engraftment following CB transplantation.
Acknowledgements
This work was supported in part by Cancer Prevention & Research Institute of Texas (CPRIT) grant #RP100469 and NIH grant #RO1-CA061508-15 (both EJS and PJS). The authors gratefully acknowledge the provision of FT-VI by America Stem Cell, Inc., Carlsbad, CA. and the assistance of Ms. Junjun Lu and Ms. Tuong-Van Nguyen (Department of Stem Cell Transplantation and Cellular Therapy) and Ms. Wendy Fang (Division of Pediatrics), University of Texas M. D. Anderson Cancer Center, Houston, TX.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest:
S.N.R. No conflict of interest to declare.
P.J.S. No conflict of interest to declare.
M.W.T. No conflict of interest to declare.
N.B. No conflict of interest to declare.
J.A.J. No conflict of interest to declare.
S.T. No conflict of interest to declare.
J-S. S. No conflict of interest to declare.
H.Y. No conflict of interest to declare.
D.S. No conflict of interest to declare.
W.K.D. No conflict of interest to declare.
D.X. No conflict of interest to declare.
L.D.S. No conflict of interest to declare.
B.S. No conflict of interest to declare.
G.D. No conflict of interest to declare.
C.M.B. No conflict of interest to declare.
L.M. Vice President, Research/Co-Founder, America Stem Cell, Inc., declares: (i) financial interest in America Stem Cell, Inc., whose product (FT-VI) was studied in the present work and (ii) patent related to the use of FT-VI.
R.E.C. A member of the Scientific and Medical Advisory Board of America Stem Cell, Inc., Carlsbad, CA.
E.J.S. A member of the Scientific and Medical Advisory Board of America Stem Cell, Inc., Carlsbad, CA.
P.A.Z-M. No conflict of interest to declare.
References
- 1.Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 2.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman E, Rocha V, Boyer-Chammard A, et al. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 4.Rocha V, Wagner JE, Jr., Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 5.Bensinger WI, Clift R, Martin P, et al. Allogeneic peripheral blood stem cell transplantation in patients with advanced hematologic malignancies: a retrospective comparison with marrow transplantation. Blood. 1996;88:2794–2800. [PubMed] [Google Scholar]
- 6.Bensinger WI, Weaver CH, Appelbaum FR, et al. Transplantation of allogeneic peripheral blood stem cells mobilized by recombinant human granulocyte colony-stimulating factor. Blood. 1995;85:1655–1658. [PubMed] [Google Scholar]
- 7.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 8.McGlave P, Bartsch G, Anasetti C, et al. Unrelated donor marrow transplantation therapy for chronic myelogenous leukemia: initial experience of the National Marrow Donor Program. Blood. 1993;81:543–550. [PubMed] [Google Scholar]
- 9.Kernan NA, Bartsch G, Ash RC, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N Engl J Med. 1993;328:593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 10.Schiller G, Feig SA, Territo M, et al. Treatment of advanced acute leukaemia with allogeneic bone marrow transplantation from unrelated donors. Br J Haematol. 1994;88:72–78. doi: 10.1111/j.1365-2141.1994.tb04979.x. [DOI] [PubMed] [Google Scholar]
- 11.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 12.Berthou C, Legros-Maida S, Soulie A, et al. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. 1995;85:1540–1546. [PubMed] [Google Scholar]
- 13.Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15:564–573. doi: 10.1016/j.bbmt.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazo IB, Gutierrez-Ramos JC, Frenette PS, et al. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katayama Y, Hidalgo A, Furie BC, et al. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102:2060–2067. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 17.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen M, Le PF, Gagnerault MC, et al. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 20.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer KM, Drager AM, van d V, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148:165–175. [PMC free article] [PubMed] [Google Scholar]
- 23.McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- 24.Peled A, Grabovsky V, Habler L, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidalgo A, Weiss LA, Frenette PS. Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. J Clin Invest. 2002;110:559–569. doi: 10.1172/JCI14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 27.Gluckman E, Rocha V, Arcese W, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32:397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Wagner JE, Barker JN, Defor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 29.Xia L, McDaniel JM, Yago T, Doeden A, McEver RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–3096. doi: 10.1182/blood-2004-02-0650. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo A, Frenette PS. Enforced fucosylation of neonatal CD34+ cells generates selectin ligands that enhance the initial interactions with microvessels but not homing to bone marrow. Blood. 2005;105:567–575. doi: 10.1182/blood-2004-03-1026. [DOI] [PubMed] [Google Scholar]
- 31.Xia L, Sperandio M, Yago T, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobzdej MM, Leppanen A, Ramachandran V, Cummings RD, McEver RP. Discordant expression of selectin ligands and sialyl Lewis x-related epitopes on murine myeloid cells. Blood. 2002;100:4485–4494. doi: 10.1182/blood-2002-06-1799. [DOI] [PubMed] [Google Scholar]
- 33.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 34.Winkler IG, Snapp KR, Simmons PJ, Levesque JP. Adhesion to E-selectin promotes growth inhibition and apoptosis of human and murine hematopoietic progenitor cells independent of PSGL-1. Blood. 2004;103:1685–1692. doi: 10.1182/blood-2003-06-1921. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg AW, Kerr WG, Hammer DA. Relationship between selectin-mediated rolling of hematopoietic stem and progenitor cells and progression in hematopoietic development. Blood. 2000;95:478–486. [PubMed] [Google Scholar]
- 36.Matsuura N, Puzon-McLaughlin W, Irie A, et al. Induction of experimental bone metastasis in mice by transfection of integrin alpha 4 beta 1 into tumor cells. Am J Pathol. 1996;148:55–61. [PMC free article] [PubMed] [Google Scholar]
- 37.Steiner D, Gelovani J, Savoldo B, et al. Noninvasive Bioluminescent Imaging Demonstrates Long-Term Multilineage Engraftment of Ex Vivo-Expanded CD34-Selected Umbilical Cord Blood Cells. Stem Cells. 2009;27:1932–1940. doi: 10.1002/stem.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laakso MM, Sutton RE. Replicative fidelity of lentiviral vectors produced by transient transfection. Virology. 2006;348:406–417. doi: 10.1016/j.virol.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 39.Kelly PF, Vandergriff J, Nathwani A, Nienhuis AW, Vanin EF. Highly efficient gene transfer into cord blood nonobese diabetic/severe combined immunodeficiency repopulating cells by oncoretroviral vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein. Blood. 2000;96:1206–1214. [PubMed] [Google Scholar]
- 40.Martin FH, Suggs SV, Langley KE, et al. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell. 1990;63:203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
- 41.Shanafelt AB, Johnson KE, Kastelein RA. Identification of critical amino acid residues in human and mouse granulocyte-macrophage colony-stimulating factor and their involvement in species specificity. J Biol Chem. 1991;266:13804–13810. [PubMed] [Google Scholar]
- 42.Yang YC, Ciarletta AB, Temple PA, et al. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986;47:3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- 43.Wagemaker G, Burger H, van Gils FC, van Leen RW, Wielenga JJ. Interleukin-3. Biotherapy. 1990;2:337–345. doi: 10.1007/BF02170083. [DOI] [PubMed] [Google Scholar]
- 44.Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 45.Matsuura N, Puzon-McLaughlin W, Irie A, Morikawa Y, Kakudo K, Takada Y. Induction of experimental bone metastasis in mice by transfection of integrin alpha 4 beta 1 into tumor cells. Am J Pathol. 1996;148:55–61. [PMC free article] [PubMed] [Google Scholar]