Abstract
Methylphenidate (MP) is a psychostimulant widely prescribed to treat Attention Deficit Hyperactivity Disorder (ADHD). Although generally well tolerated, growth deficits have been reported in children and adolescents undergoing MP treatment. This study was designed to elucidate the skeletal effects of chronic MP administration in adolescent rats. Male, 4-week-old rats received one of two doses of MP (MP-Low or MP-High) delivered for 8 hours a day via drinking water, or were untreated (water only). After 13 weeks, half were sacrificed (N=12/group) and the remaining rats were left to recover, untreated for 5 additional weeks. Femora, tibiae, and L5 vertebra were analyzed using calipers, DXA, and mechanical testing. Immediately following treatment, MP decreased femoral anterior-posterior diameter (5% and 9% for MP-Low and MP-High, respectively), femoral and tibial Bone Mineral Density (BMD) (6% and 5% for MP-High femora & tibiae, respectively), and Bone Mineral Content (BMC) (9% for MP-High femora and tibiae). In addition, femora from MP treated rats had reduced ultimate force (20% for MP-High) and energy to failure (20% and 33% for MP-Low and MP-High, respectively). However, after recovery, there were no statistically significant differences for any measured parameters. Despite these effects on the appendicular skeleton, no differences were identified between vertebral samples at either time-point. In summary, MP treatment resulted in smaller, less mineralized, and weaker bones at appendicular sites, but did not affect the axial site. Although these effects were ameliorated within 5 weeks, these data suggest that adolescents undergoing MP treatment may be at an increased risk for long bone fractures.
Keywords: methylphenidate, growth suppression, biomechanics, DXA, testosterone
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a neuropsychiatric disorder characterized by inattentiveness, impulsivity, and hyperactivity. It is most commonly diagnosed in children, with a prevalence of 6-9% in the United States, but also affects adolescents and adults [1]. The psychosocial problems associated with ADHD are profound and wide-ranging. For example, the effects of ADHD on school performance have been well studied and show strong associations between ADHD with lower grades and test scores, higher grade retention, increases in detention and expulsion, and reduced graduation [2] Outside of the school setting, ADHD is associated with higher levels of family conflict [3] and sleep disorders [4]. However, the most profound problems include increased risk for other psychiatric diagnoses, substance abuse, criminality, and suicide [1,5,6].
A wide variety of treatments have been utilized for patients with ADHD that can be broadly grouped into pharmacotherapy (stimulants), psychotherapy, and alternative therapy. In spite of widespread use (up to 64%), no alternative therapies have been demonstrated to effectively treat ADHD [7]. In contrast, some forms of psychotherapy have shown efficacy in alleviating specific ADHD symptoms, with the greatest benefits seen for targeted behavioral therapy [8]. However, the use of pharmacotherapy, alone or in combination with behavioral therapy, remains the most successful treatment for ADHD [1, 9].
Pharmacotherapy utilizes several classes of drugs [10] but the vast majority of patients with ADHD are treated with stimulants (i.e., methylphenidate [MP] and amphetamine). MP accounts for most of the prescriptions in children and adolescents [11]. While MP is generally well-tolerated and is clinically effective in 65-75% of patients, there is concern regarding potential growth deficits in children and adolescents undergoing chronic MP treatment [12].
One of the first studies reporting on the effects of MP on adolescent height found growth suppression in percentile height in patients treated with MP or dextroamphetamine when compared to non-medicated controls and that with summer holidays some growth recovery was observed [13]. More recently, in the pivotal Multimodal Treatment Study of ADHD (MTA), 7-9 year old children treated with MP exhibited growth suppression of 0.9 cm/yr over the 14-month study duration, with an additional 1.04 cm/yr in growth suppression during the 10-month follow-up phase in patients who continued medication [14]. At the 36-month follow-up, naturalistic subgroups of cases ‘Always’ or ‘Never’ treated revealed that stimulant-related growth suppression reached an apparent asymptote of about 2 cm. Even stronger suppression of growth was seen in the Preschool ADHD Treatment Study (PATS) where growth was suppressed by 1.38 cm/yr in 4-5 year old children [15]. However, as other studies have found no evidence that MP impairs adolescent growth [16], or failed to identify differences in the heights of adults previously treated with MP [17], the clinical relevance of MP induced growth suppression remains under debate.
In order to explore more precisely effects of MP on skeletal growth several studies have been performed using animal models. In one of the first such studies, treatment of neonatal and juvenile rats with MP resulted in dose dependant decreases in both body weight and length [18]. As MP is known to be anorexigenic, a follow-up experiment was performed in which untreated rats were pair-fed with MP treated rats. While pair-feeding reduced the body weights and lengths of untreated rats to levels indistinguishable to those of MP treated rats, skeletal growth rates remained lower in MP treated rats, suggesting that reduced food intake is not the sole cause of MP induced growth suppression [18]. The persistence of MP induced growth suppression following treatment cessation has also been evaluated. In a pair of studies, treatment of neonatal rats with MP reduced body weight and femoral length when assessed immediately post-treatment [19,20]. However, when assessed after a 30-day recovery period, these differences were no longer present; leading the authors to conclude that MP induced growth suppression is an acute problem that is compensated for by a ‘rebound’ in growth following MP cessation.
Although these clinical and animal data offer compelling evidence that the effects of MP on gross skeletal development may be limited in magnitude and duration, its effects on several specific and clinically more important measures of bone quality such as bone mineral density and biomechanical integrity have yet to be rigorously assessed. Given that the MP prescription rate has doubled in the US within the last decade with ~5% of children and adolescents now taking this drug [1], thorough elucidation of its effects on specific aspects of bone growth and quality is warranted. This study was designed to address this issue by testing the hypothesis that chronic MP administration to adolescent rats impairs not only bone size, but also bone mineral density, bone mineral content, and biomechanical integrity, and that these parameters are rapidly normalized following treatment cessation.
Material and Methods
Animal Study
A total of 72 male, four-week-old, Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) were obtained and individually housed at the Brookhaven National Laboratory Animal Facility with ad libitum access to food. Water was provided ad libitum prior to study initiation and following cessation of treatment. During the study water was used to deliver treatment, as described later. Lighting was maintained in a reverse 12-hour light/dark cycle, temperature was kept at 22 ± 2°C, and humidity was constant at 50 ± 10% relative humidity.
The rats were randomly divided into 3 treatment groups (n=24/group): 1) Water, a control group that received water with no MP; 2) MP-Low, a low dosage group, and; 3) MP-High, a high dose group. Rats in the Water group were allowed access to water for 8 hours each day. Rats in the MP treatment groups were administered solutions of methylphenidate hydrochloride (Sigma, St. Louis, MO) dissolved in distilled water through a recently established dual bottle 8-hour limited access protocol. This protocol involved providing water with a lower concentration of MP for the first hour of consumption (4mg/kg for MP-Low and 30mg/kg for MP-High) followed by a higher concentration for the remaining 7 hours (10mg/kg for MP-Low and 60mg/kg for MP-High). The rats had no access to fluids for the remaining 16 hours/day to ensure that they consumed the entire dose each day. This dosing protocol has been shown to result in a MP pharmacokinetic profile similar to that observed in children treated with MP [21]. Food intake and body weight were measured daily at 10am and fresh MP solutions prepared.
Following 13 weeks of treatment, half of the rats in each treatment group (N=12/group) were euthanized (Standard Protocol). Blood was collected via cardiac puncture and centrifuged to obtain serum, which was stored at −80°C until analysis. Right tibiae, left femora, and L5 vertebrae were dissected free of soft tissue, wrapped in saline soaked gauze and stored at −20°C until analysis. The other half of the rats were allowed to recover from MP treatment with ad libitum access to untreated water for 5 weeks, at which time they were euthanized (Recovery Protocol) and blood and tissue samples were collected as previously described.
All animal experiments were conducted in accordance with the National Academy of Sciences Guide for Care and Use of Laboratory Animals and were approved by the Brookhaven National Laboratory Institutional Animal Care and Use Committee prior to study initiation.
Caliper Measurements
In order to assess gross bone size, left femora and L5 vertebrae from all rats were measured with digital calipers (Mitutoyo, Aurora, IL). For the femora, length was measured by positioning the calipers on the medial-lateral (ML) axis with one end on the femoral head and the other end across the femoral condyles. Diameter was measured along the anterior-posterior (AP) and ML axes at the mid-diaphysis. For the vertebrae, height was measured by positioning the calipers across the endplates along the ML axis and diameter measurements were made across the cranial endplate along the AP and ML axes.
Dual Energy X-Ray Absorptiometry
Left femora, left tibiae, and L5 vertebrae were individually scanned by dual-energy X-ray absorptiometry (DXA) using a PIXImus II (GE-Lunar, Madison, WI) to quantify bone mineral density (BMC), bone mineral content (BMC), and area. Femora and tibiae were positioned posterior side down and scanned on the AP axis. Vertebrae were positioned caudal side down and scanned on the cranial-caudal axis. All samples were fully thawed before scanning and refrozen until biomechanical testing.
Biomechanics
Mechanical testing was performed on left femora and L5 vertebrae to determine their biomechanical properties. Prior to testing, all samples were thawed to room temperature and hydrated in phosphate buffered saline (Sigma). Femora were tested in 3-point bending by positioning them, anterior surface up, in the center of a custom designed stainless steel loading jig with an outer span of 20mm. A monotonic load to failure was applied to the anterior surface at a cross-head speed of 20mm/min, under displacement control, using an Electroforce 3200 materials testing system equipped with a 450N load cell (Bose, Eden Prairie, MN). Load and displacement data were digitally sampled at 100Hz using the WinTest software package (Bose).
The vertebral bodies were first isolated from complete L5 vertebral segments by cutting through the pedicles using a rotary cutting tool equipped with a diamond wafer blade (Dremel, Robert Bosch Tool Corp., Racine, WI). They were then tested in unconstrained uniaxial compression by positioning them, caudal surface down, between two stainless steel platens and applying a monotonic load to failure to the cranial surface at a cross-head speed of 5mm/min, using an 858 MiniBionix II equipped with a 2kN load cell (MTS, Minneapolis, MN). Load and displacement data were digitally sampled at 100Hz using the TestWorks software package (MTS).
Following testing, force versus displacement curves were plotted in Excel (Microsoft, Redmond, WA) and ultimate force, stiffness, and energy to failure were calculated using a set of custom written macros [22,23].
Serum Biomarker Analyses
Serum alkaline phosphatase (ALP) levels were quantified for all rats using a colorimetric assay. Briefly, 100μl of each sample was transferred to an optically clear 96-well plate (Fisher, Pittsburgh, PA). Next, 100μl of ALP yellow liquid ELISA substrate (Sigma) was added and the plate was incubated in the dark at 37°C for 30min. The absorbance was then read at 410nm using a Synergy 2 microplate reader (BioTek, Winooski, VT). All samples were run in triplicate and ALP activity was calculated by referencing a standard curve.
Serum carboxy-terminal collagen crosslinks (CTX) levels were assayed from 4 rats per treatment group and dosing protocol; chosen by selecting the rats with the highest, lowest, and middle two values for femoral ultimate force. Samples were analyzed using a RatLaps EIA (Immunodiagnostic Systems, Scottsdale, AZ) kit in accordance with the manufacturer’s directions. All samples were run in triplicate and CTX concentrations were calculated by referencing standard curve.
Serum testosterone from all rats was assessed using an ELISA kit (IBL, Hamburg, Germany) in accordance with the manufacturer’s protocol. Samples were run in duplicate and testosterone concentrations were calculated by referencing a standard curve.
Statistical Analyses
All data are presented as group mean ± standard error of the mean. Significant differences in body weight and food intake were assessed across time and between treatment groups using two-way ANOVA tests. When significant differences were found, pairwise comparisons were made using the Holm-Sidak method. CTX results were compared between treatment groups within dosing protocols using Kruskal-Wallis tests. All other outcome measures were compared using one-way ANOVA tests to assess significant differences between treatment groups within each dosing protocol. When significant differences were found, pairwise comparisons were made using Dunnett’s procedure. Linear regression and correlation analyses between body weight and the femoral, tibial, and biomarker results were conducted to assess the influence of body weight on these outcome measures. In addition, ANCOVA tests were conducted to determine if body weight or femoral geometric parameters significantly contributed to the differences seen in biomechanical outcomes. For all tests, p-values less than 0.05 were considered significant. These analyses were performed using SAS (SAS Institute, Cary, NC) and SigmaStat (Systat, San Jose, CA).
Results
Body Weight and Food Intake
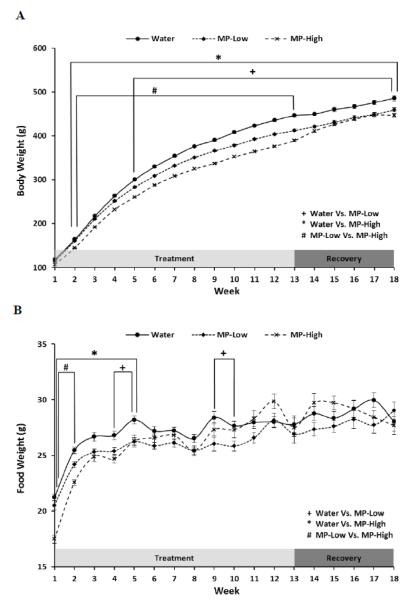
Administration of MP resulted in a dose dependent inhibition of weight gain throughout the study period (Figure 1A). Specifically, weekly average body weights of MP-High rats were ~11% lower than those of Water controls from week 2 until the end of the study at week 18. Cessation of MP led to a slight recovery of normal weight gain with MP-High progressing from a 13% deficit at the end of treatment (Week 13), to an 8% deficit at week 18. In addition, from week 2 until the end of the treatment period at week 13, the weekly average body weights of the MP-High rats were ~7% lower than those of MP-Low rats. For the MP-Low rats, average weekly body weights were ~6% lower than those of Water controls from week 5 until study completion at week 18. Little change was seen during the recovery period with MP-Low rats 8% lighter than controls at Week 13 and 5% lighter at Week 18.
Figure 1. Body Weight and Food Intake.
Line graphs showing: A) Body weight, and; B) Food intake; for the three treatment groups (Water, MP-Low, and MP-High) during the 13 weeks of treatment and 5 weeks of untreated recovery. Markers indicate average weekly weight for each group with error bars denoting SEM. Results were compared between treatment groups each week using 2-way repeated measures ANOVA, followed by pairwise comparisons using the Holm-Sidak method. All comparisons were considered significant for p-values < 0.05. Weeks in which results significantly differed are spanned by brackets and marked with symbols to identify the groups that differed.
Dose dependent decreases in food intake were also seen subsequent to MP administration. However, in contrast to body weight, these effects were concentrated in the early weeks of the study (Figure 1B). Average weekly food intake in MP-High rats decreased by ~10% from week 1 to 5 and week 1 to 2 as compared to Water and MP-Low, respectively. In the MP-Low rats, average weekly food intake was ~7% lower than in Water rats for weeks 4, 5, 9, and 10.
Bone Sizes
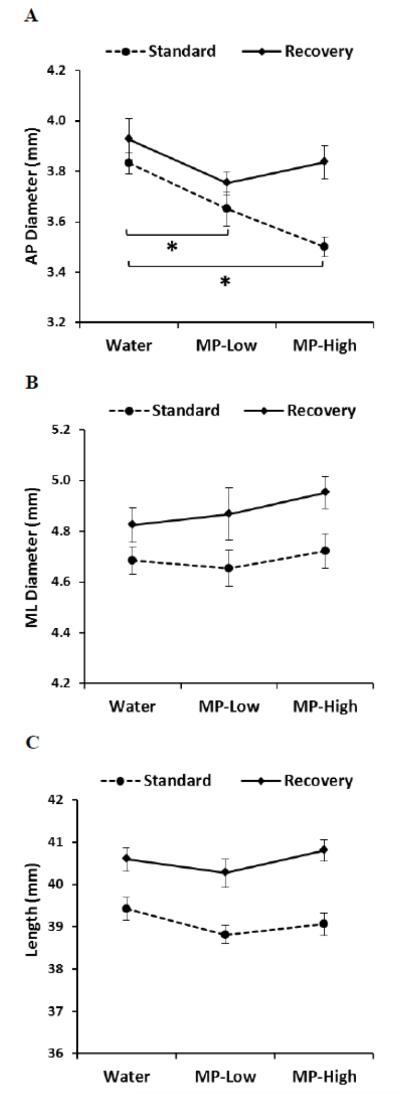
Caliper measurement of femora revealed a dose dependent decrease in AP diameter under the Standard protocol that corresponded for MP-Low to a diameter reduction of 5% and for MP-High of 9%, as compared to Water controls (Figure 2A). Five weeks later, under the Recovery protocol, these differences were abolished. Measurement of ML diameter and length revealed no differences with MP (Low or High) (Figure 2B and C).
Figure 2. Femoral Caliper Measurements.
Line graphs showing results of caliper measurements of femoral: A) Anterior-posterior (AP) diameter; B) Medial-lateral (ML) diameter, and; C) Length; for the three treatment groups (Water, MP-Low, and MP-High) analyzed immediately after 13 weeks of treatment (Standard) and following 5 weeks of untreated recovery (Recovery). Markers indicate group average with error bars denoting SEM. Results were compared between groups within each dosing protocol using ANOVA, followed by pairwise comparisons with Dunnett’s tests. All comparisons were considered significant for p-values < 0.05. Significant differences between treatment groups from the standard protocol are indicated by brackets and asterisks.
Significant positive correlations with body weight were observed under the Standard protocol with ML diameter in the MP-Low rats and with length in MP-High rats (Table 1). For the Recovery protocol, body weight was positively correlated with ML diameter and length both in MP-Low and MP-High rats (Table 1).
Table 1. Body Weight Correlations.
| Standard | Recovery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | MP-Low | MP-High | Water | MP-Low | MP-High | ||||||||
| R | p-value | R | p-value | R | p-value | R | p-value | R | p-value | R | p-value | ||
| Femora | AP Diameter | 0.534 | 0.074 | 0.624 | <0.050 | 0.368 | 0.240 | −0.129 | 0.690 | 0.267 | 0.401 | 0.422 | 0.171 |
| ML Diameter | 0.333 | 0.290 | 0.535 | 0.073 | 0.198 | 0.537 | 0.102 | 0.754 | 0.854 | <0.001 | 0.728 | <0.010 | |
| Length | 0.834 | <0.001 | 0.491 | 0.105 | 0.596 | <0.050 | 0.056 | 0.864 | 0.886 | <0.001 | 0.068 | <0.050 | |
| BMD | 0.087 | 0.789 | 0.578 | <0.050 | 0.490 | 0.106 | −0.170 | 0.597 | 0.680 | <0.050 | 0.779 | <0.010 | |
| BMC | 0.678 | <0.050 | 0.807 | 0.001 | 0.648 | <0.050 | −0.172 | 0.592 | 0.892 | <0.001 | 0.816 | 0.001 | |
| Area | 0.926 | <0.001 | 0.700 | 0.010 | 0.752 | <0.010 | −0.147 | 0.649 | 0.931 | <0.001 | 0.743 | <0.010 | |
| Ultimate Force | 0.132 | 0.682 | 0.674 | <0.050 | 0.443 | 0.150 | 0.175 | 0.585 | 0.182 | 0.592 | 0.230 | 0.497 | |
| Stiffness | 0.476 | 0.118 | 0.591 | <0.050 | 0.367 | 0.241 | −0.072 | 0.823 | 0.138 | 0.668 | 0.164 | 0.610 | |
| Energy to Failure | 0.462 | 0.131 | 0.248 | 0.437 | 0.195 | 0.543 | 0.200 | 0.534 | 0.467 | 0.147 | 0.244 | 0.470 | |
| Tibiae | BMD | 0.069 | 0.832 | 0.666 | <0.050 | 0.574 | 0.050 | −0.290 | 0.361 | 0.585 | <0.050 | 0.698 | 0.010 |
| BMC | 0.641 | <0.050 | 0.809 | 0.001 | 0.620 | <0.050 | −0.252 | 0.429 | 0.805 | <0.010 | 0.783 | <0.010 | |
| Area | 0.830 | <0.001 | 0.681 | <0.050 | 0.652 | <0.050 | −0.204 | 0.525 | 0.848 | <0.001 | 0.766 | <0.010 | |
| Serum | CTX | 0.743 | 0.257 | 0.137 | 0.863 | −0.760 | 0.240 | 0.077 | 0.923 | −0.006 | 0.994 | 0.687 | 0.313 |
| ALP | −0.266 | 0.457 | −0.270 | 0.396 | −0.554 | 0.062 | 0.036 | 0.911 | 0.325 | 0.302 | 0.041 | 0.899 | |
| Testosterone | 0.389 | 0.267 | 0.212 | 0.532 | 0.067 | 0.864 | 0.386 | 0.27 | 0.312 | 0.324 | 0.659 | <0.050 | |
This table presents the results of linear regression and correlation analyses performed between body weight at sacrifice and the caliper, DXA, biomechanics, and biomarker results from the three treatment groups (Water, MP-Low, and MP-High) analyzed immediately after 13 weeks of treatment (Standard) and following 5 weeks of untreated recovery (Recovery). Sample identities are given in the first column, followed by the outcomes, R-values, and p-values, for each treatment group and dosing protocol. Significant correlations are marked in bold.
Analysis of L5 vertebrae revealed a significant between group difference in AP diameter under the Recovery protocol, but subsequent pairwise comparisons failed to identify any significant differences (Table 2). Similarly, neither ML diameter nor vertebral height was found to differ under either dosing protocol (Table 2).
Table 2. Vertebral Properties.
| Standard | Recovery | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | MP-Low | MP-High | p-value | Water | MP-Low | MP-High | p-value | |||||||
| mean | SEM | mean | SEM | mean | SEM | mean | SEM | mean | SEM | mean | SEM | |||
| AP Diameter (mm) | 3.98 ± 0.02 | 3.90 ± 0.06 | 4.04 ± 0.06 | 0.1151 | 4.05 ± 0.07 | 3.88 ± 0.05 | 4.07 ± 0.05 | 0.0200 | ||||||
| ML Diameter (mm) | 5.11 ± 0.06 | 4.96 ± 0.07 | 5.03 ± 0.07 | 0.2968 | 5.16 ± 0.08 | 5.08 ± 0.09 | 5.22 ± 0.10 | 0.5587 | ||||||
| Height (mm) | 7.84 ± 0.13 | 7.68 ± 0.12 | 7.65 ± 0.17 | 0.5878 | 8.35 ± 0.20 | 8.06 ± 0.10 | 8.24 ± 0.09 | 0.3566 | ||||||
| BMD (g/cm2) | 0.1468 ± 0.0026 | 0.1456 ± 0.0017 | 0.1382 ± 0.0032 | 0.0524 | 0.1547 ± 0.0044 | 0.1540 ± 0.0028 | 0.1492 ± 0.0031 | 0.4864 | ||||||
| BMC (g) | 0.140 ± 0.004 | 0.133 ± 0.003 | 0.126 ± 0.004 | 0.0558 | 0.176 ± 0.013 | 0.157 ± 0.008 | 0.158 ± 0.006 | 0.3228 | ||||||
| Area (cm2) | 0.95 ± 0.02 | 0.91 ± 0.02 | 0.91 ± 0.02 | 0.1933 | 1.04 ± 0.02 | 1.03 ± 0.03 | 1.05 ± 0.02 | 0.7120 | ||||||
| Energy to Failure (mJ) | 32.73 ± 6.59 | 36.07 ± 6.04 | 26.74 ± 4.27 | 0.5117 | 23.74 ± 2.55 | 26.43 ± 1.71 | 27.62 ± 2.60 | 0.4896 | ||||||
| Stiffness (N/mm) | 2001.09 ± 199.83 | 2289.19 ± 229.79 | 2006.69 ± 372.93 | 0.7064 | 3154.65 ± 273.32 | 3585.68 ± 277.30 | 3226.79 ± 399.03 | 0.6026 | ||||||
| Ultimate Force (N) | 244.08 ± 16.37 | 275.01 ± 14.40 | 238.07 ± 25.27 | 0.3585 | 317.20 ± 14.50 | 346.40 ± 15.45 | 323.98 ± 22.68 | 0.4905 | ||||||
This table presents the results of caliper measurements, DXA scanning, and biomechanical testing of L5 vertebrae from the three treatment groups (Water, MP-Low, and MP-High) analyzed immediately after 13 weeks of treatment (Standard) and following 5 weeks of untreated recovery (Recovery). Parameters and units of measure are given in the first column, followed by group means ± SEM, and p-values arising from ANOVA comparisons between treatment groups within each dosing protocol. The only significant difference was seen for AP diameter under the Recovery protocol; however a post-hoc Dunnett’s test revealed no significant pairwise differences.
Densitometry
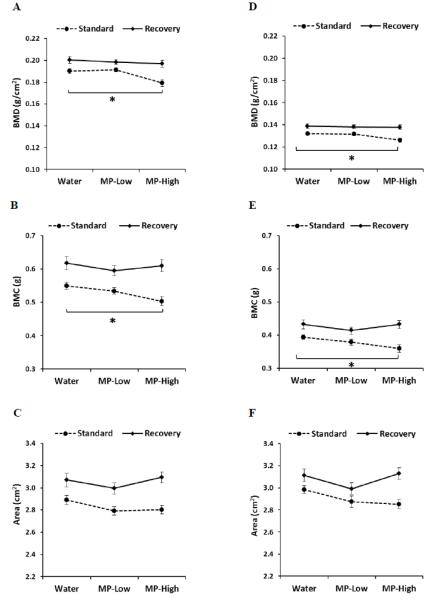
The results of the DXA analyses of femora and tibiae demonstrated similar findings. Compared to Water controls, reduced BMD and BMC were seen in MP-High rats for both bones under the Standard dosing protocol (Figure 3). Specifically, BMD was 6% and 5% lower for femora and tibia (Figure 3A and D), respectively, while BMC reductions of 9% were seen for both bones (Figure 3B and E). However, under the Recovery protocol, these parameters were no longer significantly different. Neither dosing protocol resulted in significant BMD or BMC changes in the MP-Low rats for either bone. Furthermore, no differences were seen in area for any of the samples (Figure 3C and F).
Figure 3. Femoral and Tibial DXA Analyses.
Line graphs displaying results of dual energy x-ray absorptiometry (DXA) determination of: A) Femoral bone mineral density (BMD); B) Femoral bone mineral content (BMC); C) Femoral area; D) Tibial BMD; E) Tibial BMC, and; F) Tibial area; for the three treatment groups (Water, MP-Low, and MP-High) analyzed immediately after 13 weeks of treatment (Standard) and following 5 weeks of untreated recovery (Recovery). Markers indicate group average with error bars denoting SEM. Results were compared between groups within each dosing protocol using ANOVA, followed by pairwise comparisons with Dunnett’s tests. All comparisons were considered significant for p-values < 0.05. Significant differences between treatment groups from the standard protocol are indicated by brackets and asterisks.
The correlation analysis yielded numerous significant and positive correlations with body weight. Under the Standard protocol, femoral and tibial BMC and area correlated for the Water group (Table 1). Femoral and tibial BMD, BMC, and area all correlated for MP-Low. Similarly, for MP-High all three tibial parameters were correlated with body weight, while only BMC and area were correlated in the femora. Under the Recovery protocol, correlations were seen for all three femoral and tibial parameters in the MP-Low and MP-High rats.
Consistent with the caliper measurements, no differences were found for any of the vertebral DXA outcomes (Table 2).
Biomechanical Properties
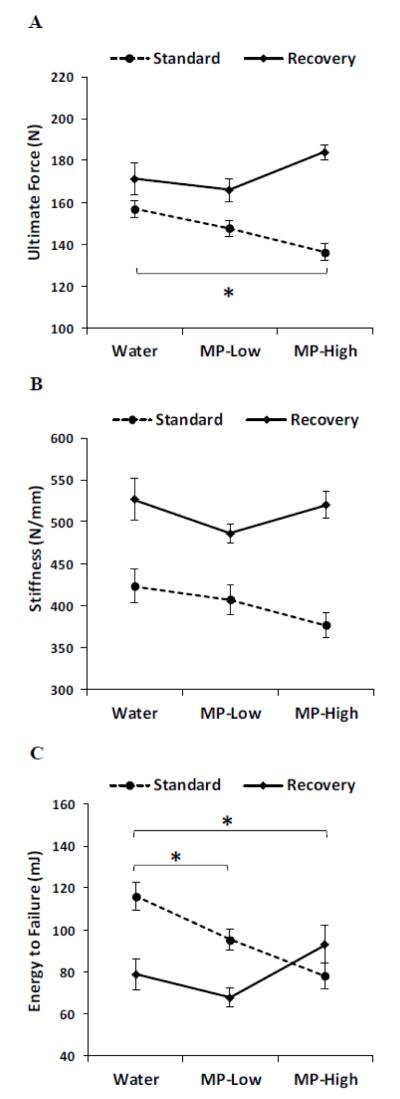
Analysis of femoral mechanical testing results under the Standard dosing protocol revealed large differences between rats treated with MP and Water controls (Figure 4). Ultimate force in MP-High rats was reduced by 13%, while energy to failure showed dose dependent reductions for MP-Low (20%) and MP-High (33%) rats as compared to Water controls (Figure 4A and C). Furthermore, a trend towards reduced stiffness was seen in the MP-High rats, though this was not significant (Figure 4B). Following the recovery phase, no differences were apparent, though the MP-High rats showed a trend towards increased ultimate force and energy to failure when compared to Water controls (Figure 4A and C).
Figure 4. Femoral Biomechanical Testing.
Line graphs showing results of femoral biomechanical testing for the parameters of: A) Ultimate force; B) Stiffness, and; C) Energy to failure; for the three treatment groups (Water, MP-Low, and MP-High) analyzed immediately after 13 weeks of treatment (Standard) and following 5 weeks of untreated recovery (Recovery). Markers indicate group average with error bars denoting SEM. Results were compared between groups within each dosing protocol using ANOVA, followed by pairwise comparisons with Dunnett’s tests. All comparisons were considered significant for p-values < 0.05. Significant differences between treatment groups from the standard protocol are indicated by brackets and asterisks.
Correlation of body weight with biomechanical properties revealed only two significant results, with ultimate force and stiffness both showing positive correlations with body weight in the MP-Low rats (Table 1). Interestingly, the ANCOVA was not significant for body weight, but did find significant effects for AP diameter on ultimate force, F(1,610) = 16, p<0.001 and stiffness F(1,2930) = 8.5, p<0.01. However, these effects were small with only 8.6% of the variance for ultimate force and 0.3% of the variance for stiffness accounted for by AP diameter (ω2 = 0.086 and 0.003, respectively).
Similar to the other outcomes, no biomechanical differences were seen between any of the vertebral samples (Table 2).
Serum Biomarkers
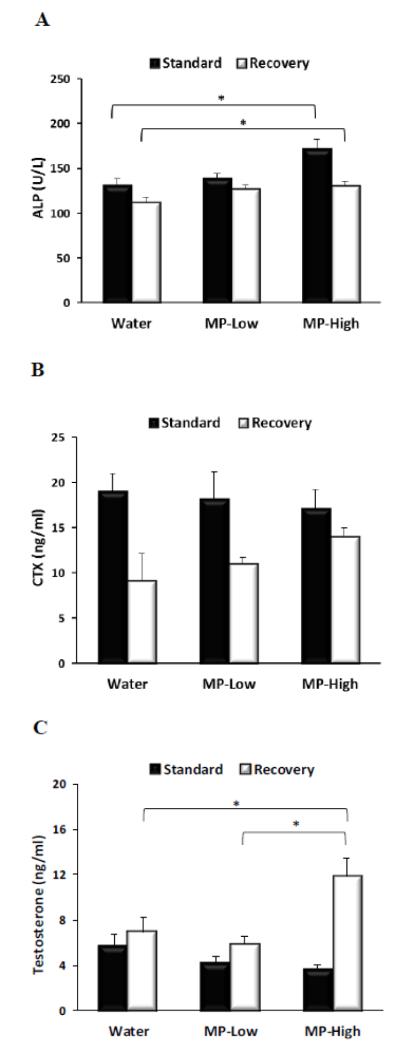
Analysis of serum ALP revealed that high dose MP treatment elevated its levels, as compared to Water controls, under both dosing protocols (Figure 5A). ALP increases with MP-High were 31% and 16% under the Standard and Recovery protocols, respectively. For MP-Low, ALP levels trended higher under both protocols, but were not significantly different (Figure 5A). In addition, ALP levels did not significantly correlate with body weight (Table 1).
Figure 5. Serum Biomarkers.
Bar graphs displaying serum levels of: A) Alkaline phosphatase (ALP); B) Carboxy-terminal collagen crosslinks (CTX) and; C) Testosterone, for the three treatment groups (Water, MP-Low, and MP-High) analyzed immediately after 13 weeks of treatment (Standard) and following 5 weeks of untreated recovery (Recovery). Markers indicate group average with error bars denoting SEM. Results were compared between groups within each dosing protocol using ANOVA (ALP and Testosterone) and Kruskal-Wallis tests (CTX), followed by pairwise comparisons with Dunnett’s tests. All comparisons were considered significant for p-values < 0.05. Significant differences between groups are indicated by brackets and asterisks.
In contrast to ALP, serum levels of CTX did not differ significantly between treatment groups under either dosing protocol (Figure 5B). However trends towards dose dependent decreases in CTX under the Standard protocol in conjunction with increases under the Recovery protocol were noted. No significant correlations were found between CTX and body weight (Table 1).
The testosterone analysis identified a trend towards a dose-dependent decrease in serum testosterone under the Standard protocol (Figure 5C). In contrast, under the Recovery protocol MP-High rats displayed a marked elevation of testosterone by 93% and 104% as compared to Water and MP-Low, respectively. Furthermore, testosterone significantly correlated with body weight for the MP-High rats under the Recovery protocol (Table 1).
Discussion
This study tested the hypothesis that chronic treatment of adolescent rats with MP significantly impairs skeletal growth, mineralization, and biomechanical integrity, and that these parameters are rapidly normalized following treatment cessation (i.e., recovery). The results of this study overwhelmingly support this hypothesis for the appendicular sites evaluated, with MP treatment demonstrating dose dependent decreases in the diameter, BMD, BMC, and biomechanical properties of both femora and tibiae, when assessed at the end of the 13-week treatment period. Furthermore, after the 5-week recovery period all of these parameters were indistinguishable from controls, indicating that skeletal recovery was achieved within 5 weeks of drug discontinuation. In contrast, MP treatment had no discernable effects on L5 vertebra for any of the outcomes evaluated, leading us to conclude that the adverse effects of MP on skeletal development are limited to appendicular sites in this model.
These findings are in general agreement with prior studies showing that MP treatment of neonatal and adolescent rats results in dose dependent inhibition of weight gain [18,19,20]. In addition, the rapid normalization of both body weight and skeletal growth upon MP cessation is also in agreement with previous reports [19, 20]. However, our results extend the basic findings of these prior studies by providing significantly more detailed measures of bone size, as well as densitometric and biomechanical data that reveal the extent to which MP treatment impairs skeletal development. Moreover, by analyzing both appendicular and axial skeletal sites, our study shows that the adverse effects of MP on skeletal development are restricted to the appendicular skeleton. One reason underlying this specificity may be preferential suppression of cortical rather than trabecular bone growth by MP. Another reason may be that MP is altering normal locomotor activity and the axial sites, which are more load-responsive, are being preferentially affected. Finally, underlying genetic and developmental factors that result in reduced variability of axial skeletal elements compared to appendicular elements may serve to protect these sites from the adverse effects of MP [24].
As MP treatment inhibited weight gain, it is possible that lower body mass in MP treated rats contributed to their impaired skeletal development. However, there are several reasons to suggest that lower body mass cannot fully account for these findings. First, despite a normalization of all measures of skeletal development at the end of the recovery period, MP-High rats remained significantly lighter than controls. Second, the majority of the differences in food intake occurred in the first 5 weeks of the study, suggesting that the effects of MP on appetite are transient and not the main cause of reduced body weight or impaired skeletal development. Furthermore, if body weight was the sole contributor to impaired skeletal development, all of the bones examined should have been similarly affected. Instead only bones from the appendicular skeleton were found to be affected. Additionally, if reduced body weight was the main factor underlying the differences seen in skeletal development, the strongest correlations should have been seen between body weight and the parameters that differed most from controls. Rather, the strongest correlations were seen for densitometric outcomes under the recovery protocol, were differences between treatments were no longer apparent. Finally, the ANCOVA showed no significant effects for body weight on femoral biomechanics, suggesting that MP impairs femoral biomechanical properties independent of its effects on body weight. Thus, although lower body weight cannot be completely ruled out as a contributor to impaired skeletal development in MP treated rats, it is not the only reason for these effects. It should also be noted that body composition was not measured in this study, making it impossible to determine if MP had differential effects on fat and lean mass, which could result in different effects on skeletal development.
One likely mechanism for the skeletal effects of MP treatment is its effects on sex hormones such as testosterone. As testosterone is vital for normal pubertal skeletal growth, as well as adult skeletal homeostasis [25], elevation of serum testosterone following MP cessation may be responsible for the rapid recovery of normal skeletal health. Moreover, while not statistically significant, reduced testosterone was seen during MP treatment suggesting that MP-induced suppression of skeletal growth may also be driven by changes in testosterone. Clinical evidence has also shown that MP can disrupt testosterone levels, as seen in a study of hormone levels in adolescents undergoing MP treatment that revealed not only significant reductions in salivary testosterone levels, but also a flattening of the normal diurnal pattern of decreasing testosterone throughout the day [26]. Taken together, these data suggest that altered testosterone levels contribute to the adverse effects of MP on skeletal development, as well as their rapid normalization following treatment cessation.
While this study only assessed testosterone as a mechanism for the skeletal effects of MP, alterations in other signaling pathways and basic physiological processes are certain to contribute to the observed effects. These could include direct effects of MP on osteoblasts, osteoclasts, and osteocytes, changes in general metabolic rates, altered nutrient absorption, differential physical activity, and acute starvation responses [27]. In addition, it is probable that MP-induced disruption of dopamine signaling also contributes to these effects because dopamine transporter (DAT) knockout mice, which have enhanced dopaminergic signaling as seen in animals treated with MP, also show significant reductions in bone density and size [28]. Complete elucidation of the mechanism by which MP impairs skeletal development will not occur until more detailed analyses of these and other possible underlying mechanisms are completed.
In addition to assessing the effects of MP directly on skeletal parameters and beginning to elucidate the mechanism by which MP affects the skeletal system, serum biomarkers of bone formation and resorption were quantified in an effort to determine if their expression levels could be used to monitor the adverse effects of MP on skeletal development. Consistent with the rapid skeletal normalization seen in MP-High rats under the recovery protocol, ALP levels were significantly increased in these animals, suggesting that it may be possible to monitor the previously reported ‘growth rebound’ effect [19,20] using this biomarker. However, ALP levels were unexpectedly elevated in MP-High rats at the end of treatment when these rats had reduced femoral size, and femoral and tibial BMD and BMC. Unfortunately, as total ALP, rather than bone specific ALP was measured, we do not know if the elevated ALP was due to alterations in bone metabolism, hepatic interference (e.g., MP metabolism), or intestinal changes (e.g., altered food intake). Furthermore, even if the increase in ALP was due to increased osteoblastic activity, an even larger induction of osteoclastic activity could have been responsible for the decreases seen in size and mineralization. However, analysis of the resorption marker, CTX, showed no differences between treatment groups. Therefore, these data indicate that while measurement of serum biomarkers may ultimately show utility in noninvasively monitoring the effects of MP on skeletal development, further research will be required to identify more relevant and clinically useful biomarkers.
While it is challenging to compare the results of this study to clinical MP studies, several basic findings are in good agreement with the clinical literature. For example, the well documented decrease in weight gain for patients taking MP [14,15,16] is clearly replicated in our animal model. Clinical studies of the phenomenon of ‘rebound growth’ following cessation of MP treatment are more limited and inconsistent. For example, one study of 76 stimulant-treated children (with 16 continued on medication and 50 withdrawn from medication for a summer drug holiday) showed that abnormally high weight gain occurred during the three months of treatment cessation, but that these gains were not sufficient to compensate for the reduced weight gain during the preceding 9 months of treatment [29]. Larger longitudinal studies (370 cases with an untreated clinical control group of 65, 147 inconsistently treated, and 158 continuously treated) have failed to show a complete rebound in height [12]. Three long-term follow-up studies failed to find differences in adult height and weight for patients treated with MP for a variable time in childhood and adolescence, but these studies, which were of children with outdated diagnostic labels before the ADHD criteria were developed, were not designed to assess height, did not always measure height, and used self-reported height in many cases [17].
This study is the first to examine the effects of MP treatment on skeletal biomechanical integrity and mineralization in an animal model and no similar clinical studies have been reported, making it difficult to assess the clinical relevance of these findings. However, given the degree of biomechanical impairment seen in the femora of MP-High rats (13% and 33% reductions in ultimate force and energy to failure, respectively) future studies should be conducted to evaluate bone density in patients undergoing MP treatment, as well as determine if they have an increased fracture incidence.
Unlike the clinical literature on studies of humans and animal studies on rats, our study failed to identify any differences in length. Clinical studies reporting on reduced growth measured total height [14,15] and prior rat studies identified reductions in both naso-anal length [18] and femoral length [19,20]. By measuring femoral length and vertebral height in this study, it was expected that the relative contribution of appendicular and axial sites would be able to be ascertained. However, no differences were seen at either site, suggesting that more vertebral levels, additional appendicular samples (i.e., radius, humerus, ulna), or naso-anal length should be measured to definitively assess the effects of MP on bone/body length in these animals. It is also possible that no differences were present and the failure to replicate prior rat studies arose due to differences in MP dosage, route of administration, rat background, or ages during treatment. However, we did see reductions in femoral AP diameter in the MP-High rats, which has not previously been reported and is indicative of reduced periosteal bone formation.
In summary, the results of this study suggest that chronic MP treatment leads to weaker, less mineralized appendicular bones. While cessation of treatment mitigated these adverse effects, these data show that bones in young rats undergoing MP treatment require less force to break and suggest that adolescents may be at an increased risk for fracture while undergoing MP treatment. In addition, as it is not yet clear if MP treatment leads to reductions in peak bone mass, these patients may also be at an increased risk for developing osteoporosis. Given the rapid rise in the number of adolescents taking MP, as well as the increasingly longer periods of time they are exposed to this drug, additional studies of its adverse skeletal effects are clearly warranted. Elucidation of the molecular mechanisms underlying these effects, as well as ways to identify and mitigate them in a timely manner would enable physicians to better tailor treatment decisions for these patients in order to effectively manage ADHD symptoms while minimizing the adverse skeletal effects of MP treatment.
Highlights.
Chronic administration of methylphenidate to young rats adversely affects appendicular skeletal development.
Chronic administration of methylphenidate to young rats does not affect axial skeletal development.
The adverse effects of methylphenidate administration to young rats were eliminated within 5 weeks of treatment cessation.
Adolescents taking methylphenidate may have an increased risk for skeletal problems.
Acknowledgements
The authors gratefully acknowledge support by: NIDA, NIAAA (Intramural Research Program, LNI), as well as the InMotion Orthopaedic Research Center in Memphis, TN. In addition, the authors thank R.J. Schroeder and G.J. Wang who provided assistance in the early phases of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dopheide JA, Pliszka SR. Attention Deficit Hyperactivity Disorder: An Update. Pharmacotherapy. 2009;29:656–679. doi: 10.1592/phco.29.6.656. [DOI] [PubMed] [Google Scholar]
- [2].Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J. Pediatr. Psychol. 2007;32:643–654. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- [3].Edwards G, Barkley RA, Laneri M, Fletcher K, Metevia L. Parent-adolescent conflict in teenagers with ADHD and ODD. J. Abnorm. Child. Psychol. 2001;29:557–572. doi: 10.1023/a:1012285326937. [DOI] [PubMed] [Google Scholar]
- [4].Konofal E, Lecendreux M, Cortese S. Sleep and ADHD. Sleep. Med. 2010;11:652–658. doi: 10.1016/j.sleep.2010.02.012. [DOI] [PubMed] [Google Scholar]
- [5].Mannuzza S, Klein RG, Moulton JL. 3rd, Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry. Res. 2008;160:237–246. doi: 10.1016/j.psychres.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].James A, Lai FH, Dahl C. Attention deficit hyperactivity disorder and suicide: a review of possible associations. Acta. Psychiatr. Scand. 2004;110:408–415. doi: 10.1111/j.1600-0447.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- [7].Weber W, Newmark S. Complementary and alternative medical therapies for attention-deficit/hyperactivity disorder and autism. Pediatr. Clin. North. Am. 2007;54:983–1006. doi: 10.1016/j.pcl.2007.09.006. [DOI] [PubMed] [Google Scholar]
- [8].Knight LA, Rooney M, Chronis-Tuscano A. Psychosocial treatments for attention-deficit/hyperactivity disorder. Curr. Psychiatry. Rep. 2008;10:412–418. doi: 10.1007/s11920-008-0066-6. [DOI] [PubMed] [Google Scholar]
- [9].MTA Cooperative Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- [10].Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- [11].Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and Treatment of Attention-Deficit/Hyperactivity Disorder in children and adolescents. J. Am. Med. Assoc. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- [12].Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, Hechtman L, Epstein JN, Pelham WE, Abikoff HB, Newcorn JH, Molina BS, Hinshaw SP, Wells KC, Hoza B, Jensen PS, Gibbons RD, Hur K, Stehli A, Davies M, March JS, Conners CK, Caron M, Volkow ND. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J. Am. Acad. Child. Adolesc. Psychiatry. 2007;46:1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- [13].Safer D, Allen R, Barr E. Depression of growth in hyperactive children on stimulant drugs. N. Engl. J. Med. 1972;287:217–220. doi: 10.1056/NEJM197208032870503. [DOI] [PubMed] [Google Scholar]
- [14].MTA Cooperative Group National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics. 2004;113:762–769. doi: 10.1542/peds.113.4.762. [DOI] [PubMed] [Google Scholar]
- [15].Swanson J, Greenhill L, Wigal T, Kollins S, Stehli A, Davies M, Chuang S, Vitiello B, Skrobala A, Posner K, Abikoff H, Oatis M, McCracken J, McGough J, Riddle M, Ghuman J, Cunningham C, Wigal S. Stimulant-related reductions of growth rates in the PATS. J. Am. Acad. Child. Adolesc. Psychiatry. 2006;45:1304–1313. doi: 10.1097/01.chi.0000235075.25038.5a. [DOI] [PubMed] [Google Scholar]
- [16].Pliszka SR, Matthews TL, Braslow KJ, Watson MA. Comparative effects of methylphenidate and mixed salts amphetamine on height and weight in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry. 2006;45:520–526. doi: 10.1097/01.chi.0000205702.48324.fd. [DOI] [PubMed] [Google Scholar]
- [17].Kramer JR, Loney J, Ponto LB, Roberts MA, Grossman S. Predictors of adult height and weight in boys treated with methylphenidate for childhood behavior problems. J. Am. Acad. Child. Adolesc. Psychiatry. 2000;39:517–524. doi: 10.1097/00004583-200004000-00022. [DOI] [PubMed] [Google Scholar]
- [18].Greeley GH, Jr, Kizer JS. The effects of chronic methylphenidate treatment on growth and endocrine function in the developing rat. J. Pharmacol. Exp. Ther. 1980;215:545–551. [PubMed] [Google Scholar]
- [19].Pizzi WJ, Rode EC, Barnhart JE. Methylphenidate and growth: demonstration of a growth impairment and a growth-rebound phenomenon. Dev. Pharmacol. Ther. 1986;9:361–368. doi: 10.1159/000457114. [DOI] [PubMed] [Google Scholar]
- [20].Pizzi WJ, Rode EC, Barnhart JE. Differential effects of methylphenidate on the growth of neonatal and adolescent rats. Neurotoxicol. Teratol. 1987;9:107–111. doi: 10.1016/0892-0362(87)90086-9. [DOI] [PubMed] [Google Scholar]
- [21].Malitsis N, Wang GJ, Swanson JM, Volkow ND, Thanos PK. Chronic Oral Methylphenidate Exposure During Adolescence: Effects on Open Field Locomotor Activity, Novel Object Recognition and Circadian Activity. Annual Meetings of Society for Neuroscience. 2008;59:6DD12. [Google Scholar]
- [22].Warden SJ, Komatsu DE, Rydberg J, Bond JL, Hassett SM. Recombinant human parathyroid hormone (PTH 1-34) and low-intensity pulsed ultrasound have contrasting additive effects during fracture healing. Bone. 2009;44:485–494. doi: 10.1016/j.bone.2008.11.007. [DOI] [PubMed] [Google Scholar]
- [23].Komatsu DE, Mary MN, Schroeder RJ, Robling AG, Turner CH, Warden SJ. Modulation of Wnt Signaling Influences Fracture Repair. J. Orthop. Res. 2010;28:928–936. doi: 10.1002/jor.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cullinane DM. Axial versus Appendicular: Constraint versus Selection. Amer. Zool. 2000;40:136–145. [Google Scholar]
- [25].Francis RM. The effects of testosterone on osteoporosis in men. Clin. Endocrinol. (Oxf) 1999;50:411–414. doi: 10.1046/j.1365-2265.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- [26].Hibel LC, Granger DA, Cicchetti D, Rogosch F. Salivary biomarker levels and diurnal variation: associations with medications prescribed to control children’s problem behavior. Child. Dev. 2007;78:927–937. doi: 10.1111/j.1467-8624.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- [27].Poulton A. Growth on stimulant medication; clarifying the confusion: a review. Arch. Dis. Child. 2005;90:801–806. doi: 10.1136/adc.2004.056952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bliziotes M, McLoughlin S, Gunness M, Fumagalli F, Jones SR, Caron MG. Bone histomorphometric and biomechanical abnormalities in mice homozygous for deletion of the dopamine transporter gene. Bone. 2000;26:15–19. doi: 10.1016/s8756-3282(99)00232-x. [DOI] [PubMed] [Google Scholar]
- [29].Safer DJ, Allen RP, Barr E. Growth rebound after termination of stimulant drugs. J. Pediatr. 1975;86:113–116. doi: 10.1016/s0022-3476(75)80720-7. [DOI] [PubMed] [Google Scholar]