Abstract
The terms “neuroacanthocytosis” (NA) and “neurodegeneration with brain iron accumulation” (NBIA) both refer to groups of genetically heterogeneous disorders, classified together due to similarities of their phenotypic or pathological findings. Even collectively, the disorders that comprise these sets are exceedingly rare and challenging to study. The NBIA disorders are defined by their appearance on brain magnetic resonance imaging, with iron deposition in the basal ganglia. Clinical features vary, but most include a movement disorder. New causative genes are being rapidly identified; however, the mechanisms by which mutations cause iron accumulation and neurodegeneration are not well understood. NA syndromes are also characterized by a progressive movement disorder, accompanied by cognitive and psychiatric features, resulting from mutations in a number of genes whose roles are also basically unknown. An overlapping feature of the two groups, NBIA and NA, is the occurrence of acanthocytes, spiky red cells with a poorly-understood membrane dysfunction. In this review we summarise recent developments in this field, specifically insights into cellular mechanisms and from animal models. Cell membrane research may shed light upon the significance of the erythrocyte abnormality, and upon possible connections between the two sets of disorders. Shared pathophysiologic mechanisms may lead to progress in the understanding of other types of neurodegeneration.
Keywords: neuroacanthocytosis, neurodegeneration, iron, NBIA
INTRODUCTION: BLOOD, BRAIN AND IRON
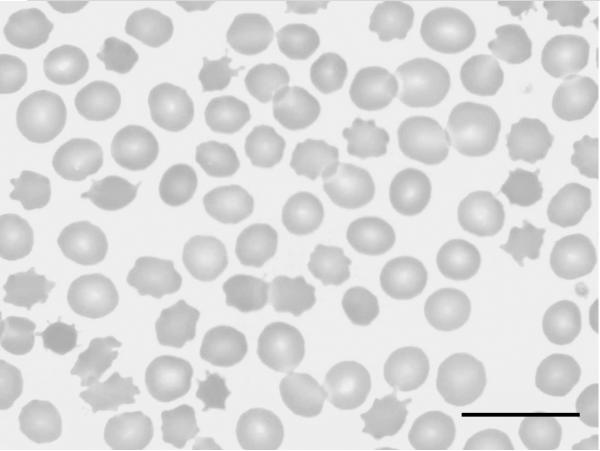
The neuroacanthocytosis (NA) syndromes (Walker et al., 2008, 2011a; Bader et al., 2011) are a group of rare neurodegenerative, genetically diverse, diseases which include the core NA disorders choreaacanthocytosis (ChAc) and McLeod syndrome (MLS), and also Huntington's disease-like 2 (HDL2), and pantothenate kinase-associated neurodegeneration (PKAN) (Table 1). The causative genes and their mutational spectra have been identified; VPS13A for ChAc (Rampoldi et al., 2001; Ueno et al., 2001; Dobson-Stone et al., 2010), XK for MLS (Ho et al., 1994; Danek et al., 2001; Jung et al., 2007), JPH3 for HDL2 (Margolis et al., 2001; Margolis, 2009), and PANK2 for PKAN (Zhou et al., 2001; Gregory and Hayflick, 2011). These diseases primarily affect the brain, particularly the basal ganglia, and are associated with central and peripheral nervous system abnormalities, including chorea, dystonia, bradykinesia, seizures, oral dyskinesia, muscle weakness, cognitive impairment, and psychiatric symptoms. Disorders of serum lipoproteins, which are not discussed here, form a distinct group of NA syndromes in which ataxia is observed, but basal ganglia disorders are not seen. NA syndromes are associated with the occurrence of “thorny” red blood cells, known as acanthocytes (Figure 1), which can be of some help for differential diagnosis. The presence of acanthocytes, however, is variable and their correct identification depends on the right preparation technique (Storch et al., 2005).
Table 1.
Causes of neuroacanthocytosis and neurodegeneration with brain iron accumulation
| Disease | Gene | Protein | Clinical features | Age of onset |
Mode of inheritance |
Acanthocytosis | |||
|---|---|---|---|---|---|---|---|---|---|
| Name | Role | ||||||||
|
Neuroacanthocytosis syndromes |
Chorea-acanthocytosis (ChAc) | VPS13A | chorein | Protein sorting and trafficking? |
Orofacial dystonia, self-mutilation (tongue, lip-biting), chorea, tics, parkinsonism, seizures, neuropathy, myopathy, behavioral compulsions, cognitive impairment, psychiatric symptoms | Late teens-early adulthood | AR | +++ | |
|
McLeod syndrome (MLS) |
XK | XK | Membrane protein; involved in transport? |
Chorea, tics, dystonia, parkinsonism, seizures, neuropathy, myopathy, behavioral compulsions cognitive impairment, psychiatric symptoms, cardiomyopathy | Mid-late adulthood | X-linked | +++ | ||
|
Huntington's disease-like 2 (HDL2) |
JPH3 | Junctophilin 3 | Regulation of calcium transport? Toxicity may be related to RNA aggregation |
Chorea, dystonia, parkinsonism, cognitive impairment, psychiatric symptoms | Inversely related to CTG repeat length, typically young-mid adulthood | AD | + | ||
|
Neurodegeneration with brain iron accumulation |
Pantothenate kinase-associated neurodegeneration (PKAN) | PANK2 | Pantothenate kinase 2 | Key regulatory enzyme in biosynthesis of coenzyme A from vitamin B5 |
Dystonia, spasticity, rigidity, retinal degeneration | Childhood, occasionally older | AR | + | |
| Phospholipase A2-associated neurodegenerat ion (PLAN) | PLA2G6 | Ca2+-independent phospholipase A2 | Catalyzes release of fatty acids from phospholipids |
Chorea, dystonia, ataxia. Classic form: neurodevelopm ental arrest, severe hypotonia, ataxia, dystonia, optic atrophy, peripheral neuropathy | Childhood | AR | − | ||
| Mitochondrial membrane protein-associated neurodegeneration (MPAN) | C19orf12 | pending | Uncertain-mitochondrial membrane-associated protein |
Spasticity, dysarthria, dystonia, parkinsonism, optic atrophy, neuropathy, psychiatric features | Childhood | AR | − | ||
| Fatty acid hydroxylase-associated neurodegeneration (FAHN) | FA2H | Fatty acid 2-hydroxylase | Catalyzes the synthesis of 2-hydroxysphingolipids |
Lower limb dystonia, ataxia, spastic quadriparesis, seizures | Childhood | AR | − | ||
| Neuroferritinopathy | FTL | Ferritin light chain | Subunit of ferritin, the major intracellular iron storage protein |
Chorea, dystonia, parkinsonism, spasticity, rigidity | Mid-late adulthood | AD | − | ||
| Aceruloplasminemia | CP | Ceruloplasmin | Copper-binding ferroxidase involved in iron transport across the cell membrane |
Chorea, dystonia, ataxia, retinal degeneration | Mid adulthood | AR | − | ||
Figure 1.
Peripheral blood smear showing significant acanthocytosis (May-Grünwald–Giemsa, ×100, scale bar = 25μm. Courtesy of Hans H. Jung, MD. Reprinted with permission from Jung HH, Ch. 7,McLeod Syndrome, in The Differential Diagnosis of Chorea, ed. Walker RH, pub. 2011 © Oxford University Press.
Neurodegeneration with brain iron accumulation (NBIA) refers to a group of rare inherited neurodegenerative diseases, characterized by a progressive movement disorder and accumulation of iron in the basal ganglia, often the globus pallidus. Several causative genes have been identified so far; pantothenate kinase 2 (PANK2), group VIA calcium-independent phospholipase A2 (PLA2G6) (Morgan et al., 2006), fatty acid hydrolase (FA2H) (Kruer et al., 2010), ferritin light chain (FTL), ceruloplasmin (CP), and, very recently, C19orf12 (Hartig et al., 2011). The specific radiologic features of the NBIA subgroups have recently been described in detail (McNeill et al., 2008a; Kruer et al., in press). The clinical symptoms associated with mutations in these genes phenotypically overlap; however, gene-specific features are also observed (Table 1). PKAN may be classified as both an NBIA and NA syndrome as both brain iron accumulation and acanthocytes are found, although acanthocytes are described in 10% or less of PKAN cases (Klopstock et al., 2004; Pellecchia et al., 2005; Hayflick et al. 2006).
A Joint International Symposium on Neuroacanthocytosis and Neurodegeneration with Brain Iron Accumulation was held in Bethesda, MD, on October 1–2, 2010 with the purpose of widening perspectives on both of these groups of disorders at the basic science level. Membrane trafficking and turnover is likely to be a factor in the pathogenesis of ChAc, as the protein involved, VPS13A (chorein), is assigned to the vacuolar protein sorting network and is associated with the late endosomal compartment in diverse organisms from yeast to mouse. Impairment of autophagy may also be implicated, as autophagosomes are closely associated with and interact with the late endosomal compartment to generate autophagolysosomes. Autophagy is known to play an important role both in neurodegeneration and in late stage erythropoiesis by removing aggregated proteins and non-functional organelles in neurons and erythroid precursor cells. In this respect it is conceivable that autophagy may be impaired in NA leading to both neurodegeneration and defective erythrocyte morphology. Abnormal iron metabolism is clearly a major factor in the NBIA disorders. Despite this, most diseases are associated with defects in pathways not known to affect iron homeostasis. Commonalities between some of the disorders in the NBIA group seem to involve mitochondrial metabolism and membrane integrity and repair. Examination of the processes, cellular and mitochondrial, leading to this final common pathway of iron dyshomeostasis, may be rewarding. Acanthocytosis is an intriguing common feature of NA and NBIA, yet its significance is not yet understood.
NEUROACANTHOCYTOSIS
Acanthocytes
Acanthocytosis is found in many patients with ChAc or MLS, with percentages varying from 5%–50 % of erythrocytes (Walker et al., 2008), but is less commonly described (about 10% of patients) in HDL2 (Walker et al., 2003) and PKAN (Hayflick et al., 2006). Determination of acanthocytosis may be challenging (Feinberg et al. 1991; Foglia, 2010). The best procedure for the detection of acanthocytes requires dilution of whole blood samples with saline/heparin, followed by incubation on a shaker and phase-contrast microscopy of wet cells (Storch et al., 2005). Dry blood smears are often inadequate. Confirmation of erythrocyte morphology by scanning electron microscopy may be helpful if available.
The reason for the occurrence of acanthocytes is not known and is very likely due to a distinct primary cause in each syndrome depending on the respective gene defect. However, it is hypothesized that each defect affects a common pathway in erythropoiesis and/or red cell membrane homeostasis, thus leading to the same phenotype. It is also hypothesized that this common pathway is responsible for both the altered red cell morphology and neurodegeneration. Because of the relative ease of access to cells from patient blood, it is a worthwhile task to identify the molecular defects leading to acanthocytosis and subsequently study their relevance in neurons. It should be noted that early works on acanthocytosis studied red cells from patients with “neuroacanthocytosis”. Results from these reports should be interpreted with the knowledge that the molecular diagnosis in these cases is absent.
The major neurodegenerative syndromes with occurrence of acanthocytes are ChAc and MLS with defects in their genes VPS13A and XK, respectively. Most mutations in the VPS13A gene lead to the absence of VPS13A/chorein in red blood cells and neurons. Pathogenic mutations in the XK gene lead to absence of the Kx antigen and low expression of Kell antigens on the red cell surface. Although acanthocyte morphology may also be caused by abnormalities in membrane lipids (Kuypers et al., 1985), in NA there is significant evidence of membrane protein and cytoskeletal abnormalities (Terada et al., 1999). Electron microscopic studies of ChAc and MLS acanthocytes revealed focal membrane skeleton changes, accumulation of spectrin at the thorn region, and fewer filaments in regions of reversed membrane curvature (Terada et al., 1999; Hosokawa et al., 1992). An abnormal accumulation of cross-linked products of tissue transglutaminase was found in red blood cells and muscle tissue of ChAc patients (Melone et al., 2002), which could cause cellular membrane distortions. The major erythrocyte membrane protein, anion exchanger AE1, also known as band 3, was found to be altered in several studies of acanthocytes. In red cells from a family with hereditary acanthocytosis not further specified, this protein showed a higher molecular mass, increased anion transport, and decreased binding to ankyrin (Kay et al., 1988). Sequence analysis revealed a mutation within the membrane domain (Bruce et al., 1993). Alternatively, in erythrocytes from ChAc patients, fast degradation of band 3, ankyrin and band 4.2 has been described (Asano et al., 1985). In a different study of ChAc red cells, band 3 also showed increased fragmentation, while the patient's serum contained an anti-brain immunoreactant (Bosman et al., 1994). Red cell protein phosphorylation and dephosphorylation is an important regulatory process for the homeostasis of red cell volume and shape (Pantaleo et al., 2010; De Franceschi et al., 2008). Band 3 and β-spectrin were found to be highly phosphorylated in acanthocytes from a ChAc patient (Olivieri et al., 1997), thus leading to weaker interactions with other cytoskeletal components. A comparative proteomics study of red cell membranes from normal controls and ChAc patients revealed differences in the tyrosine phosphorylation state of membrane proteins. Band 3, β-spectrin, β-adducin and other members of anchoring complexes were highly phosphorylated in ChAc erythrocytes (De Franceschi et al., 2011). This difference is due to abnormal activation of the Src-family kinase Lyn but independent of Syk. Increased tyrosine phosphorylation of band 3 may alter its interaction with the junctional complexes and thus play a role in the generation of acanthocyte morphology. Interestingly, the Src-family kinases Lyn and Fyn are important regulators of cerebral N-methyl-D-aspartate receptors (NMDARs) that are implicated in motor activity (Salter and Kalia, 2004; Umemori et al., 2003). Despite overactive Lyn in ChAc red cells, the phosphatidylinositol 3-kinase (PI3K) subunit p85 (VPS34) showed decreased phosphorylation (Föller et al., 2012) leading to deactivation of downstream components Rac1 and PAK1 and depolymerization of cortical actin. Moreover, in K562 erythroid cells, silencing of VPS13A or PAK1 inhibition decreased the phosphorylation of Bcl2-Antagonist of cell Death (BAD) thereby inducing Bcl2-dependent apoptosis. Hence, VPS13A was shown to be a novel regulator of cytoskeletal architecture and cell survival, thus explaining red cell misshape and neurodegeneration in ChAc (Föller et al., 2012).
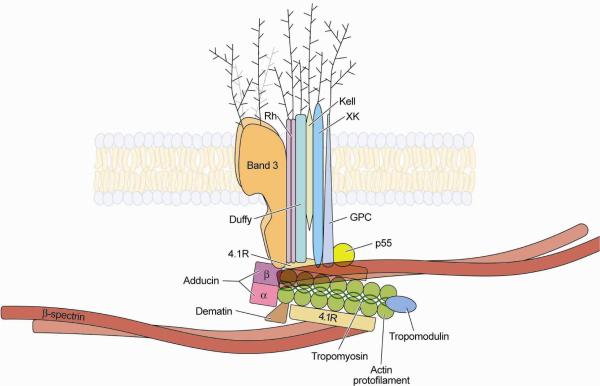
MLS red blood cells, which lack Kx/Kell antigens, show decreased deformability; they are rigid and have decreased surface area. Their membranes show intrinsic membrane stiffness suggesting that Kx/Kell proteins are required for the maintenance of the normal physical function of red cell skeletal proteins (Ballas et al. 1990). The Kx/Kell complex is part of a large red cell membrane protein-cytoskeleton complex, known as 4.1R complex (Figure 2) (Mohandas and Gallagher, 2008). This junctional complex associates with the major cytoskeletal proteins, spectrin and actin, and interacts with inner membrane lipids to play a role in mechanical stability (Manno et al., 2002; An et al., 2005; An et al., 2006). Band 3 is also part of a second macromolecular membrane protein complex, comprising the Rh-associated glycoprotein (RhAG) and others, which is associated with spectrin via ankyrin and protein 4.2 (Mohandas and Gallagher, 2008). These multiprotein complexes are formed during erythropoiesis and remodeled during reticulocyte maturation (Liu et al., 2010).
Figure 2.
Diagram of relationships of Kell and XK, illustrating their relationships to other red cell membrane proteins. Courtesy of Mohandas Narla, DSc.
Erythroblast enucleation is a critical step in erythropoiesis because the membrane proteins must distribute between the extruded nucleus and the membrane that now forms the shell of the reticulocyte. Aberrant protein sorting during this process leads to morphological changes of the red blood cell (Salomao et al, 2010). It is therefore likely that acanthocyte formation is based on the unbalanced distribution of membrane proteins and/or cytoskeleton during enucleation.
In the case of the Kx/Kell complex, deficiency of this part of the cytoskeleton-attached 4.1R complex could clearly lead to changes of red cell shape. In the case of VPS13A, the defect may impair endosomal trafficking during enucleation (Keerthivasan et al., 2010), or subsequently during the massive autophagic activity leading to red cell maturation (Sandoval et al., 2008; Zhang et al., 2009). It is conceivable that lack of transport of proteases to the late endosomal compartment could impair the ordered autophagic maturation of the red cell. The formation of acanthocytes in HDL2 and PKAN is still enigmatic.
Chorea-acanthocytosis
ChAc (OMIM #200150) is characterized by a progressive movement disorder, cognitive and behavioral changes, myopathy and chronically increased muscle creatine kinase (CK) in serum (Bader et al., 20011; Walker et al., 2011a). The movement disorder is mostly limb chorea, but some individuals present with parkinsonism. Dystonia is common and affects the oral region, especially the tongue, causing dysarthria and dysphagia. Habitual tongue and lip biting are characteristic. Seizures are observed in about half of ChAc patients. ChAc is a chronically progressive disease with a mean age of onset of 32 years (range 8–62) leading to disability within a few years. The diagnosis of ChAc is based primarily on clinical findings, characteristic neuroimaging findings of caudate nucleus atrophy, and evidence of muscle disease. Although the disorder is named for erythrocyte acanthocytosis, this feature is variable for reasons not yet understood. Acanthocytes are present in 5%–50% of the red cell population, may appear late during the course of the disease (Sorrentino et al., 1999) or may be absent (Bayreuther et al., 2010). Increased serum CK is observed in the majority of affected individuals and is a useful diagnostic feature. Muscle biopsy reveals central nuclei and atrophic fibers. For differential diagnosis, Western blot analysis of red cells with anti-VPS13A/chorein (Dobson-Stone et al., 2004) is available (www.eurohd.net/html/na/network/docs/chorein-wb-info.pdf). Genetic testing is at present limited and costly due to the large gene size, however, next generation sequencing will overcome this limitation (Walker et al., 2011b)..
Molecular genetics and pathology of ChAc
ChAc is an autosomal recessive disease caused by mutations in the CHAC gene, now renamed VPS13A to acknowledge its similarity with the Vps13/Soi1 yeast gene. The CHAC/VPS13A locus (OMIM *605978) was identified by linkage studies of 11 families in a 6 cM region of chromosome 9q21–22 (Rubio et al., 1997). This result was confirmed by homozygosity-by-descent analysis in offspring from consanguineous marriages. The gene comprises 73 exons in a genomic region of 250 kb (Rampoldi et al., 2001). The transcript has a full-length sequence of 11,262 bp and codes for a protein with 3174 amino acids. A splice variant containing exons 1–69 encodes a 3095 amino acid protein. In the reported 11 families (Rubio et al., 1997), 16 different mutations were identified in CHAC/VPS13A demonstrating that this is the gene that, when mutated, causes ChAc (Rampoldi et al., 2001). The gene was independently identified by fine linkage analysis and haplotype comparison of 4 ChAc patients from 3 Japanese kindreds (Ueno et al., 2001). Homozygosity for a 260-bp deletion was found in the patients, whereas the unaffected parents were heterozygous for the deletion. The gene contained 69 exons and the deduced protein of 3096 amino acids was named chorein. The 260-bp deletion was present in the coding region and resulted in a frame shift and production of a truncated protein (Ueno et al., 2001).
In a large study of 43 patients, 57 different mutations were identified in CHAC/VPS13A (Dobson-Stone et al., 2002). In 7 patients, only one heterozygous mutation was found; in 4 patients, no disease mutation was found, possibly due to undetected, small deletions. In a Japanese family, initially reported to have autosomal dominant inheritance of ChAc (Saiki et al., 2003; Ishida et al. 2009), the second VPS13A mutation was subsequently reported (Tomiyasu et al., 2011) refuting this assumption regarding inheritance (Bader et al., 2009).
In 11 affected members of 5 apparently unrelated French Canadian ChAc families, a single deletion of exons 70–73 was identified in the CHAC/VPS13A gene (Dobson-Stone et al., 2005). Haplotype analysis indicated a founder effect. A list of 95 pathogenic mutations is presented in a recent review (Dobson-Stone et al., 2010), with some recent additions (Tomiyasu et la. 2011).
The CHAC/VPS13A gene belongs to a family of 4 related genes: VPS13A through VPS13D, on chromosomes 9q21, 8q22, 15q21, and 1p36, respectively (Velayos-Baeza et al., 2004). VPS13B (COH1) is altered in individuals with Cohen syndrome (OMIM 216550), a rare autosomal recessive disorder characterized by non-progressive psychomotor retardation and microcephaly, retinal dystrophy, neutropenia, and characteristic facial features (Kolehmainen et al., 2003). An animal model of the disease in dogs (Border collies with a small exon 19 VPS13B deletion) is mainly characterized by bone marrow abnormalities with deficiency in segmented blood neutrophils, the so-called “trapped neutrophil syndrome”, but also occasional circulating nucleated erythrocytes (Shearman and Wilton, 2011). No human disorders have yet been associated with the VPS13C or VPS13D genes. All four human VPS13 genes have multiple splicing variants.
A mouse model of ChAc has been developed with a deletion of VPS13A exons 60–61, which shows acanthocytosis and late-onset motor disturbance but no involuntary movements (Tomemori et al., 2005). Brain pathology indicated apoptotic cells in the striatum. Levels of homovanillic acid, a dopamine metabolite, were reduced in the midbrain (Tomemori et al., 2005). These mice had significantly higher levels of gephyrin, a GABAA receptor-anchoring protein, and GABRG2, the GABAA receptor γ2 subunit, in the striatum and hippocampus, suggesting that loss of chorein may lead to a compensatory upregulation of these proteins to prevent striatal degeneration (Kurano et al., 2006). With an antibody against a VPS13A peptide, protein expression was studied in mouse tissue and found in brain, testis, kidney, spleen, muscle, heart, lung, ovary, with lower expression in other tissues (Kurano et al., 2007). The highest expression level was found in testis, where it plays an essential role, as male ChAc mice are infertile. Preliminary data show high chorein expression in human testis (Bader et al. unpublished). Chorein was seen throughout all parts of the brain. Analysis of fractionated brain tissue showed chorein mainly in the microsomal and synaptosomal pellet. In accordance with the immunoblot data, chorein was found in all parts of the brain and in testis by immunohistochemical analysis (Kurano et al., 2007). Chorein was identified in Sertoli cells and spermatocytes, leading to comparison of chorein with VPS54, mutation of which causes motor neuron disease and defective spermiogenesis in mouse (Schmitt-John et al., 2005). VPS54 is a component of the GARP tethering complex (Bonifacino and Hierro, 2011) which is involved in retrograde endosome-TGN transport (Pérez-Victoria et al., 2008; 2010). Thus, the biochemical and histochemical data of Kurano et al. (2007) support a possible role of chorein in vesicle trafficking.
Function of VPS13A/chorein
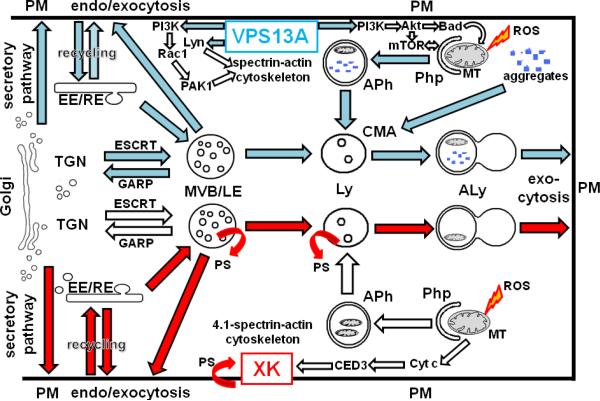
Little is known about the function of VPS13A/chorein. There are no conserved domains or motifs in the sequence to indicate a specific function (Rampoldi et al., 2001). However, the N- and C-terminal regions show the highest conservation suggesting that they play a role as binding domains in intermediate filament proteins and tethering components. The yeast homologue, Vps13p (Soi1p), forms high molecular weight complexes associated with vesicle membranes. This protein is required for proper intracellular trafficking of certain transmembrane proteins (Kex2p, Ste13p, Vps10p) from the trans-Golgi network (TGN) to the prevacuolar compartment (PVC) and recycling back to TGN (Redding et al., 1996; Brickner and Fuller, 1997). Taking into account that the human late endosomal (LE) compartment is the equivalent of PVC, this cycling pathway resembles the human TGN-to-LE pathway, which is characterized by the presence of mannose-6-phosphate receptor. Thus, VPS13A/chorein may control one or more steps in the cycling of proteins from the TGN to early and late endosomes and back, and possibly to lysosomes and the plasma membrane. Using a yeast in vitro assay for the delivery of protease Kex2p from TGN to PVC, it was shown that Vps13p was directly required for clathrin- GGA (Golgi-localising, Gamma-adaptin ear domain homology, ARF-binding protein)--dependent trafficking. With membranes from vps13 null strains, the TGN to PVC trafficking was blocked but could be rescued by addition of wildtype Vps13p (Fuller and De, 2010). A summary diagram of the proposed VPS13A trafficking pathways and functions is shown in Figure 3.
Figure 3. Hypothetical model of VPS13A and XK trafficking pathways and functions.
VPS13A is a peripheral membrane protein likely to follow the secretory pathway via Golgi and trans-Golgi network (TGN) to the plasma membrane (PM). VPS13A may be endocytosed thus coating and/or tethering early or recycling endosomes (EE/RE). It may then translocate to multivesicular bodies (MVB), late endosomes (LE) and lysosomes (Ly), respectively. Alternatively, it may take the direct route from TGN to MVB that is regulated by ESCRT proteins or in the retrograde direction regulated by GARP proteins. Lack of VPS13A may impair the function of the MVB/LE/Ly compartment and the associated autophagic flux via phagophores (Php), autophagosomes (APh) and autolysosomes (ALy), thus leading to lysosomal storage disease-like conditions. Likewise, chaperone-mediated autophagy (CMA) will be impaired, which leads to inefficient elimination of toxic protein aggregates. Moreover, inefficient mitophagy of reactive oxygen radical (ROS)-damaged mitochondria (MT) will lead to cell death. In ChAc erythrocytes, the Src-family kinase Lyn is overactive thus leading to aberrant PM-cytoskeleton association, whereas phosphatidylinositol 3-kinase (PI3K/VPS34) activity is low. Downstream signaling via Rac1 and PAK1 leads to depolymerization of cortical actin filaments thus explaining the red cell shape change. Low PI3K activity reduces phosphorylation of Proteinkinase B (PKB/AKT) and Bcl2-Antagonist of cell Death (BAD) thus inducing Bcl2-dependent apoptosis. AKT is an activator of the mammalian Target of Rapamycin (mTOR), which is a key regulator of autophagy. The XK protein is an integral membrane protein at the PM of erythroid cells and perinuclear endosomes in neurons. It is hypothesized to be involved in the maintenance of phospholipid asymmetry, particularly of phosphatidylserine (PS). PS plays an important role in apoptotic signaling, endocytic sorting and recycling, retrograde membrane traffic, and may play a role in the synthesis of sphingolipids. Due to the similarity of XK with CED8, it is hypothesized that XK may be involved in cytochrome c (Cyt c)-induced caspase activation downstream of CED3. VPS13A pathways are highlighted in blue, while XK pathways are marked in red.
Large orthologous proteins were not only identified in the yeast Saccharomyces cerevisiae and in Schizosaccharomyces pombe (Soi1p/Vps13p), but also in Caenorhabditis elegans, Drosophila melanogaster, Arabidopsis thaliana, and Dictyostelium discoideum (TipC) (Rampoldi et al., 2001; Ueno et al., 2001). In Tetrahymena thermophila, TtVPS13A was identified as a phagosomal protein (Jacobs et al., 2006). A GFP-tagged TtVPS13A fusion protein was found to associate with the phagosome membrane during the entire phagocytosis cycle. The TtVPS13A knockout displayed impaired phagocytosis and delayed digestion of phagosomal contents (Samaranayake et al., 2011). These data suggest that human VPS13A may also play a role in phagocytosis or related pathways such as autophagy and lysosomal degradation.
Interacting partners of human VPS13A have not yet been identified. Although several human homologues of the furin-like protease Kex2p are known, experiments to co-precipitate them with VPS13A were not successful (Velayos-Baeza et al., 2008). Trafficking of endosomes along certain pathways is regulated by various tethering complexes such as the CORVET, exocyst, GARP, and HOPS complexes (Brown and Pfeffer, 2010; Bröcker et al., 2010). The ESCRT complexes (ESCRT-0, -I, -II, -III and Vps4) are involved in the sorting of cargo proteins along the multivesicular body (MVB) pathway (Saksena et al., 2007; Saksena and Emr, 2009; Hurley and Hanson, 2010). ESCRT complexes are also involved in neurodegeneration, specifically in a type of frontotemporal dementia described in a single family with CHMP2B mutations (Urwin et al., 2010).
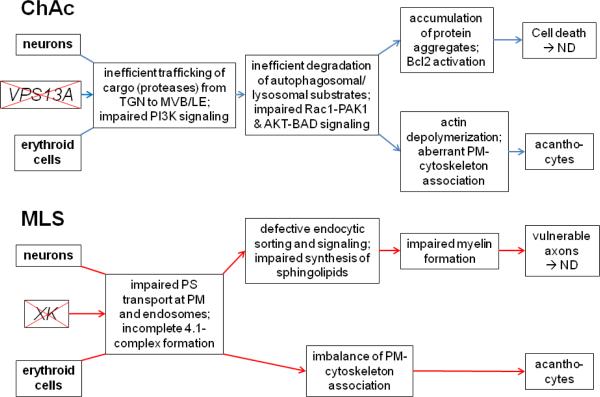
Neurons require fast re-uptake of neurotransmitters and synaptic vesicle membranes for optimal function, in addition, neuronal endocytosis is important for down-regulation of certain receptors, channels or pumps. These membrane proteins are recognized by arrestin-related trafficking adaptors (ARTs) that contain multiple PPXY (PY) motifs at the C-terminus. PY motifs are required for the recruitment of a Nedd4-like ubiquitin ligase that modifies the ARTs and membrane protein cargo (Lin et al., 2008). The ubiquitinated cargo is internalized and targeted to the MVB pathway for degradation. Defects in the ESCRT machinery result in the accumulation of membranes, misfolded protein aggregates, ubiquitinated protein inclusions, and defects in autophagic clearance (Saksena and Emr, 2009). These features are characteristic for the majority of neurodegenerative disorders, including Huntington's and Parkinson's diseases. A hypothetical scheme of ChAc pathogenesis is shown in Figure 4.
Figure 4. Hypothetical scheme of NA pathogenesis.
In chorea-acanthocytosis (ChAc), dysfunctional or absent VPS13A may lead to inefficient transport of proteases from the trans-Golgi network (TGN) to the late endosomal (MVB/LE) compartment. Lack of these proteases may cause the inefficient degradation of autophagosomal/lysosomal substrates thus leading to accumulation of toxic protein complexes or aggregates in lysosomes or cytosol. Moreover, impaired phosphatidylinositol 3-kinase (PI3K) signaling via downstream components Rac1 and PAK1 leads to cortical actin depolymerization and also affects AKT-BAD signaling thus causing Bcl2 activation, apoptosis and neurodegeneration (ND). In erythroid cells, actin depolymerization and inefficient degradation of proteins and organelles may cause an aberrant PM- cytoskeleton association that may lead to acanthocyte formation. Overactive Lyn kinase will also interfere with PM-cytoskeleton interaction. In McLeod syndrome (MLS), absent XK protein may cause inefficient phospholipid transport, particularly of phosphatidylserine (PS), at the PM and in endosomes. This leads to defective endocytic sorting and signaling and may impair sphingolipid synthesis that is important for myelin formation. Reduced myelin will result in vulnerable axons and ND. The possible involvement of XK in an apoptotic pathway may also induce cell death and ND. In erythroid cells, the absence of XK causes incomplete protein 4.1 complex (Figure 2) formation. In addition, impaired synthesis of sphingolipids may lead to imbalance of the PM-cytoskeleton association that may result in acanthocyte formation.
The family of VPS proteins that shares an involvement in sorting of yeast vacuoloes is clearly heterogeneous in terms of function. It is nevertheless of considerable clinical interest that another member of this family, VPS35, plays a role in the pathogenesis of both Parkinson's and Alzheimer's diseases (Vilariño-Güell et al., 2011; Zimprich et al., 2011; Sullivan et al., 2011). VPS35 is part of the so-called retromer that mediates retrograde transport from endosomes to the trans-Golgi network (McGough and Cullen, 2011).
McLeod syndrome
MLS (OMIM #314850) is an X-linked, multisystem disorder with central nervous system, neuromuscular, and hematologic manifestations in males (Danek et al., 2001; 2004; Jung et al., 2007). Neuromuscular symptoms include nonspecific myopathy with weakness and atrophy, and elevated serum levels of the muscle isoform of CK (Marsh et al., 1981; Jung et al., 2007). Although MLS myopathy was originally denoted as “benign”, a recent study showed that this is not the case (Hewer et al., 2007):half of all patients died from MLS-related complications.
The hematologic features of MLS are red blood cell acanthocytosis, compensated hemolysis, and the McLeod blood group phenotype resulting from the absence of Kx antigen (XK protein) and weak expression of Kell blood group antigens. The Kell blood group system can cause strong reactions to transfusions of incompatible blood and severe anemia in newborns of Kell-negative mothers (Lee et al., 2000). Heterozygous females have mosaicism for the Kell antigens and acanthocytosis but usually lack basal ganglia neurodegeneration and neuromuscular symptoms (Jung et al., 2007).
The diagnosis of MLS is based on clinical and hematologic findings. XK is the only gene currently known to be associated with MLS (Jung et al., 2007).
Molecular genetics and pathology of McLeod syndrome
The McLeod phenotype is caused by mutation in the XK gene leading to the lack of XK protein, which carries the Kx epitope. The XK gene contains three exons and is located in the chromosome region Xp21.1. The majority of XK mutations comprise deletions, nonsense mutations, or splice-site mutations predicting absent or truncated XK protein suggesting loss of function (Ho et al., 1994). Larger X-chromosomal deletions including the XK gene may result in a contiguous gene syndrome, comprising X-linked chronic granulomatous disease (CGD; OMIM 306400), Duchenne muscular dystrophy (DMD; OMIM 310200), and X-linked retinitis pigmentosa (RP3; OMIM 300389) (Brown et al., 1996; El Nemer et al., 2000). The XK-associated red cell membrane protein Kell is encoded by the KEL gene (OMIM 110900), composed of 19 exons, located at chromosome 7q33. Deletion of the KEL gene is not associated with MLS or other hereditary diseases.
XK is a membrane protein consisting of 444 amino acids that is predicted to have 10 transmembrane domains (Ho et al., 1994) and has structural characteristics of a transport protein. In red cells, XK forms a complex with Kell protein (Figure 5) (Lee et al., 1991; Russo et al., 1998). Together with band 3, Rh, Duffy, and glycophorin C, these proteins are part of the 4.1R-associated multiprotein complex (Figure 2) (Mohandas and Gallagher, 2008) that is connected to the actin-spectrin cytoskeleton and regulated by phosphorylation (Gauthier et al., 2011). XK is widely expressed in various tissues, especially in brain and skeletal muscle, whereas Kell is primarily expressed in complex with XK in erythroid cells, testis, and skeletal muscle (Russo et al., 2000). In situ hybridization histochemistry (ISHH) and RT-PCR of mouse tissues showed that XK is expressed in brain with high amounts in the pontine region, olfactory lobe, and cerebellum (Lee et al., 2007). Coexpression of Kell and XK in erythroid tissues and the different expressions in non-erythroid tissues suggest that XK may have a complementary hematological function with Kell and a separate role in other tissues (Lee et al., 2007). ISHH and immunohistochemistry of rodent and human brain also revealed the independent localization of XK and Kell, XK being expressed in neurons throughout the whole brain, whereas Kell expression was restricted to the red cells in cerebral vessels (Clapéron et al., 2007). In contrast to the localization of XK on the red cell membrane, neuronal XK is located in intracellular compartments such as ER, Golgi vesicles, and endosomes, suggesting a cell specific trafficking pattern and function. A summary diagram of proposed XK trafficking pathways and functions is illustrated in Figure 3.
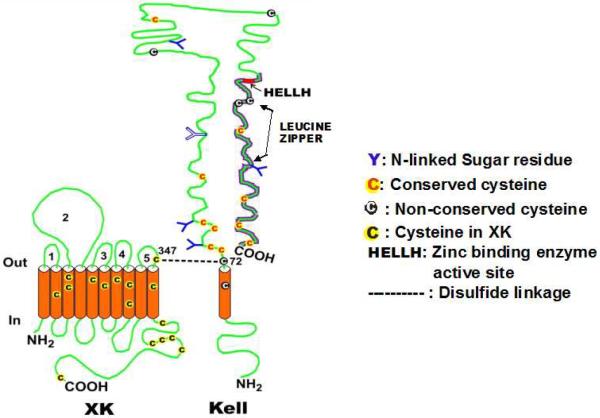
Figure 5. Kell and XK complex showing multiple cysteine residues in the transmembrane regions (TMRs) of XK and ecto-domain of Kell protein.
Cysteine residues are marked by “C”; the disulfide linkage between Kell Cys72 and XK Cys347 is shown by a dotted line. Putative N-glycosylation sites on the Kell glycoprotein are marked “Y”. The location of the zinc-binding, enzymatic active site of Kell is shown as HELLH. The C-terminal domain of Kell, depicted as a thick line, is conserved in the M13 family of zinc endopeptidases. Courtesy of Soohee Lee, PhD. Reprinted with permission from Lee S “The value of DNA analysis for antigens of the Kell and Kx blood group systems” Transfusion 2007; 47, Supplement S1:32S-39S.
XK shows weak similarity to the C. elegans protein CED8, a membrane protein with 10 transmembrane domains that plays a role as a cell death effector downstream of the caspase CED3 (Stanfield and Horvitz, 2000). CED8 and XK may act as transporters involved in maintenance of membrane phospholipid asymmetry, possibly for phosphatidylserine, which is an early marker of apoptosis and signal for cell engulfment. A defect in membrane lipid equilibrium between inner and outer membrane leaflets may also explain the acanthocytic morphology. The hypothetical scheme of MLS pathogenesis is shown in Figure 4.
The Kell protein, also known as CD238 antigen, is a highly polymorphic type II red cell membrane glycoprotein of 93 kDa (732 amino acids) with a short cytoplasmic N-terminus and a large extracellular domain that gives rise to over 30 different alloantigens (Lee et al., 2000). In the red cell membrane, Kell is bound to XK by a disulfide bond between Kell Cys-72 and XK Cys-347 close to the extracellular surface. Kell is an enzymatically active member of the large family of zinc-dependent endopeptidases, the neprilysin (M13) subfamily of mammalian neutral endopeptidases (Lee, 1997) including endothelin converting enzyme-1 (ECE-1). ECE-1, a disulfide-linked homodimer type II membrane glycoprotein, converts big endothelins 1–3 (big ET1–3) into the biologically active peptides ET1–3, with a preference for big ET1. The propeptide proET1 is processed to big ET1 by a furin-like convertase (Denault et al., 1995). Similar to ECE-1, the soluble, extracellular domain of Kell cleaves big ET3 to generate the 21-amino acid active ET3 peptide (Lee et al., 1999; 2000). Endothelins are vasoactive peptides derived from endothelium; however, ET3 is expressed in trophoblasts and placental stem villi (Onda et al., 1990). In mouse embryos, ET3 plays a role as an axonal guidance cue for developing sympathetic neurons (Makita et al., 2008). Acting through the G protein-coupled receptor, EDNRA, ET3 directs extensions of axons from the superior cervical ganglion to a preferred intermediate target, the external carotid artery, which serves as the gateway to select targets (Makita et al., 2008). EDNRB acts as an anti-apoptotic neuronal survival factor in the dentate gyrus in rodents and man, both during postnatal development and under pathological conditions (Ehrenreich et al., 2000). These findings establish a previously unknown mechanism of axonal pathfinding involving vascular-derived endothelins, and have broad implications for endothelins as general mediators of axonal growth.
The expressions of Kell glycoprotein and XK are each affected by the absence of the other partner. McLeod red cells, which lack XK, have reduced Kell antigen expression. The complete lack of Kell blood group antigens is known as Kell null (K0)-phenotype (Lee et al., 2000; 2001). Red cells from K0 phenotypes have less XK protein, however, the serologically determined Kx antigen increases, possibly due to unmasking of the Kx epitope when Kell is missing (Lee et al., 2000; 2007). Red cells of Kell knockout (KO) mice lack Kell glycoprotein and ECE-3 activity, and have reduced levels of XK, as in human K0 red cells, but XK levels in non-erythroid tissues are unchanged (Zhu et al., 2009). These mice display very discrete motor abnormalities (decline in forelimb strength, hindlimb foot splay in the drop test, falls on the rotarod test) yet in humans, lack of Kell protein - in contrast to the McLeod situation - so far has only been associated with absent ECE activity and transfusion risks but no other abnormalities (Yang 2011).
Huntington's disease-like 2
HDL2 was first described by Margolis and colleagues (Margolis et al., 2001) as an autosomal dominant neurodegenerative disease associated with CAG repeat expansion. The mutation leading to HDL2 is a CTG/CAG repeat expansion on chromosome 16q24.3, in the gene encoding junctophilin-3 protein (JPH3; Holmes et al., 2001).
JPH3 is a member of a conserved family of membrane proteins, the junctophilins (Takeshima et al., 2000), which are components of junctional complexes between the plasma membrane (PM) and the endoplasmic/sarcoplasmic reticulum (ER/SR). They appear to mediate cross-talk of PM components with calcium channels in the ER/SR membrane and are thus involved in signal transduction. JPH3 contains an N-terminal PM-binding domain and a C-terminal hydrophobic domain spanning the ER/SR membrane. In contrast to the other junctophilins, which are expressed in skeletal muscle and heart, JPH3 is predominantly expressed in the brain.
HDL2 presents in midlife with abnormalities of movement, psychiatric syndromes, weight loss, and dementia progressing to death over 10 to 20 years (Margolis and Rudnicki, 2008; Margolis, 2009). It is a rare disease that so far has only been detected in individuals of African ancestry. In South Africa, HDL2 is almost as common as HD (Krause et al., 2002; Margolis et al., 2004) suggesting a South African origin of HDL2.
Molecular genetics and pathology of Huntington's disease-like 2
The CTG/CAG repeat expansion in HDL2 resides in JPH3 exon 2A which is not part of the full-length JPH3 transcript. At least three JPH3 splice variants containing exon 2A have been identified, with the repeat region in frame to encode polyalanine or polyleucine, or falling within the 3' untranslated region. In a fraction of HDL2 patients acanthocytosis was detected on peripheral blood smear (Walker et al., 2003), thus HDL2 is included among the NA syndromes. The potential role of JPH3 in red blood cell morphology remains to be determined. HDL2 is clinically, genetically, and neuropathologically very similar to Huntington's disease (HD). One of the pathologic hallmarks of HDL2 is the presence of protein inclusions which are detectable with antibodies known to be at least partially selective for expanded polyglutamine (Holmes et al., 2001; Rudnicki et al., 2008). The presence of these inclusions in HDL2 brains has led to the hypothesis that expanded polyglutamine tracts, potentially encoded by a cryptic gene on the DNA strand antisense to JPH3, are the key to the pathogenesis of HDL2.
Recently, a mouse model of HDL2 has been generated by introducing a bacterial artificial chromosome (BAC) transgene containing the entire human JPH3 gene with a repeat of > 120 CTG/CAG triplets (Wilburn et al., 2011). A CAG repeat-containing transcript from the strand antisense to JPH3 was detected in these mice, along with expression of JPH3 transcripts containing the expanded repeat. Bi-directional transcription appears to be common in mammalian genome (Lindberg and Lundeberg, 2010) and was detected in the loci involved in a number of trinucleotide repeat diseases, including myotonic dystrophy 1 (Cho et al., 2005), spinocereballar ataxia type 8 (Moseley et al., 2006), Fragile × syndrome (Ladd et al., 2007), spinocerebellar ataxia 7 (Sopher et al., 2011) and HD (Chung et al., 2011). The HDL2 BAC mice accumulate nuclear inclusions similar to those observed in HDL2 patients, and develop a progressive motor phenotype. Inclusions and motor abnormalities are also found in a variant of this mouse model in which a mutation has been introduced to block expression of JPH3, so that only the cryptic antisense gene is expressed. Together, these results suggest that the polyglutamine tract encoded by the cryptic antisense gene is sufficient to cause the observed neuropathological and motor phenotypes in the mouse model. Whether this applies to the human disease is less clear. Normal antisense JPH3 transcripts were identified in human control and HDL2 brains, however no expanded antisense transcripts and no expanded polyglutamine-containing tracts have been detected in HDL2 brains (Seixas et al., in press).
While low levels of expanded polyglutamine tracts, undetectable using conventional methods, may be expressed in HDL2 brains, it seems unlikely that this would be sufficient to fully explain the pathogenesis of HDL2. Indeed, there is growing evidence that an RNA gain-of-function mechanism also contributes to HDL2 pathogenesis. JPH3 exon 2A transcripts with the expansion are toxic to both neuronal and nonneuronal cells (Rudnicki et al., 2007). JPH3 transcripts with an expanded repeat aggregate in HDL2 brain to form foci. These RNA foci appear to sequester splicing factors, with potential pathogenic significance. Further, and more speculatively, this aggregation of JPH3 may lead to loss of expression of JPH3 protein, with subsequent toxicity via loss of function (Rudnicki et al., 2007; Rudnicki et al., 2008). Overall, HDL2 appears to be a complex disease, with bidirectional transcription leading to toxic RNA, toxic protein, and potential loss of normal function.
NEURODEGENERATION WITH BRAIN IRON ACCUMULATION
Pantothenate kinase-associated neurodegeneration
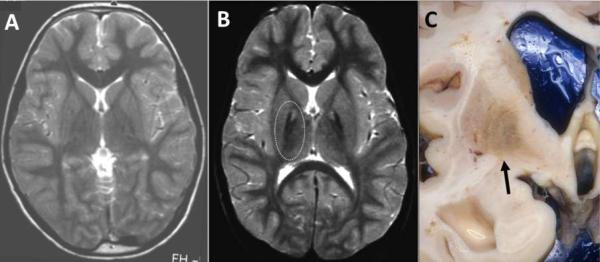
In 2001 the first NBIA gene, coding for pantothenate kinase 2 (PANK2), was identified in 32 of 38 individuals with classical symptoms of NBIA who carried two mutant alleles in the PANK2 gene (Zhou et al., 2001). This disorder is now referred to as pantothenate kinase-associated neurodegeneration (PKAN) and accounts for approximately 50% of NBIA cases (Zhou et al., 2001; Hayflick et al., 2003; Gregory et al., 2009). PKAN can be subdivided in two categories; classic PKAN and atypical PKAN, based on the age at onset and rate of progression of the disease. While this categorization remains useful, the phenotypic spectrum is, in fact, a continuum. In general the age of onset correlates with the severity of the mutation, and patients with two null mutations have an early onset, usually before the age of 6 (Hartig et al., 2006). Clinical features of classic PKAN are severe dystonia, dysarthria, and occasional acanthocytosis. In about two thirds of patients, clinical pigmentary retinopathy occurs (Egan et al., 2005). In later onset or atypical PKAN, neuropsychiatric symptoms are frequently reported and include cognitive decline, depression, emotional lability and impulsivity. Parkinsonism is common in atypical PKAN (Hayflick et al., 2003). On T2-weighted MR images, there is a distinct pattern of signal hyperintensity (fluid accumulation or edema representing tissue damage) surrounded with hypointensity (representing high levels of iron) in the globus pallidus, called the `eye-of-the-tiger' sign (Kruer et al., in press) (Figure 6A,B). In post mortem tissue at low power, frank iron deposition can sometimes be seen (Figure 6C), while at high power, neuroaxonal spheroids showing intense staining of ubiquitin were reported, representing axonal degeneration (Kruer et al., 2011). The observed pathology was mainly limited to the globus pallidus. It is unclear why the globus pallidus seems to be most affected as human PANK2 is widely expressed in other tissues.
Figure 6. MRI in PKAN.
T2-weighted MRI of control (A) and subject with PKAN (B), showing a central area of signal hyperintensity (fluid accumulation or edema representing tissue damage) surrounded with hypointensity (representing high levels of iron; outlined by the dashed oval) in the globus pallidus. (C) Gross view of a coronal section through the basal ganglia from a subject with PKAN, showing iron deposition (arrow) in the globus pallidus.
Molecular genetics and pathology of PKAN
The human genome contains 4 distinct genes coding for pantothenate kinase: PANK1, PANK2, PANK3 and PANK4. Only mutations in PANK2 are associated with PKAN. Human PANK1, 2 and 3 are highly structurally similar to Pank1, 2 and 3 in mice, whereas PANK4/ Pank4 are both structurally related to PANK genes in S. cerevisiae and C. elegans (Zhou et al., 2001). Pantothenate kinase is an enzyme required for the first conversion step of the de novo biosynthesis route of coenzyme A (CoA) starting from Vitamin B5 (or pantothenate) (Leonardi et al., 2005). CoA is an essential cofactor for over 100 metabolic reactions and is required for fatty acid metabolism, Krebs cycle, and amino acid synthesis among others (Leonardi et al., 2005). It is not currently understood why mutations in PANK2 lead to the specific symptoms of PKAN, nor what the specific functions of the 4 distinct mammalian proteins are, whether there exists redundancy of their specific functions, or whether they have additional functions besides their essential role in CoA biosynthesis. It is also unclear whether or not CoA levels are influenced in different tissues or in different subcellular compartments when specific PANK genes are disturbed in humans. In order to address these questions, animal models are required; however recent work shows that there may be differences in the specific functions of the pantothenate kinase proteins between mice and humans.
Although the mouse Pank2 gene is structurally highly similar to human PANK2 and the regulatory properties of the PanK2 enzymes from both species are comparable (Leonardi et al., 2007b), recent work from available mouse models suggest that the specific function of human and mouse PanK2 in tissue homeostasis may differ (Leonardi et al., 2007b). Pank2KO mice do not show impaired locomotor behavior, decreased life span or iron accumulation (Kuo et al., 2005). This may in theory be due to the ability of mouse PanK1 and 3 to compensate for the loss of mouse PanK2. It is unlikely that PanK4 compensates for loss of PanK2 in mice as PanK4 does not show enzymatic activity (Zhang et al., 2007). Another explanation for the different consequences of defective pantothenate kinase in humans and mice may be due to differences in the subcellular localization in the two species. In humans PANK2 is localized in mitochondria (Kotzbauer et al., 2005; Hortnagel et al., 2003) whereas localization of mouse PanK2 has been reported in mitochondria (Kuo et al., 2005) as well as cytoplasm (Leonardi et al., 2007b). In humans, localization of PANK2 may be linked to a distinctive mitochondrial function, which when disturbed leads to neurodegeneration. It has not been investigated whether the other mouse PanKs localize to mitochondria. The tissue specific abundance of the pantothenate kinase proteins in human and mice also differs. PANK2 is the most abundant form in human brain, whereas PanK3 is the most abundant form in murine brain (Leonardi et al., 2007b).
Pank1 KO mice have also been generated in order to study the consequences of disrupted de novo synthesis of CoA (Leonardi et al., 2010a). Pank1KO mice have lower CoA levels in the liver, and during fasting, fatty acid oxidation was impaired resulting in accumulation of long chain acyl-CoAs, acyl-carnitines and triglycerides, seen as lipid droplets. Gluconeogenesis was also impaired during fasting. Pank1 KO mice do not show any signs of locomotor defects or neurodegeneration. This study demonstrated that lower CoA levels induced by disruption of the main cytosolic pantothenate kinase results in defects in fatty acid oxidation. In human brain PANK2 is expressed relatively abundantly, therefore it may be possible that in PKAN patients, brain fatty acid oxidation is compromised, leading to lipid dyshomeostasis with resulting neuronal abnormalities (Figure 7).
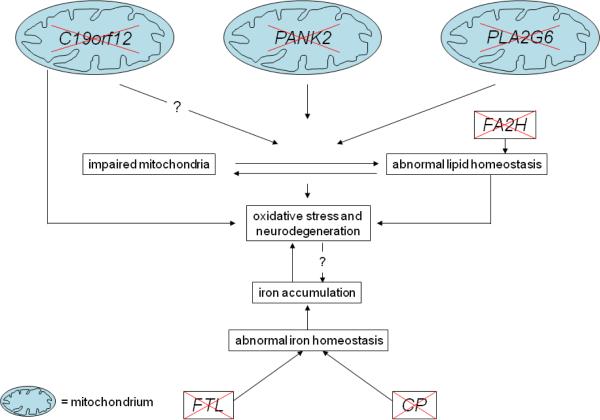
Figure 7. Hypothetical scheme of neurodegeneration in NBIA disorders.
Impaired mitochondrial function in PKAN, PLAN, and MPAN is associated with oxidative stress and iron deposition. This combination may lead to neurodegeneration. A similar order of events is suggested to occur in FAHN with impaired lipid homeostasis as a starting point. In neuroferritinopathy and aceruloplasminemia, aberrant iron homeostasis and accumulation also result in neurodegeneration.
Additional insights concerning consequences of disturbed CoA metabolism and possible links to neurodegeneration came from models in the fruitfly (Drosophila melanogaster). The fruitfly possesses only one pank gene, referred to as fumble, fbl (Afshar et al., 2001) or as dPANK/fbl (Bosveld et al., 2008). This gene encodes five isoforms of pantothenate kinase, one of which contains a mitochondrial targeting signal and localizes to mitochondria (Wu et al., 2009). dPANK/fbl homozygous mutants show severely disrupted mitochondria, progressive locomotor defects, neurodegeneration, and male and female sterility (Afshar et al., 2001; Bosveld et al., 2008; Rana et al., 2010; Wu et al., 2009). No signs of iron accumulation, however, were observed. The mitochondrial isoform of fruitfly pank seems to be the essential one, because overexpression of only this isoform but not the others showed a complete rescue of all phenotypic features (Wu et al., 2009). Overexpression of human PANK2 in a dPANK/fbl mutant background also resulted in a more complete rescue compared to overexpression of human PANK3 or PANK4 (human PANK1 was not tested) (Wu et al., 2009). These data suggest that mitochondrially-localized pantothenate kinase is able to compensate for the other forms. These data obtained from human, mice and Drosophila strongly suggest that disturbance of mitochondrially-localized forms are strongly linked to neurodegeneration. The work of Leonardi et al. (2007a) provided compelling biochemical evidence that the mitochondrial localization of Pank2 is important to upregulate CoA biosynthesis when demand for mitochondrial β-oxidation is increased. In addition, the presence of a specific mitochondrial fatty acid synthesis pathway that is required for the maintenance of phospholipids of the mitochondrial membrane has been described (Schneider et al., 1995; Schneider et al., 1997). Together, it may be that disturbed function of pantothenate kinase 2 leads to defects in fatty acid synthesis and β-oxidation, leading to lipid dyshomeostasis especially in mitochondria and neurodegeneration. The link to iron accumulation remains unknown.
Neuroaxonal dystrophy
Neuroaxonal dystrophies (NAD) are neurodegenerative disorders characterized by the presence of spheroids in degenerating axons throughout the nervous system. Upon electron microscopic examination an accumulation of membranes with tubulovesicular structures is observed within the spheroids (Liu et al., 1974; Kimura, 1991). Spheroids can also be detected at the light microscopic level using hematoxylin and eosin staining. Classically, NAD is a progressive disease beginning in infancy (INAD), with neurodevelopmental arrest and severe hypotonia, followed by ataxia, dystonia, optic atrophy, peripheral neuropathy and a general intellectual and motor decline. In many patients suffering from NAD, cerebellar atrophy and accumulation of iron in basal ganglia is observed (Gregory and Hayflick, 2011). In 2006, a causative gene for INAD, PLA2G6 was identified (Morgan et al., 2006). PLA2G6 encodes iPLA2-VI, a calcium-independent phospholipase A2 (also referred to as iPLA2 or iPLA2β). Some patients lack mutations in PLA2G6, thus there must be other causative genes for INAD (Morgan et al., 2006).
Examination of post mortem tissue demonstrated neuroaxonal spheroids with intense staining of ubiquitin as well as a-synuclein positive Lewy bodies, dystrophic neurites and neurofibrillary tangles (Gregory et al. 2008; Paisan-Ruiz et al., 2011). These features are also found in Alzheimer's and Parkinson's diseases and may suggest that INAD and these more common diseases share a similar pathogenesis. Whereas in PKAN the globus pallidus is mainly affected, in INAD spheroids, gliosis and neuronal loss are also observed in the substantia nigra pars reticulata, cerebellar white matter, nucleus gracilis and dentate nucleus (Paisan-Ruiz et al., 2011). Indeed axonal degeneration is seen throughout the central and peripheral nervous systems.
PLA2G6 mutations have also been discovered in cases of early-onset parkinsonism with phenotypes including dystonia, dementia, and frontotemporal atrophy but not necessarily brain iron accumulation (Paisan-Ruiz et al., 2009; Kauther et al., 2011; Yoshino et al., 2010).
Molecular genetics and pathology of neuroaxonal dystrophy
Phospholipase A2 enzymes catalyze the hydrolysis of glycerophospholipids at the sn-2 position and form free fatty acids such as arachidonic acid and lysophospholipids (Winstead et al., 2000; Burke and Dennis,2009; Schaloske and Dennis, 2006). A major function of iPLA2 is to mediate phospholipid remodeling, thus disrupted function of iPLA2 will result in altered lipid homeostasis. Membrane phospholipids may undergo peroxidation by reactive oxygen species (ROS). Mitochondria are considered the most important cellular source for ROS (Cadenas, 2004). The peroxidized fatty acid residues in cells can be selectively cleaved and replaced with native fatty acids (van Ginkel and Sevanian, 1994). iPLA2 is found in mitochondria, and overexpression of iPLA2 protects these organelles from oxidative stress induced by staurosporine (Seleznev et al., 2006; Williams and Gottlieb, 2002). It is possible that iPLA2 may be required to protect mitochondria from peroxidative damage, by replacing peroxidized fatty acid residues, and when disturbed this leads to dysfunctional mitochondria and neurodegeneration (Figure 7).
The mouse model of INAD (iPLA2β-KO) develops age-dependent neurological impairment and ubiquitin-positive spheroids containing tubulovesicular membranes, similar to those observed in INAD (Malik et al., 2008). Mitochondrial remnants were also observed within the membranes although no abnormal mitochondria were reported. In the iPLA2β-KO mouse ubiquitinated proteins were observed enriched in the more insoluble fractions, indicating increased pathologic protein accumulation. Another similarity with humans was the accumulation of α-synuclein; however, despite the remarkable neuropathological similarities, rather disappointingly, the mouse model did not show brain iron accumulation. An earlier onset, more severe murine phenotype associated with specific mutations in the Pla2g6 gene (Wada et al., 2009) may serve as a better model of human disease.
Studies of autophagy were performed by crossing the iPLA2 KO mouse (Zhao et al., 2010) with a transgenic mouse expressing GFP-LC3, which labels autophagosomes. After a 2-day starvation period, only few autophagic puncta were seen in the wild-type brain, while there was a dramatic increase of these markers of autophagy in the iPLA2 KO brain (Ma, 2010). This may result in the accumulation of aggregated material leading to neurodegeneration in INAD and NBIA.
Fatty acid hydroxylase-associated neurodegeneration
Another subtype of NBIA is caused by mutations in the gene encoding fatty acid-2 hydroxylase, FA2H, (Kruer et al., 2010). Clinical symptoms of FAHN (fatty acid hydroxylase-associated neurodegeneration) begin typically with childhood-onset focal dystonia of the lower limbs. Then patients develop progressive ataxia and dysmetria followed by spastic tetraparesis and optic atrophy. Intellectual impairment and seizures are common later in the course of disease. In addition to basal ganglia iron accumulation, brain MRI demonstrates white matter involvement in this distinctive form of NBIA.
Molecular genetics and pathology of FAHN
Fatty acid hydroxylase is required for the formation of 2-hydroxy fatty acids (hFA) (Alderson et al., 2004; Eckhardt et al., 2005), which are among the most important building blocks of sphingolipids in mammals (for review see Hama et al., 2010). Based on this it is likely that mutations in FA2H affect the synthesis of sphingolipids, with resultant neurodegeneration (Figure 7). Of note, CoA is also required, along with ATP, to synthesize hFA-dihydroceramide, which is an intermediate product in the biosynthesis of hFA-sphingolipids.
A mouse model of FAHN, which had absent 2-hydroxylated sphingolipids in brain and peripheral nerves, shows axonal and myelin sheath degeneration and impaired locomotor skills, but only at older ages (22 months). These results indicate that 2-hydroxylated sphingolipids are not required for the development and formation of myelinated structures; however, they are required for long-term maintenance in ageing mice (Zoller et al., 2008). It was not determined whether iron accumulates in brain in FA2H KO mice.
Neuroferritinopathy
The only autosomal dominant form of NBIA, neuroferritinopathy (NFT) is caused by mutations in the ferritin light chain gene, FTL (Curtis et al., 2001; Vidal et al., 2004; Mancuso et al., 2005). NFT leads to a complex neurological phenotype characterized by adult onset of parkinsonism, chorea, dystonia, spasticity, ataxia, dementia and autonomic features. Iron and ferritin positive elements were observed in brain tissue such as in the caudate nucleus, putamen, globus pallidus, thalamus and dentate nucleus. Cavitations, not seen in other forms of NBIA, occur in the basal ganglia (Curtis et al., 2001; McNeill et al., 2008a). A mouse model has recently been developed (Vidal et al., 2008), which exhibits signs of increased oxidative stress and DNA damage, particularly in mitochondria (Deng et al., 2010), with ferritin inclusion bodies in the brain similar to those seen in human NFT (Barbeito et al., 2009).
Molecular genetics and pathology of neuroferritinopathy
Ferritins are heteropolymers composed of light (FTL) and heavy subunits (FTH1) (Harrison and Arosio, 1996) and are required for normal iron homeostasis. Disease-causing mutations lead to disruption of the C terminus of the FTL polypeptide and this may alter the tertiary structure and stability, affecting the function of ferritin leading to abnormal iron homeostasis. Ferritins have a dual function, playing a role in iron detoxification as well as in iron reserve. While iron is essential for various cellular processes such as neuronal development, and electron transport (Beard and Connor, 2003), it may also be toxic, generating reactive free radicals that eventually induce lipid peroxidation, DNA damage and protein modifications. Ferritins are therefore key proteins in maintaining the balance of iron levels. In a mouse model, mutant transcripts of FTL were expressed in an otherwise wildtype background resulting in a progressive decrease in locomotor performance, shorter life span, dysregulation of iron metabolism, and in various neuronal and glial inclusions (Vidal et al., 2008). Although the mouse phenotype is more marked than that seen in humans, most abnormalities of the mouse model resemble the pathology observed in NFT (Vidal et al., 2004; Mancuso et al., 2005). The more severe features in the mouse model are most likely due to the use of an exogenous promoter to drive mutant FTL expression, whereas in patients the endogenous promoter regulates expression of the affected allele. The results of the mouse studies demonstrate that indeed one affected FTL gene induces a dominant form of neuroferritinopathy. In addition, there was damage to mitochondrial but not nuclear DNA (Deng et al., 2010), likely leading to abnormal mitochondrial function and neurodegeneration (Figure 7).
It has been demonstrated that heteropolymers containing mutated or truncated light subunits show a reduced capacity to incorporate iron and decreased stability, supporting the dominant negative action of mutations and explaining the dominant transmission of the disease (Baraibar et al., 2008; Baraibar et al., 2010; Luscieti et al., 2010).
Aceruloplasminemia
Patients with autosomal recessive aceruloplasminemia (ACP) lack the ferroxidase activity of the multi-copper oxidase ceruloplasmin, due to mutations in the ceruloplasmin gene (CP). This results in iron deposition in the central nervous system, and also in the liver, pancreas and other viscera. Clinical symptoms may include ataxia, chorea, parkinsonism, dysarthria and progressive dementia, as well as retinopathy (McNeill et al., 2008b; Xu et al., 2004; Miyajima, 2003). Neuroimaging typically demonstrates widespread iron deposition in basal ganglia nuclei. Diabetes mellitus is typical. In all patients with CP mutations evaluated so far, serum ceruloplasmin was undetectable, ferritin was elevated and serum copper and iron levels were low (McNeill et al., 2008b; Xu et al., 2004; Miyajima, 2003).
Molecular genetics and pathology of aceruloplasminemia
Ceruloplasmin (Cp) is a protein of the α2-globulin fraction of human blood serum and contains 95% of serum copper (Harris and Gitlin, 1996). Cp expressed by hepatocytes is released in the serum, but it is also expressed in brain where Cp is expressed as a specialized glycosyl-phosphatidyl-inositol (GPI)-anchored membrane bound form in astroglia (reviewed in Vassilev et al., 2005; McNeill et al., 2008b). Cp oxidizes Fe2+ to Fe3+ and was named ferro-O2-oxidoreductase. It plays a crucial role in the mobilization of iron from tissues (Osaki, 1966; Osaki et al., 1966; 1971). Reported mutations lead to abolished protein synthesis or disruption of ferroxidase function, with inhibition of iron efflux from neuronal tissue (McNeill et al., 2008b). De Domenico and coworkers reported an additional mechanism of Cp and showed that expression of wild type GPI-anchored Cp is required for normal stabilization of the iron exporter ferroportin. Loss of function mutations in Cp destabilize ferroportin proteins, resulting in decreased iron transport towards the plasma and increased storage of intercellular iron (De Domenico et al., 2007). These data explain how mutations in Cp can lead to iron depositions, what the exact mechanisms remains to be elucidated, for more extensive reviews on this subject see (McNeill and Chinnery, 2011; Texel et al., 2008. Iron deposition has been detected in astrocytes and neurons in the basal ganglia, thalamus and cerebral and cerebellar cortices of ACP affected individuals (Kono and Miyajima, 2006). Increased concentrations of Fe2+ result in acceleration of hydroxyl radical (HO.) production in the presence of hydrogen peroxide (H2O2), the Fenton reaction, likely resulting in increased oxidative stress (Yoshida et al., 2000). Increased oxidative stress will in turn lead to lipid dyshomeostasis and neurodegeneration (Figure 7), as demonstrated by increased lipid peroxidation in the brains of ACP patients (Kono and Miyajima, 2006).
A mouse model of ACP has been generated and CP−/− adult mice show a progressive accumulation of iron in reticuloendothelial cells and hepatocytes (Harris et al., 1999) and in later studies loss of CP in mice was shown to result also in abnormal locomotor behavior, increased iron deposition in the cerebellum and the brainstem, coinciding with increased lipid peroxidation (Patel et al., 2002).
Mitochondrial membrane protein associated neurodegeneration
The most recently-discovered subtype of NBIA is caused by mutations in C19orf12, a gene coding for a protein which co-localizes with mitochondria, explaining the acronym MPAN (mitochondrial membrane protein associated-neurodegeneration) (Hartig et al., 2011). Compared with PKAN patients, the age of onset is later in MPAN-affected individuals and the disease progresses more slowly. In all reported cases T2-weighted MRI revealed hypointensities in the globus pallidus and substantia nigra, indicative of high levels of iron. Psychiatric signs such as impulsive or compulsive behavior, depression and emotional lability were common (Hartig et al., 2011), as is common also in PKAN. Histopathological examination revealed iron-containing deposits, axonal spheroids, α-synuclein-positive Lewy bodies and Lewy body-like inclusions, sparse Lewy neurites and hyperphosphorylated tau-containing neuronal inclusions in various regions of the brain (Hartig et al., 2011).
Molecular genetics and pathology of MPAN
The affected gene in MPAN codes for a protein that is expressed in mitochondria (Figure 7), based on evidence from GFP-tagged protein studies and specific antibody staining. The cellular function of C19orf12 is currently unknown, however in silico analysis predicts a transmembrane region (Hartig et al., 2011). The encoded protein is highly conserved throughout evolution enabling future functional studies in animal models.
IRON AND NEURODEGENERATION
Iron accumulation in the brain is a hallmark of many neurodegenerative diseases. It is found not only in NBIA diseases but also in Parkinson's and Alzheimer's diseases (Zecca et al., 2004), and in Huntington's disease (Bartzokis et al., 2007). Huntingtin plays a role in the iron trafficking pathway and metabolism (Lumsden et al., 2007; Hilditch-Maguire et al., 2000). Moreover, it is clear that iron progressively accumulates in the brain with normal aging, and that iron-induced oxidative stress can cause neurodegeneration (Zecca et al., 2004). Recently, our understanding of iron regulatory mechanisms and their relevance to neurodegeneration has greatly improved. The iron regulatory proteins 1 and 2 (IRP1 and IRP2) regulate the expression of numerous proteins involved in cellular iron metabolism (Rouault, 2006). When cells are iron-depleted, IRP1 and 2 bind to stem-loops in transcripts known as iron-responsive elements (IREs). IRP binding to IREs at the 3'-end stabilizes transferrin receptor mRNA but represses transcripts with IREs at the 5'-end such as ferritin H and L chains. IRP1 is an iron-sulfur protein that functions as an aconitase in iron-replete cells while the homologous IRP2 is degraded concomitantly. The IRP2 KO mouse presents with mild anemia, erythropoietic protoporphyria, and adult-onset neurodegeneration, apparently resulting from functional iron deficiency within neurons and red cell precursors. IRP2 KO mice show Fe3+ accumulation in cerebral white matter, and axonal degeneration with clumping of neurofilaments and myelin invaginations. In populations of degenerating neurons, ferritin colocalizes with iron accumulations, and iron-laden oligodendrocytes accumulate ubiquitin-positive inclusions, demonstrating that misregulation of iron metabolism leads to neurodegeneration (LaVaute et al., 2001). IRP2 KO mice offer a unique example of spontaneous adult-onset, slowly progressive neurodegeneration. Using this mouse model to identify compounds preventing neurodegeneration, the membrane-permeable radical scavenger, 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPOL) was identified. TEMPOL appeared to prevent neurodegeneration in IRP2 KO mice by recruiting the IRE-binding activity of IRP1 (Ghosh et al., 2008). In humans with NBIA disorders, reducing iron overload by chelators is unlikely to be effective at stopping neurodegeneration, thus agents such as TEMPOL may be novel therapeutic options. Recently, important regulatory mechanisms of iron metabolism have been elucidated; the FBXL5-mediated IRP2 degradation (Moroishi et al., 2011) and the role of hepcidin in ferroportin regulation (Kaplan et al., 2011; Zhang et al., 2011). These mechanisms are relevant for iron metabolism in neurons and in erythropoiesis.
IMPAIRED AUTOPHAGY AS A MECHANISM FOR NEURODEGENERATION
An increasing amount of data implicates dysregulation of autophagy in the pathophysiology of neurodegenerative diseases (Yue et al., 2009; Wong and Cuervo, 2010; García-Arencibia et al., 2010; Mariño et al., 2011). In neurons, autophagy is tightly controlled by restricting autophagosome formation. Under normal conditions, basal autophagy plays a critical role in protein quality control and organelle homeostasis. Under pathological conditions, such as stress or injury, autophagy is induced and autophagosomes are synthesized locally resulting in their accumulation in axons and axon terminals. When clearance of autophagosomes is impaired, autophagy is impaired, resulting in neurodegeneration. In a conditional autophagy-related gene 7 (atg7) KO mouse, progressive dystrophy and degeneration of Purkinje cell axon terminals was observed before dendritic tree atrophy and cell death (Komatsu et al., 2007). No autophagosomes were found, however, there were aberrant membrane structures and organelles in dystrophic swellings. In both atg5 and atg7 KO mice, ubiquitin-associated inclusion bodies were identified in neurons, suggesting that autophagy plays a role in protein clearance and maintenance of axonal homeostasis by removal of autophagic cargo (Yue et al., 2009). Another mechanism of regulation of autophagy is by association of the Beclin 1-VPS34 complex with either Atg14L or the RUN domain and cysteine-rich domain containing protein “Rubicon” (Zhong et al., 2009).
Studies of the role of autophagy-related component Atg9, a six-transmembrane domain membrane protein, in autophagosome formation in the yeast S. cerevisiae, found that it was concentrated in a novel compartment, termed Atg9 reservoirs, comprising clusters of vesicles and tubules derived from the secretory pathway, and often adjacent to mitochondria (Cebollero and Reggiori, 2009). These Atg9 reservoirs translocate en bloc next to the vacuole to form the phagophore assembly site (PAS) and generate the autophagosome precursor, the phagophore (Mari et al., 2010). Atg1, Atg13, and phosphatidylinositol-3-phosphate are involved in the further rearrangement of these initial membranes. Thus, Atg9-positive compartments are important for the de novo formation of PAS and the sequestering vesicle that are hallmarks of autophagy. A regulatory link between mitochondrial function and autophagy was recently identified in yeast (Graef and Nunnari, 2011). Both functions are repressed by activated protein kinase A (PKA). Normal mitochondrial respiration deactivates PKA, whereas mitochondrial dysfunction activates PKA and thereby suppresses autophagic flux. .
AN INTEGRATIVE HYPOTHESIS
Neurodegeneration is often accompanied by compromised autophagy as mentioned above. Neuronal autophagy is essential for the elimination of accumulated and aggregated proteins (Yamamoto and Simonsen, 2011) or dysfunctional organelles such as mitochondria (Batlevi and La Spada, 2011). The selective chaperone-mediated autophagy (CMA) is important for degradation of specific proteins like α-synuclein (Koga and Cuervo, 2011). Thus, autophagy has an important role in cellular turnover, metabolism and protection (Wong and Cuervo, 2010; Mariño et al., 2011). Along the autophagic flux, autophagosomes (APh) are in contact with mitochondria (MT), lipid droplets, endosomes and multivesicular bodies (MVB) until they finally fuse with lysosomes (Ly), to form autolysosomes (ALy), as shown in Figure 3. Multiple factors are necessary for a smooth flow. The metabolic state of a cell is also important, because autophagy is activated when the metabolic regulator mTOR is inhibited (by rapamycin or starvation) and vice versa. This regulation is currently exploited for therapy of neurodegenerative diseases (Ravikumar et al., 2004; Fleming et al. 2011). Autophagy is important, not only for the maintenance of neurons but also for late stage erythropoiesis. After expulsion of the erythroblast nucleus, all mitochondria, ribosomes and other organelles are cleared by autophagy (Ney 2011). Subsequently, the reticulocyte plasma membrane undergoes a dramatic rearrangement. It is therefore conceivable that impaired autophagy may lead to imperfect or deformed erythrocytes such as acanthocytes.
The ChAc-associated protein VPS13A apparently plays a role in vesicle tethering along the recycling or late endosomal (RE/LE) pathway, possibly interacting with ESCRT or retrograde GARP components. Late endosomes/lysosomes will eventually fuse with autophagosomes to autolysosomes. Absence or dysfunction of VPS13A may thus interfere with autolysosomal degradation of cargo and may lead to accumulation of aggregates. The function of MLS-associated XK membrane protein is unknown but it may play a role in a CED-8-like apoptotic pathway (Stanfield and Horvitz, 2000) that may also intersect with autophagic flux. XK has been proposed to act as a phosphatidylserine (PS) transporter, thus causing the appearance of exofacial PS when absent. Exofacial PS is an apoptotic marker and “eat-me” signal for macrophages. Deficiency of XK may change the apoptotic pathway or PS-disequilibrium at the plasma membrane, both of which may lead to neuronal death. In MLS reticulocytes, autophagic clearance may be hampered and PS may create acanthocyte membrane domains. NBIA-associated proteins PANK2, PLA2G6, and FA2H are enzymes involved in lipid metabolism. PANK2 is necessary for CoA synthesis, PLA2G6 for phospholipid turnover and supply of arachidonic acid, and FA2H for the formation of 2-hydroxy galactolipids in myelin. Deficiency of these enzymes may interfere with the synthesis of sphingolipids and could affect the ceramide pool composition (Kruer at al., 2010). Sphingolipids are part of membrane microdomains or lipid rafts that are thought to act as signaling platforms. Changes in microdomain components may thus disturb or disrupt essential signal transduction pathways. Aggregation of these components may generate macrodomains in the plasma membrane. Accumulation of modified sphingolipids in the endosomal pathway will eventually lead to lysosomal storage disorders. Iron deposition in the brain of NBIA patients may be due to accumulation of free Fe(2+) in astroglial mitochondria. Stress-induced up-regulation of heme oxidase, HO-1, in astroglia may degrade heme to Fe(2+), which may be deposited in oxidatively stressed mitochondria leading to cell death.
CONCLUDING REMARKS
Following the identification of various causative genes for NBIA, and the recent development of animal models, some early, cautious, remarks can be made regarding a common underlying theme for NBIA pathogenesis. For all NBIA subtypes, with the exception of FAHN, studies in patient material or in animal models demonstrate a strong association with oxidative stress. This may be due to aberrant lipid homeostasis, iron homeostasis, mitochondrial functioning or a combination of the three. Further studies, additional animal models, identification of causative genes of the idiopathic forms of NBIA are required to fully understand the basic mechanisms of NBIA and to develop rational therapies.
Is iron simply a biomarker of NBIA disease or is it pathogenic? For most forms of NBIA, iron accumulation is secondary and its role in pathogenesis remains unclear. Almost certainly iron chelation will not prevent the primary neurodegenerative process. At the same time, massive amounts of intra- and extra-cellular iron are predicted to t be deleterious and are strongly suspected to contribute to pathogenesis. While iron chelation may have a role in experimental therapies for NBIA, efforts to overcome the primary defects remain the therapeutic goal.
Membrane dysfunction appears to offer a tantalizing hint for understanding the two core NA syndromes, ChAc and MLS. In both, a protein is absent from red cells and most likely also from neuronal membranes. In contrast, the acanthocytosis of PKAN is probably best understood on the basis of membrane lipid abnormalities. In any case, circulating erythrocytes offer the welcome possibility of a model system for NA and NBIA.
For future research, it is of interest that following the description of the McLeod antigen variant of red cells by blood banks, it took sixteen years before their exotic shapes were noticed (Wimer et al., 1977). Acanthocytes should thus be assiduously sought in the newer NBIA and NA syndromes. Also, since the rare Kell null subjects so far have not been properly examined outside of the hematology clinic, we propose that they be studied in greater detail by neurologists in case there are subtle neurological findings related to Kell/Kx dysfunction. Finally, although MLS may increasingly be understood as a delayed variant of ChAc with slower evolution of the multi-organ changes (Chauveau et al.,. 2011; Gantenbein et al., 2011), it is of particular note that cardiomyopathy is not found in ChAc, given that the assumed membrane dysfunction of NA probably also affects muscle cells.
Acanthocytic deformation of red cells is an intriguing feature shared by many of the conditions discussed above, and may possibly provide an essential clue for the understanding of their pathophysiology. The acanthocytic protrusion of parts of the cell membrane appears to be the result of disorganized membrane-cytoskeleton interactions due to defective endosomal trafficking and sorting, autophagic flux and autolysosomal degradation in late stage erythropoiesis. The protrusions and thorns of acanthocytes could perhaps be viewed as an arrested state among a multitude of continuously changing states of membrane deformation, thus potentially providing insights into basic mechanisms of cell biology.
Acknowledgements
This work would have not been possible without the tireless efforts of Glenn and Ginger Irvine of the Advocacy for Neuroacanthocytosis Patients, and Patty Wood of the NBIA Disorders Association.
Funding This article reflects presentations and discussion at a meeting entitled “Brain, Blood and Iron: Joint International Symposium on Neuroacanthocytosis and Neurodegeneration with Brain Iron Accumulation” which was held in Bethesda, MD, USA on Oct 1–2 2010. This meeting was supported by the National Institute of Neurological Diseases and Stroke; the Office of Rare Diseases Research, and the National Institute of Child Health and Human Development [1R13NS067922-01], and also by the Movement Disorder Society, Advocacy for Neuroacanthocytosis Patients (UK), Associazione Italiana Sindromi Neurodegenerative da Accumulo di Ferro (AISNAF) (Italy), NBIA Disorders Association (USA), Hoffnungsbaum e.V. (Germany) and GlaxoSmithKline. Funding by an ERA-Net E-Rare project, “European Multidisciplinary Initiative on Neuroacanthocytosis (EMINA)”, is gratefully acknowledged by AD (BMBF/DLR grant 01GM1003), RP (FWF grant I 438-B13), and OCMS (ZonMW grant 113301162).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afshar K, Gonczy P, DiNardo S, Wasserman SA. fumble encodes a pantothenate kinase homolog required for proper mitosis and meiosis in Drosophila melanogaster. Genetics. 2001;157:1267–1276. doi: 10.1093/genetics/157.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson NL, Rembiesa BM, Walla MD, Bielawska A, Bielawski J, Hama H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J Biol Chem. 2004;279:48562–48568. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- An X, Debnath G, Guo X, et al. Identification and functional characterization of protein 4.1R and actin-binding sites in erythrocyte beta spectrin: regulation of the interactions by phosphatidylinositol-4,5-bisphosphate. Biochemistry. 2005;44:10681–10688. doi: 10.1021/bi047331z. [DOI] [PubMed] [Google Scholar]
- An X, Zhang X, Debnath G, Baines AJ, Mohandas N. Phosphatidylinositol-4,5-biphosphate (PIP2) differentially regulates the interaction of human erythrocyte protein 4.1 (4.1R) with membrane proteins. Biochemistry. 2006;45:5725–5732. doi: 10.1021/bi060015v. [DOI] [PubMed] [Google Scholar]
- Asano K, Osawa Y, Yanagisawa N, Takahashi Y, Oshima M. Erythrocyte membrane abnormalities in patients with amyotrophic chorea with acanthocythosis. Part 2. Abnormal degradation of membrane proteins. J Neurol Sci. 1985;68:161–173. doi: 10.1016/0022-510x(85)90097-8. [DOI] [PubMed] [Google Scholar]
- Bader B, Danek A, Walker RH. Chorea-acanthocytosis. In: Walker RH, editor. The Differential Diagnosis of Chorea. Oxford University Press; New York, NY: 2011. Chap 6. 2011. [Google Scholar]
- Bader B, Velayos-Baeza A, Walker RH, Danek A. Dominant transmission of chorea-acanthocytosis with VPS13A mutations remains speculative. Acta Neuropathol. 2009;117:95–96. doi: 10.1007/s00401-008-0418-7. [DOI] [PubMed] [Google Scholar]
- Bader B, Arzberger T, Heinsen H, Dobson-Stone C, Kretzschmar HA, Danek A. Neuropathology of Chorea-Acanthocytosis. In: Walker RH, Saiki S, Danek A, editors. Neuroacanthocytosis Syndromes II. Springer-Verlag; Berlin Heidelberg, Germany: 2008. pp. 187–195. [Google Scholar]
- Ballas SK, Bator SM, Aubuchon JP, Marsh WL, Sharp DE, Toy EM. Abnormal membrane physical properties of red cells in McLeod syndrome. Transfusion. 1990;30:722–727. doi: 10.1046/j.1537-2995.1990.30891020333.x. [DOI] [PubMed] [Google Scholar]
- Barbeito AG, Garringer HJ, Baraibar MA, Gao X, Arredondo M, Nunez MT, et al. Abnormal iron metabolism and oxidative stress in mice expressing a mutant form of the ferritin light polypeptide gene. J Neurochem. 2009;109:1067–1078. doi: 10.1111/j.1471-4159.2009.06028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraibar MA, Barbeito AG, Muhoberac BB, Vidal R. Iron-mediated aggregation and a localized structural change characterize ferritin from a mutant light chain polypeptide that causes neurodegeneration. J Biol Chem. 2008;283:31679–31689. doi: 10.1074/jbc.M805532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraibar MA, Muhoberac BB, Garringer HJ, Hurley TD, Vidal R. Unraveling of the E-helices and disruption of 4-fold pores are associated with iron mishandling in a mutant ferritin causing neurodegeneration. J Biol Chem. 2010;285:1950–1956. doi: 10.1074/jbc.M109.042986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S. Myelin breakdown and iron changes in Huntington's disease: pathogenesis and treatment implications. Neurochem Res. 2007;32:1655–1664. doi: 10.1007/s11064-007-9352-7. [DOI] [PubMed] [Google Scholar]
- Batlevi Y, La Spada AR. Mitochondrial autophagy in neural function, neurodegenerative disease, neuron cell death, and aging. Neurobiol Dis. 2011;43:46–51. doi: 10.1016/j.nbd.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayreuther C, Borg M, Ferrero-Vacher C, Chaussenot A, Lebrun C. Choreo-acanthocytose sans acanthocytes. Revue Neurologique. 2010;166:100–103. doi: 10.1016/j.neurol.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Hierro A. Transport according to GARP: receiving retrograde cargo at the trans-Golgi network. Trends Cell Biol. 2011;21(3):159–67. doi: 10.1016/j.tcb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman GJ, Bartholomeus IG, De Grip WJ, Horstink MW. Erythrocyte anion transporter and antibrain immunoreactivity in chorea-acanthocytosis. A contribution to etiology, genetics, and diagnosis. Brain Res Bull. 1994;33:523–528. doi: 10.1016/0361-9230(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Bosveld F, Rana A, van der Wouden PE, Lemstra W, Ritsema M, Kampinga HH, et al. De novo CoA biosynthesis is required to maintain DNA integrity during development of the Drosophila nervous system. Hum Mol Genet. 2008;17:2058–2069. doi: 10.1093/hmg/ddn105. [DOI] [PubMed] [Google Scholar]
- Brickner JH, Fuller RS. SOI1 encodes a novel, conserved protein that promotes TGN-endosomal cycling of Kex2p and other membrane proteins by modulating the function of two TGN localization signals. J Cell Biol. 1997;139:23–36. doi: 10.1083/jcb.139.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröcker C, Engelbrecht-Vandré S, Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Curr Biol. 2010;20:R943–52. doi: 10.1016/j.cub.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Brown J, Dry KL, Edgar AJ, Pryde FE, Hardwick LJ, Aldred MA, Lester DH, Boyle S, Kaplan J, Dufier JL, Ho MF, Monaco AM, Musarella MA, Wright AF. Analysis of three deletion breakpoints in Xp21.1 and the further localization of RP3. Genomics. 1996;37:200–210. doi: 10.1006/geno.1996.0543. [DOI] [PubMed] [Google Scholar]
- Brown FC, Pfeffer SR. An update on transport vesicle tethering. Mol Membr Biol. 2010;27:457–461. doi: 10.3109/09687688.2010.501765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce LJ, Kay MM, Lawrence C, Tanner MJ. Band 3 HT, a human red-cell variant associated with acanthocytosis and increased anion transport, carries the mutation Pro-868-->Leu in the membrane domain of band 3. Biochem J. 1993;293:317–320. doi: 10.1042/bj2930317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25:17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Cebollero E, Reggiori F. Regulation of autophagy in yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2009;1793:1413–1421. doi: 10.1016/j.bbamcr.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Chauveau M, Damon-Perriere N, Latxague C, Spampinato U, Jung H, Burbaud P, Tison F. Head drops are also observed in McLeod syndrome. Movement Disorders. 2011;26(8):1562–1563. doi: 10.1002/mds.23605. [DOI] [PubMed] [Google Scholar]
- Cho DH, Thienes CP, Mahoney SE, Analau E, Filippova GN, Tapscott SJ. Antisense transcription and heterochromatin at the DM1 CTG repeats are constrained by CTCF. Mol Cell. 2005 Nov 11;20(3):483–9. doi: 10.1016/j.molcel.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Chung DW, Rudnicki DD, Lan Y, Margolis RL. A natural antisense transcript at the Huntington's disease repeat locus regulates HTT expression. Hum Mol Gen. 2011;20(17):3467–77. doi: 10.1093/hmg/ddr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapéron A, Hattab C, Armand V, Trottier S, Bertrand O, Ouimet T. The Kell and XK proteins of the Kell blood group are not co-expressed in the central nervous system. Brain Res. 2007;1147:12–24. doi: 10.1016/j.brainres.2007.01.106. [DOI] [PubMed] [Google Scholar]
- Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet. 2001;28:350–354. doi: 10.1038/ng571. [DOI] [PubMed] [Google Scholar]
- Danek A, Sheesley L, Tierney M, Uttner I, Grafman J. Cognitive and neuropsychiatric findings in McLeod syndrome and in chorea-acanthocytosis. In: Danek A, editor. Neuroacanthocytosis Syndromes. Springer; Dordrecht, The Netherlands: 2004. pp. 95–115. [Google Scholar]
- Danek A, Rubio JP, Rampoldi L, Ho M, Dobson-Stone C, Tison F, Symmans WA, Oechsner M, Kalckreuth W, Watt JM, Corbett AJ, Hamdalla HH, Marshall AG, Sutton I, Dotti MT, Malandrini A, Walker RH, Daniels G, Monaco AP. McLeod neuroacanthocytosis: genotype and phenotype. Ann Neurol. 2001;50:755–764. doi: 10.1002/ana.10035. [DOI] [PubMed] [Google Scholar]
- Danek A, Tison F, Rubio J, Oechsner M, Kalckreuth W, Monaco AP. The chorea of McLeod syndrome. Mov Disord. 2001;16:882–889. doi: 10.1002/mds.1188. [DOI] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26:2823–2831. doi: 10.1038/sj.emboj.7601735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi L, Tomelleri C, Matte A, Brunati AM, Bovee-Geurts PH, Bertoldi M, Lasonder E, Tibaldi E, Danek A, Walker RH, Jung HH, Bader B, Siciliano A, Ferru E, Mohandas N, Bosman GJCGM Erythrocyte membrane changes of chorea-acanthocytosis are the result of altered Lyn kinase activity. Blood. 2011;118(20):5652–63. doi: 10.1182/blood-2011-05-355339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi L, Biondani A, Carta F, Turrini F, Laudanna C, Deana R, Brunati AM, Turretta L, Iolascon A, Perrotta S, Elson A, Bulato C, Brugnara C. PTPepsilon has a critical role in signaling transduction pathways and phosphoprotein network topology in red cells. Proteomics. 2008;8:4695–4708. doi: 10.1002/pmic.200700596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denault JB, Claing A, D'Orléans-Juste P, Sawamura T, Kido T, Masaki T, Leduc R. Processing of proendothelin-1 by human furin convertase. FEBS Lett. 1995;362:276–280. doi: 10.1016/0014-5793(95)00249-9. [DOI] [PubMed] [Google Scholar]
- Deng X, Vidal R, Englander EW. Accumulation of oxidative DNA damage in brain mitochondria in mouse model of hereditary ferritinopathy. Neurosci Lett. 2010;479:44–48. doi: 10.1016/j.neulet.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson-Stone C, Rampoldi L, Bader B, Velayos-Baeza A, Walker RH, Danek A, Monaco AP. Chorea-acanthocytosis. GeneReviews. 2010 ( http://www.ncbi.nlm.nih.gov/books/NBK1387/)
- Dobson-Stone C, Danek A, Rampoldi L, Hardie RJ, Chalmers RM, Wood NW, Bohlega S, Dotti MT, Federico A, Shizuka M, Tanaka M, Watanabe M, Ikeda Y, Brin M, Goldfarb LG, Karp BI, Mohiddin S, Fananapazir L, Storch A, Fryer AE, Maddison P, Sibon I, Trevisol-Bittencourt PC, Singer C, Caballero IR, Aasly JO, Schmierer K, Dengler R, Hiersemenzel LP, Zeviani M, Meiner V, Lossos A, Johnson S, Mercado FC, Sorrentino G, Dupre N, Rouleau GA, Volkmann J, Arpa J, Lees A, Geraud G, Chouinard S, Nemeth A, Monaco AP. Mutational spectrum of the CHAC gene in patients with chorea-acanthocytosis. Eur J Hum Genet. 2002;10:773–781. doi: 10.1038/sj.ejhg.5200866. [DOI] [PubMed] [Google Scholar]
- Dobson-Stone C, Velayos-Baeza A, Filippone LA, Westbury S, Storch A, Erdmann T, Wroe SJ, Leenders KL, Lang AE, Dotti MT, Federico A, Mohiddin SA, Fananapazir L, Daniels G, Danek A, Monaco AP. Chorein detection for the diagnosis of chorea-acanthocytosis. Ann Neurol. 2004;56:299–302. doi: 10.1002/ana.20200. [DOI] [PubMed] [Google Scholar]
- Dobson-Stone C, Velayos-Baeza A, Jansen A, Andermann F, Dubeau F, Robert F, Summers A, Lang AE, Chouinard S, Danek A, Andermann E, Monaco AP. Identification of a VPS13A founder mutation in French Canadian families with chorea-acanthocytosis. Neurogenetics. 2005;6:151–158. doi: 10.1007/s10048-005-0220-9. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Yaghootfam A, Fewou SN, Zoller I, Gieselmann V. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem J. 2005;388:245–254. doi: 10.1042/BJ20041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan RA, Weleber RG, Hogarth P, Gregory A, Coryell J, Westaway SK, Gitschier J, Das S, Hayflick SJ. Neuro-ophthalmologic and electroretinographic findings in pantothenate kinase-associated neurodegeneration (formerly Hallervorden-Spatz syndrome) Am J Ophthalmol. 2005;140:267–274. doi: 10.1016/j.ajo.2005.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Nau TR, Dembowski C, Hasselblatt M, Barth M, Hahn A, Schilling L, Siren A-L, Bruck W. Endothelin b receptor deficiency is associated with an increased rate of neuronal apoptosis in the dentate gyrus. Neuroscience. 2000;95:993–1001. doi: 10.1016/s0306-4522(99)00507-2. [DOI] [PubMed] [Google Scholar]
- El Nemer W, Colin Y, Collec E, Gane P, Cartron JP, Kim CL. Analysis of deletions in three McLeod patients: exclusion of the XS locus from the Xp21.1-Xp21.2 region. Eur J Immunogenet. 2000;27:29–33. doi: 10.1046/j.1365-2370.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- Feinberg TE, Cianci CD, Morrow JS, Pehta JC, Redman CM, Huima T, Koroshetz WJ. Diagnostic tests for choreoacanthocytosis. Neurology. 1991;41:1000–1006. doi: 10.1212/wnl.41.7.1000. [DOI] [PubMed] [Google Scholar]
- Fleming A, Noda T, Yoshimori T, Rubinsztein DC. Chemical modulators of autophagy as biological probes and potential therapeutics. Nat Chem Biol. 2011;7:9–17. doi: 10.1038/nchembio.500. [DOI] [PubMed] [Google Scholar]
- Föller M, Hermann A, Gu S, Alesutan I, Qadri SM, Borst O, Schmidt EM, Schiele F, Müller Vom Hagen J, Saft C, Schöls L, Lerche H, Stournaras C, Storch A, Lang F. Chorein-sensitive polymerization of cortical actin and suicidal cell death in chorea-acanthocytosis. FASEB J. doi: 10.1096/fj.11-198317. in press. [DOI] [PubMed] [Google Scholar]
- Foglia A. The acanthocyte-echinocyte differential: The example of chorea-acanthocytosis. Swiss Med Wkly. 2010;16 doi: 10.4414/smw.2010.13039. [DOI] [PubMed] [Google Scholar]
- Fuller RS, De M. A direct role for Vps13p in vesicular transport from the TGN to late endosome in yeast. Proceedings of the Brain, Blood and Iron symposium. 2010;16 [Google Scholar]
- Gantenbein AR, Damon-Perrière N, Bohlender JE, Chauveau M, Latxague C, Miranda M, Jung HH, Tison F. Feeding dystonia in McLeod syndrome. Movement Disorders. 2011;26:2123–6. doi: 10.1002/mds.23843. [DOI] [PubMed] [Google Scholar]
- García-Arencibia M, Hochfeld WE, Toh PP, Rubinsztein DC. Autophagy, a guardian against neurodegeneration. Semin Cell Dev Biol. 2010;21:691–698. doi: 10.1016/j.semcdb.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier E, Guo X, Mohandas N, An X. Phosphorylation-dependent perturbations of the 4.1R-associated multiprotein complex of the erythrocyte membrane. Biochemistry. 2011;50:4561–4567. doi: 10.1021/bi200154g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MC, Tong WH, Zhang D, Ollivierre-Wilson H, Singh A, Krishna MC, Mitchell JB, Rouault TA. Tempol-mediated activation of latent iron regulatory protein activity prevents symptoms of neurodegenerative disease in IRP2 knockout mice. Proc Natl Acad Sci U S A. 2008;105:12028–12033. doi: 10.1073/pnas.0805361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef M, Nunnari J. Mitochondria regulate autophagy by conserved signalling pathways. EMBO J. 2011;30:2101–2114. doi: 10.1038/emboj.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Hayflick SJ. Genetics of neurodegeneration with brain iron accumulation. Curr Neurol Neurosci Rep. 2011;11:254–261. doi: 10.1007/s11910-011-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Polster BJ, Hayflick SJ. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet. 2009;46:73–80. doi: 10.1136/jmg.2008.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Westaway SK, Holm IE, Kotzbauer PT, Hogarth P, Sonek S, Coryell JC, Nguyen TM, Nardocci N, Zorzi G, Rodriguez D, Desguerre I, Bertini E, Simonati A, Levinson B, Dias C, Barbot C, Carrilho I, Santos M, Malik I, Gitschier J, Hayflick SJ. Neurodegeneration associated with genetic defects in phospholipase A2. Neurology. 2008;71(18):1402–9. doi: 10.1212/01.wnl.0000327094.67726.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H. Fatty acid 2-hydroxylation in mammalian sphingolipid biology. Biochim Biophys Acta. 2010;1801:405–414. doi: 10.1016/j.bbalip.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ZL, Gitlin JD. Genetic and molecular basis for copper toxicity. Am J Clin Nutr. 1996;63:836S–841S. doi: 10.1093/ajcn/63.5.836. [DOI] [PubMed] [Google Scholar]
- Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Hartig MB, Iuso A, Haack T, Kmiec T, Jurkiewicz E, Heim K, Roeber S, Tarabin V, Dusi S, Krajewska-Walasek M, Jozwiak S, Hempel M, Winkelmann J, Elstner M, Oexle K, Klopstock T, Mueller-Felber W, Gasser T, Trenkwalder C, Tiranti V, Kretzschmar H, Schmitz G, Strom TM, Meitinger T, Prokisch H. Absence of an orphan mitochondrial protein, c19orf12, causes a distinct clinical subtype of neurodegeneration with brain iron accumulation. Am J Hum Genet. 2011;89(4):543–50. doi: 10.1016/j.ajhg.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig MB, Hortnagel K, Garavaglia B, Zorzi G, Kmiec T, Klopstock T, Rostasy K, Svetel M, Kostic VS, Schuelke M, Botz E, Weindl A, Novakovic I, Nardocci N, Prokisch H, Meitinger T. Genotypic and phenotypic spectrum of PANK2 mutations in patients with neurodegeneration with brain iron accumulation. Ann Neurol. 2006;59:248–56. doi: 10.1002/ana.20771. [DOI] [PubMed] [Google Scholar]
- Hayflick SJ, Westaway SK, Levinson B, Zhou B, Johnson MA, Ching KH, Gitschier J. Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med. 2003;348:33–40. doi: 10.1056/NEJMoa020817. [DOI] [PubMed] [Google Scholar]
- Hayflick SJ. Neurodegeneration with brain iron accumulation: from genes to pathogenesis. Semin Pediatr Neurol. 2006;13:182–185. doi: 10.1016/j.spen.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Hewer E, Danek A, Schoser BG, Miranda M, Reichard R, Castiglioni C, Oechsner M, Goebel HH, Heppner FL, Jung HH. McLeod myopathy revisited: more neurogenic and less benign. Brain. 2007;130:3285–3296. doi: 10.1093/brain/awm269. [DOI] [PubMed] [Google Scholar]
- Hilditch-Maguire P, Trettel F, Passani LA, Auerbach A, Persichetti F, MacDonald ME. Huntingtin: an iron-regulated protein essential for normal nuclear and perinuclear organelles. Hum Mol Genet. 2000;9:2789–2797. doi: 10.1093/hmg/9.19.2789. [DOI] [PubMed] [Google Scholar]
- Ho M, Chelly J, Carter N, Danek A, Crocker P, Monaco AP. Isolation of the gene for McLeod syndrome that encodes a novel membrane transport protein. Cell. 1994;77:869–880. doi: 10.1016/0092-8674(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Ho MF, Chalmers RM, Davis MB, Harding AE, Monaco AP. A novel point mutation in the McLeod syndrome gene in neuroacanthocytosis. Ann Neurol. 1996;39:672–675. doi: 10.1002/ana.410390518. [DOI] [PubMed] [Google Scholar]
- Holmes SE, O'Hearn E, Rosenblatt A, Callahan C, Hwang HS, Ingersoll-Ashworth RG, Fleisher A, Stevanin G, Brice A, Potter NT, Ross CA, Margolis RL. A repeat expansion in the gene encoding junctophilin-3 is associated with Huntington disease-like 2. Nat Genet. 2001;29:377–378. doi: 10.1038/ng760. [DOI] [PubMed] [Google Scholar]
- Hortnagel K, Prokisch H, Meitinger T. An isoform of hPANK2, deficient in pantothenate kinase-associated neurodegeneration, localizes to mitochondria. Hum Mol Genet. 2003;12:321–327. doi: 10.1093/hmg/ddg026. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Omoto K, Kanaseki T, Sugi Y, Wakamatsu H, Hamaguchi K. Studies on the erythrocyte membrane skeleton in a patient with chorea-acanthocytosis - theoretical speculation on the mechanism of neurological involvement. No To Shinkei. 1992;44:739–744. [PubMed] [Google Scholar]
- Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat Rev Mol Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida C, Makifuchi T, Saiki S, Hirose G, Yamada M. A neuropathological study of autosomal-dominant chorea-acanthocytosis with a mutation of VPS13A. Acta Neuropathol. 2009;117:85–94. doi: 10.1007/s00401-008-0403-1. [DOI] [PubMed] [Google Scholar]
- Jackowski S, Garcia M, Leonardi R, Rock O. CoA metabolism and consequences of reduced CoA. Proceedings of the Brain, Blood and Iron symposium. 2010;14 [Google Scholar]
- Jacobs ME, DeSouza LV, Samaranayake H, Pearlman RE, Siu KW, Klobutcher LA. The Tetrahymena thermophila phagosome proteome. Eukaryot Cell. 2006;5:1990–2000. doi: 10.1128/EC.00195-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HH, Danek A, Dobson-Stone C, Lee S. McLeod neuroacanthocytosis syndrome. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews. University of Washington, Seattle GeneReviews; Seattle (WA): 2007. http://www.ncbi.nlm.nih.gov/books/NBK1354/ [Google Scholar]
- Jung HH, Danek A, Frey BM. McLeod syndrome: a neurohaematological disorder. Vox Sang. 2007;93:112–121. doi: 10.1111/j.1423-0410.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- Kaplan J, Ward DM, De Domenico I. The molecular basis of iron overload disorders and iron-linked anemias. Int J Hematol. 2011;93:14–20. doi: 10.1007/s12185-010-0760-0. [DOI] [PubMed] [Google Scholar]
- Kay MM, Bosman GJ, Lawrence C. Functional topography of band 3: specific structural alteration linked to functional aberrations in human erythrocytes. Proc Natl Acad Sci U S A. 1988;85:492–496. doi: 10.1073/pnas.85.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthivasan G, Small S, Liu H, Wickrema A, Crispino JD. Vesicle trafficking plays a novel role in erythroblast enucleation. Blood. 2010;116:3331–3340. doi: 10.1182/blood-2010-03-277426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauther KM, Höft C, Rissling I, Oertel WH, Möller JC. The PLA2G6 gene in early-onset Parkinson's disease. Movement Disorders. Mov Disord. 2011;26:2415–7. doi: 10.1002/mds.23851. [DOI] [PubMed] [Google Scholar]
- Klopstock T, Elstner M, Malandrini A. Acanthocytes in pantothenate kinase associated neurodegeneration. In: Danek A, editor. Neuroacanthocytosis Syndromes. Springer; Heidelberg, Germany: 2004. pp. 67–70. [Google Scholar]
- Kolehmainen J, Black GC, Saarinen A, Chandler K, Clayton-Smith J, Traskelin AL, Perveen R, Kivitie-Kallio S, Norio R, Warburg M, Fryns JP, de la Chapelle A, Lehesjoki AE. Cohen syndrome is caused by mutations in a novel gene, COH1, encoding a transmembrane protein with a presumed role in vesicle-mediated sorting and intracellular protein transport. Am J Hum Genet. 2003;72:1359–1369. doi: 10.1086/375454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S. Terminal axon pathology in infantile neuroaxonal dystrophy. Pediatr Neurol. 1991;7:116–120. doi: 10.1016/0887-8994(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Koga H, Cuervo AM. Chaperone-mediated autophagy dysfunction in the pathogenesis of neurodegeneration. Neurobiol Dis. 2011;43:29–37. doi: 10.1016/j.nbd.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono S, Miyajima H. Molecular and pathological basis of aceruloplasminemia. Biol Res. 2006;39:15–23. doi: 10.4067/s0716-97602006000100003. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Truax AC, Trojanowski JQ, Lee VM. Altered neuronal mitochondrial coenzyme A synthesis in neurodegeneration with brain iron accumulation caused by abnormal processing, stability, and catalytic activity of mutant pantothenate kinase 2. J Neurosci. 2005;25:689–698. doi: 10.1523/JNEUROSCI.4265-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A, Temlett J, Van der Meyden K, Ross CA, Callahan C, Margolis RL. CAG/CTG repeat expansions at the HDL2 locus are a common cause of Huntington disease in black South Africans. Am J Hum Genet. 2002;71:528S. [Google Scholar]
- Kruer MC, Boddaert N, Schneider SA, Houlden H, Bhatia KP, Gregory A, Anderson JC, Rooney WD, Hogarth P, Hayflick SJ. Neuroimaging features of neurodegeneration with brain iron accumulation. AJNR Am J Neuroradiol. doi: 10.3174/ajnr.A2677. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruer MC, Hiken M, Gregory A, Malandrini A, Clark D, Hogarth P, et al. Novel histopathologic findings in molecularty-confirmed pantothenate kinase-associated neurodegeneration. Brain. 2011;134:947–958. doi: 10.1093/brain/awr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruer MC, Paisan-Ruiz C, Boddaert N, Yoon MY, Hama H, Gregory A, et al. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA) Ann Neurol. 2010;68:611–618. doi: 10.1002/ana.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Duncan JL, Westaway SK, Yang H, Nune G, Xu EY, et al. Deficiency of pantothenate kinase 2 (Pank2) in mice leads to retinal degeneration and azoospermia. Hum Mol Genet. 2005;14:49–57. doi: 10.1093/hmg/ddi005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurano Y, Nakamura M, Ichiba M, Matsuda M, Mizuno E, Kato M, Izumo S, Sano A. Chorein deficiency leads to upregulation of gephyrin and GABAA receptor. Biochem Biophys Res Commun. 2006;351:438–442. doi: 10.1016/j.bbrc.2006.10.070. [DOI] [PubMed] [Google Scholar]
- Kurano Y, Nakamura M, Ichiba M, Matsuda M, Mizuno E, Kato M, Agemura A, Izumo S, Sano A. In vivo distribution and localization of chorein. Biochem Biophys Res Commun. 2007;353:431–435. doi: 10.1016/j.bbrc.2006.12.059. [DOI] [PubMed] [Google Scholar]
- Kuypers FA, van Linde-Sibenius Trip M, Roelofsen B, Op den Kamp JA, Tanner MJ, Anstee DJ. The phospholipid organisation in the membranes of McLeod and Leach phenotype erythrocytes. FEBS Lett. 1985;184:20–24. doi: 10.1016/0014-5793(85)80644-x. [DOI] [PubMed] [Google Scholar]
- Ladd PD, Smith LE, Rabaia NA, Moore JM, Georges SA, Hansen RS, Hagerman RJ, Tassone F, Tapscott SJ, Filippova GN. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet. 2007 Dec 15;16(24):3174–87. doi: 10.1093/hmg/ddm293. [DOI] [PubMed] [Google Scholar]
- LaVaute T, Smith S, Cooperman S, Iwai K, Land W, Meyron-Holtz E, Drake SK, Miller G, Abu-Asab M, Tsokos M, Switzer R, 3rd, Grinberg A, Love P, Tresser N, Rouault TA. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet. 2001;27:209–214. doi: 10.1038/84859. [DOI] [PubMed] [Google Scholar]
- Lee S. Molecular basis of Kell blood group phenotypes. Vox Sang. 1997;73:1–11. doi: 10.1046/j.1423-0410.1997.7310001.x. [DOI] [PubMed] [Google Scholar]
- Lee S. The value of DNA analysis for antigens of the Kell and Kx blood group systems. Transfusion. 2007;47(Suppl. S1):32S–39S. doi: 10.1111/j.1537-2995.2007.01308.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Lin M, Mele A, Cao Y, Farmar J, Russo D, Redman C. Proteolytic processing of big endothelin-3 by the Kell blood group protein. Blood. 1999;94:1440–1450. [PubMed] [Google Scholar]
- Lee S, Russo D, Redman C. The Kell blood group system: Kell and XK membrane proteins. Semin Hematol. 2000;37:113–121. doi: 10.1016/s0037-1963(00)90036-2. [DOI] [PubMed] [Google Scholar]
- Lee S, Russo DC, Reiner AP, Lee JH, Sy MY, Telen MJ, Judd WJ, Simon P, Rodrigues MJ, Chabert T, Poole J, Jovanovic-Srzentic S, Levene C, Yahalom V, Redman CM. Molecular defects underlying the Kell null phenotype. J Biol Chem. 2001;276:27281–27289. doi: 10.1074/jbc.M103433200. [DOI] [PubMed] [Google Scholar]
- Lee S, Sha Q, Wu X, Calenda G, Peng J. Expression Profiles of Mouse Kell, XK, and XPLAC mRNA. J Histochem Cytochem. 2007;55:365–377. doi: 10.1369/jhc.6A7126.2006. [DOI] [PubMed] [Google Scholar]
- Lee S, Zambas ED, Marsh WL, Redman CM. Molecular cloning and primary structure of Kell blood group protein. Proc Natl Acad Sci USA. 1991;88:6353–6357. doi: 10.1073/pnas.88.14.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi R, Zhang YM, Rock CO, Jackowski S. Coenzyme A: back in action. Prog Lipid Res. 2005;44:125–53. doi: 10.1016/j.plipres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Leonardi R, Rock CO, Jackowski S, Zhang YM. Activation of human mitochondrial pantothenate kinase 2 by palmitoylcarnitine. Proc Natl Acad Sci U S A. 2007a;104:1494–9. doi: 10.1073/pnas.0607621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi R, Zhang YM, Lykidis A, Rock CO, Jackowski S. Localization and regulation of mouse pantothenate kinase 2. FEBS Lett. 2007b;581:4639–44. doi: 10.1016/j.febslet.2007.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi R, Rehg JE, Rock CO, Jackowski S. Pantothenate kinase 1 is required to support the metabolic transition from the fed to the fasted state. PLoS One. 2010a;5:e11107. doi: 10.1371/journal.pone.0011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi R, Zhang YM, Yun MK, Zhou R, Zeng FY, Lin W, et al. Modulation of pantothenate kinase 3 activity by small molecules that interact with the substrate/allosteric regulatory domain. Chem Biol. 2010b;17:892–902. doi: 10.1016/j.chembiol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Lindberg J, Lundeberg J. The plasticity of the mammalian transcriptome. Genomics. 2010 Jan;95(1):1–6. doi: 10.1016/j.ygeno.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Liu J, Guo X, Mohandas N, Chasis JA, An X. Membrane remodeling during reticulocyte maturation. Blood. 2010;115:2021–2027. doi: 10.1182/blood-2009-08-241182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HM, Larson M, Mizuno Y. An analysis of the ultrastructural findings in infantile neuroaxonal dystrophy (Seitelberger's disease) Acta Neuropathol. 1974;27:201–213. doi: 10.1007/BF00687630. [DOI] [PubMed] [Google Scholar]
- Lumsden AL, Henshall TL, Dayan S, Lardelli MT, Richards RI. Huntingtin-deficient zebrafish exhibit defects in iron utilization and development. Hum Mol Genet. 2007;16:1905–1920. doi: 10.1093/hmg/ddm138. [DOI] [PubMed] [Google Scholar]
- Luscieti S, Santambrogio P, Langlois d'Estaintot B, Granier T, Cozzi A, Poli M, et al. Mutant ferritin L-chains that cause neurodegeneration act in a dominant-negative manner to reduce ferritin iron incorporation. J Biol Chem. 2010;285:11948–11957. doi: 10.1074/jbc.M109.096404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZA. Alteration of autophagy in the brain of the INAD animal model. Proceedings of the Brain, Blood and Iron symposium. 2010;15 [Google Scholar]
- Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik I, Turk J, Mancuso DJ, Montier L, Wohltmann M, Wozniak DF, et al. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am J Pathol. 2008;172:406–416. doi: 10.2353/ajpath.2008.070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso M, Davidzon G, Kurlan RM, Tawil R, Bonilla E, Di Mauro S, et al. Hereditary ferritinopathy: a novel mutation, its cellular pathology, and pathogenetic insights. J Neuropathol Exp Neurol. 2005;64:280–294. doi: 10.1093/jnen/64.4.280. [DOI] [PubMed] [Google Scholar]
- Manno S, Takakuwa Y, Mohandas N. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc Natl Acad Sci U S A. 2002;99:1943–1948. doi: 10.1073/pnas.042688399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RL, O'Hearn E, Rosenblatt A, Willour V, Holmes SE, Franz ML, Callahan C, Hwang HS, Troncoso JC, Ross CA. A disorder similar to Huntington's disease is associated with a novel CAG repeat expansion. Ann Neurol. 2001;50:373–380. doi: 10.1002/ana.1312. [DOI] [PubMed] [Google Scholar]
- Margolis RL, Holmes SE, Rosenblatt A, Gourley L, O'Hearn E, Ross CA, Seltzer WK, Walker RH, Ashizawa T, Rasmussen A, Hayden M, Almqvist EW, Harris J, Fahn S, MacDonald ME, Mysore J, Shimohata T, Tsuji S, Potter N, Nakaso K, Adachi Y, Nakashima K, Bird T, Krause A, Greenstein P. Huntington's Disease-like 2 (HDL2) in North America and Japan. Ann Neurol. 2004;56:670–674. doi: 10.1002/ana.20248. [DOI] [PubMed] [Google Scholar]
- Margolis RL, Holmes SE, Rudnicki DD, O'Hearn E, Ross CA, Pletnikova O, Troncoso JC. Huntington's disease-like 2. In: Wells R, Ashizawa T, editors. Genetic Instabilities and Neurological Diseases. Academic Press; San Diego: 2006. pp. 261–273. [Google Scholar]
- Margolis RL, Rudnicki DD. Huntington's Disease-Like 2. In: Walker RH, Saiki S, Danek A, editors. Neuroacanthocytosis Syndromes II. Springer-Verlag; Berlin Heidelberg, Germany: 2008. pp. 59–73. [Google Scholar]
- Margolis RL. Huntington Disease-Like 2. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews. University of Washington; Seattle (WA): 2004. updated 2009. [Google Scholar]
- Mari M, Griffith J, Rieter E, Krishnappa L, Klionsky DJ, Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 2010;190:1005–1022. doi: 10.1083/jcb.200912089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño G, Madeo F, Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Curr Opin Cell Biol. 2011;23:198–206. doi: 10.1016/j.ceb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Marsh WL, Marsh NJ, Moore A, Symmans WA, Johnson CL, Redman CM. Elevated serum creatine phosphokinase in subjects with McLeod syndrome. Vox Sang. 1981;40:403–411. doi: 10.1111/j.1423-0410.1981.tb00728.x. [DOI] [PubMed] [Google Scholar]
- McNeill A, Birchall D, Hayflick SJ, Gregory A, Schenk JF, Zimmerman EA, Shang H, Miyajima H, Chinnery PF. T2* and FSE MRI distinguishes four subtypes of neurodegeneration with brain iron accumulation. Neurology. 2008a;70:1614–1619. doi: 10.1212/01.wnl.0000310985.40011.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Pandolfo M, Kuhn J, Shang H, Miyajima H. The neurological presentation of ceruloplasmin gene mutations. Eur Neurol. 2008b;60:200–205. doi: 10.1159/000148691. [DOI] [PubMed] [Google Scholar]
- McGough IJ, Cullen PJ. Recent Advances in Retromer Biology. Traffic. 2011;12(8):963–71. doi: 10.1111/j.1600-0854.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- McNeill A, Chinnery PF. Neurodegeneration with brain iron accumulation. Handb Clin Neurol. 2011;100:161–172. doi: 10.1016/B978-0-444-52014-2.00009-4. [DOI] [PubMed] [Google Scholar]
- Melone MA, Di Fede G, Peluso G, Lus G, Di Iorio G, Sampaolo S, Capasso A, Gentile V, Cotrufo R. Abnormal accumulation of tTGase products in muscle and erythrocytes of chorea-acanthocytosis patients. J Neuropathol Exp Neurol. 2002;61:841–848. doi: 10.1093/jnen/61.10.841. [DOI] [PubMed] [Google Scholar]
- Miyajima H. Aceruloplasminemia, an iron metabolic disorder. Neuropathology. 2003;23:345–350. doi: 10.1046/j.1440-1789.2003.00521.x. [DOI] [PubMed] [Google Scholar]
- Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008 Nov 15;112(10):3939–48. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006;38:752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Nishiyama M, Takeda Y, Iwai K, Nakayama KI. The FBXL5-IRP2 axis is integral to control of iron metabolism in vivo. Cell Metab. 2011;14:339–351. doi: 10.1016/j.cmet.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Moseley ML, Zu T, Ikeda Y, Gao W, Mosemiller AK, Daughters RS, Chen G, Weatherspoon MR, Clark HB, Ebner TJ, Day JW, Ranum LP. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet. 2006 Jul;38(7):758–69. doi: 10.1038/ng1827. [DOI] [PubMed] [Google Scholar]
- Ney PA. Normal and disordered reticulocyte maturation. Curr Opin Hematol. 2011;18:152–157. doi: 10.1097/MOH.0b013e328345213e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri O, De Franceschi L, Bordin L, Manfredi M, Miraglia del Giudice E, Perrotta S, De Vivo M, Guarini P, Corrocher R. Increased membrane protein phosphorylation and anion transport activity in chorea-acanthocytosis. Haematologica. 1997;82:648–653. [PubMed] [Google Scholar]
- Onda H, Ohkubo S, Ogi K, Kosaka T, Kimura C, Matsumoto H, Suzuki N, Fujino M. One of the endothelin gene family, endothelin 3 gene, is expressed in the placenta. FEBS Lett. 1990;261:327–330. doi: 10.1016/0014-5793(90)80583-5. [DOI] [PubMed] [Google Scholar]
- Osaki S. Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase (ceruloplasmin) J Biol Chem. 1966;241:5053–5059. [PubMed] [Google Scholar]
- Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746–2751. [PubMed] [Google Scholar]
- Osaki S, Johnson DA, Frieden E. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J Biol Chem. 1971;246:3018–3023. [PubMed] [Google Scholar]
- Paisan-Ruiz C, Bhatia KP, Li A, Hernandez D, Davis M, Wood NW, Hardy J, Houlden H, Singleton A, Schneider SA. Characterization of PLA2G6 as a Locus for Dystonia-Parkinsonism. Ann Neurol. 2009;65:19–23. doi: 10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Li A, Schneider SA, Holton JL, Johnson R, Kidd D, et al. Widespread Lewy body and tau accumulation in childhood and adult onset dystonia-parkinsonism cases with PLA2G6 mutations. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo A, De Franceschi L, Ferru E, Vono R, Turrini F. Current knowledge about the functional roles of phosphorylative changes of membrane proteins in normal and diseased red cells. J Proteomics. 2010;73:445–455. doi: 10.1016/j.jprot.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellecchia MT, Valente EM, Cif L, Salvi S, Albanese A, Scarano V, Bonuccelli U, Bentivoglio AR, D'Amico A, Marelli C, Di Giorgio A, Coubes P, Barone P, Dallapiccola B. The diverse phenotype and genotype of pantothenate kinase-associated neurodegeneration. Neurology. 2005;64:1810–1812. doi: 10.1212/01.WNL.0000161843.52641.EC. [DOI] [PubMed] [Google Scholar]
- Pérez-Victoria FJ, Mardones GA, Bonifacino JS. Requirement of the human GARP complex for mannose 6-phosphate-receptor-dependent sorting of cathepsin D to lysosomes. Mol Biol Cell. 2008;19:2350–2362. doi: 10.1091/mbc.E07-11-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Victoria FJ, Abascal-Palacios G, Tascón I, Kajava A, Magadán JG, Pioro EP, Bonifacino JS, Hierro A. Structural basis for the wobbler mouse neurodegenerative disorder caused by mutation in the Vps54 subunit of the GARP complex. Proc Natl Acad Sci U S A. 2010;107:12860–12865. doi: 10.1073/pnas.1004756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampoldi L, Dobson-Stone C, Rubio JP, Danek A, Chalmers RM, Wood NW, Verellen C, Ferrer X, Malandrini A, Fabrizi GM, Brown R, Vance J, Pericak-Vance M, Rudolf G, Carre S, Alonso E, Manfredi M, Nemeth AH, Monaco AP. A conserved sorting-associated protein is mutant in chorea-acanthocytosis. Nat Genet. 2001;28:119–120. doi: 10.1038/88821. [DOI] [PubMed] [Google Scholar]
- Rana A, Seinen E, Siudeja K, Muntendam R, Srinivasan B, van der Want JJ, et al. Pantethine rescues a Drosophila model for pantothenate kinase-associated neurodegeneration. Proc Natl Acad Sci U S A. 2010;107:6988–6993. doi: 10.1073/pnas.0912105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Redding K, Brickner JH, Marschall LG, Nichols JW, Fuller RS. Allele-specific suppression of a defective trans-Golgi network (TGN) localization signal in Kex2p identifies three genes involved in localization of TGN transmembrane proteins. Mol Cell Biol. 1996;16:6208–6217. doi: 10.1128/mcb.16.11.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- Rubio JP, Danek A, Stone C, Chalmers R, Wood N, Verellen C, Ferrer X, Malandrini A, Fabrizi GM, Manfredi M, Vance J, Pericak-Vance M, Brown R, Rudolf G, Picard F, Alonso E, Brin M, Németh AH, Farrall M, Monaco AP. Chorea-acanthocytosis: genetic linkage to chromosome 9q21. Am J Hum Genet. 1997;61:899–908. doi: 10.1086/514876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki DD, Holmes SE, Lin MW, Thornton CA, Ross CA, Margolis RL. Huntington's disease-like 2 is associated with CUG repeat-containing RNA foci. Ann Neurol. 2007;61:272–282. doi: 10.1002/ana.21081. [DOI] [PubMed] [Google Scholar]
- Rudnicki DD, Pletnikova O, Vonsattel JP, Ross CA, Margolis RL. A comparison of huntington disease and huntington disease-like 2 neuropathology. J Neuropathol Exp Neurol. 2008;67:366–374. doi: 10.1097/NEN.0b013e31816b4aee. PMID: 18379432. [DOI] [PubMed] [Google Scholar]
- Russo D, Redman C, Lee S. Association of XK and Kell blood group proteins. J Biol Chem. 1998;273:13950–13956. doi: 10.1074/jbc.273.22.13950. [DOI] [PubMed] [Google Scholar]
- Russo D, Wu X, Redman CM, Lee S. Expression of Kell blood group protein in nonerythroid tissues. Blood. 2000;96:340–346. [PubMed] [Google Scholar]
- Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Saksena S, Emr SD. ESCRTs and human disease. Biochem Soc Trans. 2009;37:167–172. doi: 10.1042/BST0370167. [DOI] [PubMed] [Google Scholar]
- Salomao M, Chen K, Villalobos J, Mohandas N, An X, Chasis JA. Hereditary spherocytosis and hereditary elliptocytosis: aberrant protein sorting during erythroblast enucleation. Blood. 2010;116:267–269. doi: 10.1182/blood-2010-02-264127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Samaranayake HS, Cowan AE, Klobutcher LA. Vacuolar Protein Sorting Protein 13A, TtVPS13A, localizes to the Tetrahymena thermophila phagosome membrane and is required for efficient phagocytosis. Eukaryot Cell. 2011;10:1207–1218. doi: 10.1128/EC.05089-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki S, Sakai K, Kitagawa Y, Saiki M, Kataoka S, Hirose G. Mutation in the CHAC gene in a family of autosomal dominant chorea-acanthocytosis. Neurology. 2003;61:1614–1616. doi: 10.1212/01.wnl.0000096172.26601.02. Correction published; Neurology. 2011, 77:701. [DOI] [PubMed] [Google Scholar]
- Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Schmitt-John T, Drepper C, Mussmann A, Hahn P, Kuhlmann M, Thiel C, Hafner M, Lengeling A, Heimann P, Jones JM, Meisler MH, Jockusch H. Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat Genet. 2005;37:1213–1215. doi: 10.1038/ng1661. [DOI] [PubMed] [Google Scholar]
- Schneider R, Brors B, Massow M, Weiss H. Mitochondrial fatty acid synthesis: a relic of endosymbiontic origin and a specialized means for respiration. FEBS Lett. 1997;407:249–252. doi: 10.1016/s0014-5793(97)00360-8. [DOI] [PubMed] [Google Scholar]
- Schneider R, Massow M, Lisowsky T, Weiss H. Different respiratory-defective phenotypes of Neurospora crassa and Saccharomyces cerevisiae after inactivation of the gene encoding the mitochondrial acyl carrier protein. Curr Genet. 1995;29:10–17. doi: 10.1007/BF00313188. [DOI] [PubMed] [Google Scholar]
- Seixas AI, Holmes SE, Takeshima H, Pavlovich A, Sachs N, Pruitt JL, Silveira I, Ross CA, Margolis RL, Rudnicki DD. Loss of junctophilin-3 contributes to Huntington's disease-like 2 pathogenesis. Ann Neurol. doi: 10.1002/ana.22598. in press. [DOI] [PubMed] [Google Scholar]
- Seleznev K, Zhao C, Zhang XH, Song K, Ma ZA. Calcium-independent phospholipase A2 localizes in and protects mitochondria during apoptotic induction by staurosporine. J Biol Chem. 2006;281:22275–22288. doi: 10.1074/jbc.M604330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman JR, Wilton AN. A canine model of Cohen syndrome: Trapped Neutrophil Syndrome. BMC Genomics. 2011;12:258. doi: 10.1186/1471-2164-12-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopher BL, Ladd PD, Pineda VV, Libby RT, Sunkin SM, Hurley JB, Thienes CP, Gaasterland T, Filippova GN, La Spada AR. CTCF regulates ataxin-7 expression through promotion of a convergently transcribed, antisense noncoding RNA. Neuron. 2011 Jun 23;70(6):1071–84. doi: 10.1016/j.neuron.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino G, De Renzo A, Miniello S, Nori O, Bonavita V. Late appearance of acanthocytes during the course of chorea-acanthocytosis. J Neurol Sci. 1999;163:175–178. doi: 10.1016/s0022-510x(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Stanfield GM, Horvitz HR. The ced-8 gene controls the timing of programmed cell deaths in C. elegans. Mol Cell. 2000;5:423–433. doi: 10.1016/s1097-2765(00)80437-2. [DOI] [PubMed] [Google Scholar]
- Storch A, Kornhass M, Schwarz J. Testing for acanthocytosis: A prospective reader-blinded study in movement disorder patients. J Neurol. 2005;252:84–90. doi: 10.1007/s00415-005-0616-3. [DOI] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Sullivan CP, Jay AG, Stack EC, Pakaluk M, Wadlinger E, Fine RE, Wells JM, Morin PJ. Retromer disruption promotes amyloidogenic APP processing. Neurobiology of Disease. 2011;430:338–45. doi: 10.1016/j.nbd.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Fujii Y, Ueda H, Kato Y, Baba T, Hayashi R, Ohno S. Ultrastructural changes of erythrocyte membrane skeletons in chorea- acanthocytosis and McLeod syndrome revealed by the quick-freezing and deep-etching method. Acta Haematol. 1999;101:25–31. doi: 10.1159/000040917. [DOI] [PubMed] [Google Scholar]
- Texel SJ, Xu X, Harris ZL. Ceruloplasmin in neurodegenerative diseases. Biochem Soc Trans. 2008;36:1277–1281. doi: 10.1042/BST0361277. [DOI] [PubMed] [Google Scholar]
- Tomiyasu A, Nakamura M, Ichiba M, Ueno S, Saiki S, Morimoto M, Kobal J, Kageyama Y, Inui T, Wakabayashi K, Yamada T, Kanemori Y, Jung HH, Tanaka H, Orimo S, Afawi Z, Blatt I, Aasly J, Ujike H, Babovic-Vuksanovic D, Josephs KA, Tohge R, Rodrigues GR, Dupré N, Yamada H, Yokochi F, Kotschet K, Takei T, Rudzińska M, Szczudlik A, Penco S, Fujiwara M, Tojo K, Sano A. Novel pathogenic mutations and copy number variations in the VPS13A gene in patients with chorea-acanthocytosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156(5):620–31. doi: 10.1002/ajmg.b.31206. [DOI] [PubMed] [Google Scholar]
- Tomemori Y, Ichiba M, Kusumoto A, Mizuno E, Sato D, Muroya S, Nakamura M, Kawaguchi H, Yoshida H, Ueno S, Nakao K, Nakamura K, Aiba A, Katsuki M, Sano A. A gene-targeted mouse model for chorea-acanthocytosis. J Neurochem. 2005;92:759–766. doi: 10.1111/j.1471-4159.2004.02924.x. [DOI] [PubMed] [Google Scholar]
- Ueno S, Maruki Y, Nakamura M, Tomemori Y, Kamae K, Tanabe H, Yamashita Y, Matsuda S, Kaneko S, Sano A. The gene encoding a newly discovered protein, chorein, is mutated in chorea-acanthocytosis. Nat Genet. 2001;28:121–122. doi: 10.1038/88825. [DOI] [PubMed] [Google Scholar]
- Umemori H, Ogura H, Tozawa N, Mikoshiba K, Nishizumi H, Yamamoto T. Impairment of N-methyl-D-aspartate receptor-controlled motor activity in LYN-deficient mice. Neuroscience. 2003;118:709–713. doi: 10.1016/s0306-4522(03)00025-3. [DOI] [PubMed] [Google Scholar]
- Urwin H, Authier A, Nielsen JE, Metcalf D, Powell C, Froud K, Malcolm DS, Holm I, Johannsen P, Brown J, Fisher EM, van der Zee J, Bruyland M, FReJA Consortium. Van Broeckhoven C, Collinge J, Brandner S, Futter C, Isaacs AM. Disruption of endocytic trafficking in frontotemporal dementia with CHMP2B mutations. Hum Mol Genet. 2010;19:2228–38. doi: 10.1093/hmg/ddq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginkel G, Sevanian A. Lipid peroxidation-induced membrane structural alterations. Methods Enzymol. 1994;233:273–288. doi: 10.1016/s0076-6879(94)33031-x. [DOI] [PubMed] [Google Scholar]
- Vassiliev V, Harris ZL, Zatta P. Ceruloplasmin in neurodegenerative diseases. Brain Res Brain Res Rev. 2005;49:633–640. doi: 10.1016/j.brainresrev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Velayos-Baeza A, Vettori A, Copley RR, Dobson-Stone C, Monaco AP. Analysis of the human VPS13 gene family. Genomics. 2004;84:536–549. doi: 10.1016/j.ygeno.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Velayos-Baeza A, Levecque C, Dobson-Stone C, Monaco AP. The Function of Chorein. In: Walker RH, Saiki S, Danek A, editors. Neuroacanthocytosis Syndromes II. Springer-Verlag; Berlin Heidelberg, Germany: 2008. pp. 87–105. [Google Scholar]
- Vidal R, Ghetti B, Takao M, Brefel-Courbon C, Uro-Coste E, Glazier BS, et al. Intracellular ferritin accumulation in neural and extraneural tissue characterizes a neurodegenerative disease associated with a mutation in the ferritin light polypeptide gene. J Neuropathol Exp Neurol. 2004;63:363–680. doi: 10.1093/jnen/63.4.363. [DOI] [PubMed] [Google Scholar]
- Vidal R, Miravalle L, Gao X, Barbeito AG, Baraibar MA, Hekmatyar SK, et al. Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci. 2008;28:60–67. doi: 10.1523/JNEUROSCI.3962-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño-Güell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, Behrouz B, Melrose HL, Hentati E, Puschmann A, Evans DM, Conibear E, Wasserman WW, Aasly JO, Burkhard PR, Djaldetti R, Ghika J, Hentati F, Krygowska-Wajs A, Lynch T, Melamed E, Rajput A, Rajput AH, Solida A, Wu RM, Uitti RJ, Wszolek ZK, Vingerhoets F, Farrer MJ. VPS35 Mutations in Parkinson Disease. The American Journal of Human Genetics. 2011;89(1):162–7. doi: 10.1016/j.ajhg.2011.06.001. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Yasuda T, Miura I, Watabe K, Sawa C, Kamijuku H, Kojo S, Taniguchi M, Nishino I, Wakana S, Yoshida H, Seino K. Establishment of an improved mouse model for infantile neuroaxonal dystrophy that shows early disease onset and bears a point mutation in Pla2g6. Am J Pathol. 2009;175(6):2257–63. doi: 10.2353/ajpath.2009.090343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RH, Jung HH, Danek A. Neuroacanthocytosis. In: Wiener WJ, Tolosa E, editors. Handbook of Clinical Neurology, Vol 100 (3rd series) Hyperkinetic Disorders. pub. Elsevier; Amsterdam: 2011a. pp. 141–151. Chapter 7. [DOI] [PubMed] [Google Scholar]
- Walker RH, Morgello S, Davidoff-Feldman B, Melnick A, Walsh MJ, Shashidharan P, Brin MF. Autosomal dominant chorea-acanthocytosis with polyglutamine-containing neuronal inclusions. Neurology. 2002;58:1031–1037. doi: 10.1212/wnl.58.7.1031. [DOI] [PubMed] [Google Scholar]
- Walker RH, Rasmussen A, Rudnicki D, Holmes SE, Alonso E, Matsuura T, Ashizawa T, Davidoff-Feldman B, Margolis RL. Huntington's disease-like 2 can present as chorea-acanthocytosis. Neurology. 2003;61:1002–1004. doi: 10.1212/01.wnl.0000085866.68470.6d. [DOI] [PubMed] [Google Scholar]
- Walker RH, Saiki S, Danek A, editors. Neuroacanthocytosis Syndromes II. Springer-Verlag; Berlin Heidelberg: 2008. [Google Scholar]
- Walker RH, Schulz VP, Tikhonova IR, Mahajan MC, Mane S, Arroyo Muniz M, Gallagher PG. Genetic diagnosis of neuroacanthocytosis disorders using exome sequencing. Mov Disord. 2011b doi: 10.1002/mds.24020. in press. [DOI] [PubMed] [Google Scholar]
- Wilburn B, Rudnicki DD, Zhao J, Weitz TM, Cheng Y, Gu X, Greiner E, Park CS, Wang N, Sopher BL, La Spada AR, Osmand A, Margolis RL, Sun YE, Yang XW. An Antisense CAG Repeat Transcript at JPH3 Locus Mediates Expanded Polyglutamine Protein Toxicity in Huntington's Disease-like 2 Mice. Neuron. 2011;70:427–440. doi: 10.1016/j.neuron.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SD, Gottlieb RA. Inhibition of mitochondrial calcium-independent phospholipase A2 (iPLA2) attenuates mitochondrial phospholipid loss and is cardioprotective. Biochem J. 2002;362:23–32. doi: 10.1042/0264-6021:3620023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimer BM, Marsh WL, Taswell HF, Galey WR. Haematological changes associated with the McLeod phenotype of the Kell blood group system. British Journal of Haematology. 1977;36:219–224. doi: 10.1111/j.1365-2141.1977.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A(2): structure and function. Biochim Biophys Acta. 2000;1488:28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Li C, Lv S, Zhou B. Pantothenate kinase-associated neurodegeneration: insights from a Drosophila model. Hum Mol Genet. 2009;18:3659–3672. doi: 10.1093/hmg/ddp314. [DOI] [PubMed] [Google Scholar]
- Xu X, Pin S, Gathinji M, Fuchs R, Harris ZL. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Ann N Y Acad Sci. 2004;1012:299–305. doi: 10.1196/annals.1306.024. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Simonsen A. The elimination of accumulated and aggregated proteins: A role for aggrephagy in neurodegeneration. Neurobiol Dis. 2011;43:17–28. doi: 10.1016/j.nbd.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Li L, Kuo YF, Hung YS, Yu LC, Hung CS, Tsai SJ, Lin KS, Chu DC. Genetic and functional analyses describe a novel 730delG mutation in the KEL gene causing K(0) phenotype in a Taiwanese blood donor. Transfus Med. 2011 doi: 10.1111/j.1365-3148.2011.01084.x. in press. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kaneko K, Miyajima H, Tokuda T, Nakamura A, Kato M, et al. Increased lipid peroxidation in the brains of aceruloplasminemia patients. J Neurol Sci. 2000;175:91–95. doi: 10.1016/s0022-510x(00)00295-1. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Tomiyama H, Tachibana N, Ogaki K, Li Y, Funayama M, Hashimoto T, Takashima S, Hattori N. Phenotypic spectrum of patients with PLA2G6 mutation and PARK14-linked parkinsonism. Neurology. 2010;75:1356–61. doi: 10.1212/WNL.0b013e3181f73649. [DOI] [PubMed] [Google Scholar]
- Yu LC, Twu YC, Chang CY, Lin M. Molecular basis of the Kell-null phenotype: a mutation at the splice site of human KEL gene abolishes the expression of Kell blood group antigens. J Biol Chem. 2001;276:10247–10252. doi: 10.1074/jbc.M009879200. [DOI] [PubMed] [Google Scholar]
- Yue Z, Friedman L, Komatsu M, Tanaka K. The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases. Biochim Biophys Acta. 2009;1793:1496–1507. doi: 10.1016/j.bbamcr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zhang DL, Senecal T, Ghosh MC, Ollivierre-Wilson H, Tu T, Rouault TA. Hepcidin regulates ferroportin expression and intracellular iron homeostasis of erythroblasts. Blood. 2011;118:2868–2877. doi: 10.1182/blood-2011-01-330241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Randall MS, Loyd MR, Dorsey FC, Kundu M, Cleveland JL, Ney PA. Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood. 2009;114:157–164. doi: 10.1182/blood-2008-04-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Rock CO, Jackowski S. Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters. J Biol Chem. 2005;280:32594–32601. doi: 10.1074/jbc.M506275200. [DOI] [PubMed] [Google Scholar]
- Zhang YM, Rock CO, Jackowski S. Biochemical properties of human pantothenate kinase 2 isoforms and mutations linked to pantothenate kinase-associated neurodegeneration. J Biol Chem. 2006;281:107–114. doi: 10.1074/jbc.M508825200. [DOI] [PubMed] [Google Scholar]
- Zhang YM, Chohnan S, Virga KG, Stevens RD, Ilkayeva OR, Wenner BR, et al. Chemical knockout of pantothenate kinase reveals the metabolic and genetic program responsible for hepatic coenzyme A homeostasis. Chem Biol. 2007;14:291–302. doi: 10.1016/j.chembiol.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhang X, Zhao C, Choi J, Shi J, Song K, Turk J, Ma ZA. Protection of pancreatic beta-cells by group VIA phospholipase A(2)-mediated repair of mitochondrial membrane peroxidation. Endocrinology. 2010;151:3038–3048. doi: 10.1210/en.2010-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet. 2001;28:345–349. doi: 10.1038/ng572. [DOI] [PubMed] [Google Scholar]
- Zhu X, Rivera A, Golub MS, Peng J, Sha Q, Wu X, Song X, Kumarathasan P, Ho M, Redman CM, Lee S. Changes in red cell ion transport, reduced intratumoral neovascularization, and some mild motor function abnormalities accompany targeted disruption of the Mouse Kell gene (Kel) Am J Hematol. 2009;84:492–498. doi: 10.1002/ajh.21453. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Benet-Pagès A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, Reinthaler E, Harutyunyan A, Kralovics R, Peters A, Zimprich F, Brücke T, Poewe W, Auff E, Trenkwalder C, Rost B, Ransmayr G, Winkelmann J, Meitinger T, Strom TM. A Mutation in VPS35, Encoding a Subunit of the Retromer Complex, Causes Late-Onset Parkinson Disease. Am J of Hum Gen. 2011;89(1):168–75. doi: 10.1016/j.ajhg.2011.06.008. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller I, Meixner M, Hartmann D, Bussow H, Meyer R, Gieselmann V, et al. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J Neurosci. 2008;28:9741–9754. doi: 10.1523/JNEUROSCI.0458-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]