Abstract
Psoriasis vulgaris is a complex disease characterized by alterations in growth and differentiation of epidermal keratinocytes as well as marked increase in leukocyte populations. Lesions are known to contain alterations in mRNAs encoding more than 1000 products, but only a very small number of these transcripts have been localized to specific cell types or skin regions. In this study, we used laser capture microdissection (LCM) and gene array analysis to study the gene expression of cells in lesional epidermis and dermis, compared with corresponding non-lesional resions. Using this approach, we detected >1800 differentially expressed gene products in the epidermis or dermis of psoriasis lesions. These results established sets of genes that are differentially expressed between epidermal and dermal compartments, as well as between non-lesional and lesional psoriasis skin. One of our findings involved the local production of CCL19, a lymphoid organizing chemokine, and its receptor CCR7 in psoriatic dermal aggregates, along with the presence of gene products LAMP3/DC-LAMP and CD83, which typify mature DCs. Gene expression patterns obtained with LCM and microarray analysis along with T cell and DC detection by immune staining suggest a possible mechanism for lymphoid organization via CCL19/CCR7 in diseased skin.
Introduction
Valuable insights into the pathogenic processes of various skin diseases can be obtained by studying differentially expressed genes (DEGs) via cDNA microarray analysis. However, whole-tissue analysis has limitations, including its inability to localize mRNAs to specific cell types. In addition, gene products specific to “minority” cell types may be lost by dilution with mRNAs from more dominant cell types. Our group developed cellular genomic maps of the skin by using in vitro cultured cells of various types to localize in vivo gene changes to specific cell types (Haider et al. 2008). However, there are important differences between cultured cells and their in vivo counterparts. For example, keratinocytes undergo two differentiation programs in vivo - homeostatic growth and regenerative maturation - while cultured keratinocytes reflect predominantly the regenerative phenotype (Mansbridge and Knapp 1987; Kennedy-Crispin et al. 2012). Additionally, cultured keratinocytes are less differentiated than their in vivo counterparts. Inflammatory skin disease like psoriasis and atopic dermatitis also contain several types of dendritic cells (DCs) that cannot be found in normal skin and blood and are distinct from those differentiated from monocytes in vitro with specific cytokines.
Given that psoriasis vulgaris is a complex disease involving distinct cells and tissues (marked alterations in growth, differentiation, and patterning of the epidermis as well as tissue infiltration by T cells, DCs, and other myeloid leukocytes), it would be desirable to localize disease-related genes specifically to the epidermis or immune components. Numerous prior studies have used bulk-tissue homogenates to establish major differences in gene expression between diseased and non-diseased states (Bowcock et al. 2001; Zhou et al. 2003; Yao et al. 2008; Suárez-Fariñas et al. 2010; Gudjonsson et al. 2010). Although specific studies differ in total number of DEGs, the psoriasis transcriptome has consistent alterations in >1000 transcripts between lesional and non-lesional skin. Only a relatively small number of DEGs has been localized to specific cells or regions of psoriasis lesions in prior studies, e.g., by immune staining for protein products.
We therefore sought to expand our understanding of cell/region-specific molecular changes in psoriasis vulgaris through the use of laser capture microdissection (LCM) combined with cDNA microarray analysis. LCM is a technique used to isolate subpopulations of cells from tissue sections under direct microscopic visualization (Epsina et al. 2006), but its application to gene expression analysis has been limited by the difficulty of recovering sufficient amounts of RNA for whole transcriptome analysis (Clément-Ziza et al. 2009). There has also been the concern that multiply amplified mRNA products may not reflect the correct abundance of transcripts as detected by single amplification methods used in whole tissue analysis. We chose to perform regional gene expression analysis on psoriasis, because 1) prior bulk-tissue studies provide a strong reference gene set for comparison to LCM-derived transcripts and 2) distinct cellular and genomic pathways should exist within epidermal and dermal/immune tissue compartments. Our results established marked differences in gene expression between epidermis and dermis of non-lesional psoriasis skin as well as between diseased epidermis and dermis of lesional skin. Specifically, leukocyte-rich dermal inflammatory regions in psoriasis lesions contained high-level expression of a number of genes, as well as corresponding proteins, that direct lymphoid organization and structure. This suggests a mechanism for the formation and persistence of T cell mediated inflammation in diseased skin. Extensive experiments presented in supplementary results and discussion (SRD) evaluate potential technical limitations of LCM methodology that must be considered before it can be applied widely to other skin diseases.
Results
LCM localizes genes selectively expressed in non-lesional epidermis and dermis
We performed LCM to collect cells in the epidermis (EPI), papillary dermis (PD), reticular dermis (RD), and dermal inflammatory cell aggregates (ICs) in frozen sections of skin, as shown in Figure S1c-d. The potential quality of RNA of our samples and the efficacy of the double amplification are summarized in Figure S1a-b and S1e. We also assessed the linearity of double amplification in Figure S1f. The PCA-plot and heatmap (Figure S2a-b) illustrating unsupervised clustering of expression values for all samples clearly show that there are no outliers and that the major source of variation in the samples is the sample type (i.e. Epidermis, Dermis, and Bulk), even when samples from the same patient are used.
To evaluate the specificity of LCM to localize gene products, we compared gene expression profiles of EPI and RD in non-lesional skin. In EPI, 1151 probe-sets were up-regulated and 1471 were down-regulated. Table 1a-b show the top 25 up- and down-regulated DEGs, respectively. Known keratinocyte-related genes such as KRT1, KRT2, KRT5, DSC1, and DSC3, as well as melanocyte-related genes TYRP1 and DCT, were found in EPI. In RD, we found higher expression of collagens (COL3A1 and COL6A3), immune related genes (CXCL12, IGHA1, and LYZ), and vascular endothelial cell and leukocyte transendothelial migration-related genes (SPARCL1, PECAM1, and VCAM1). We can thus clearly separate gene expression in different regions of the skin combining LCM and cDNA microarray analysis. The complete list of DEGs between EPI and RD of non-lesional skin is shown in Table S1.
Table 1. Short lists of DEGs in psoriasis non-lesional EPI vs. non-lesional RD (LCM)*.
| a. Top 25 Up-regulated genes in psoriasis non-lesional EPI vs. non-lesional RD | ||||
|---|---|---|---|---|
| Probe Set ID | FCH | FDR | Symbol | Description |
| 205900_at | 252.28 | <0.01 | KRT1 | keratin 1 |
| 205694_at | 244.41 | <0.01 | TYRP1 | tyrosinase-related protein 1 |
| 207908_at | 243.36 | <0.01 | KRT2 | keratin 2 |
| 206400_at | 153.37 | 0.01 | LGALS7 / 7B | lectin, galactoside-binding, soluble, 7 / 7B |
| 206032_at | 139.60 | 0.02 | DSC3 | desmocollin 3 |
| 207324_s_at | 130.69 | <0.01 | DSC1 | desmocollin 1 |
| 204379_s_at | 92.21 | <0.01 | FGFR3 | fibroblast growth factor receptor 3 |
| 207955_at | 91.10 | <0.01 | CCL27 | chemokine (C-C motif) ligand 27 |
| 213506_at | 88.21 | <0.01 | F2RL1 | coagulation factor II (thrombin) receptor-like 1 |
| 201131_s_at | 82.44 | 0.01 | CDH1 | cadherin 1, type 1, E-cadherin (epithelial) |
| 207109_at | 81.63 | <0.01 | POU2F3 | POU class 2 homeobox 3 |
| 204455_at | 78.56 | 0.01 | DST | dystonin |
| 221854_at | 76.68 | <0.01 | PKP1 | plakophilin 1 |
| 213929_at | 70.43 | 0.01 | EXPH5 | exophilin 5 |
| 206276_at | 66.07 | <0.01 | LY6D | lymphocyte antigen 6 complex, locus D |
| 205337_at | 64.29 | 0.01 | DCT | dopachrome tautomerase |
| 209570_s at | 63.15 | <0.01 | D4S234E | DNA segment on chr 4 - 234 expressed sequence |
| 206642_at | 58.40 | 0.01 | DSG1 | desmoglein 1 |
| 205807_s_at | 57.30 | <0.01 | TUFT1 | tuftelin 1 |
| 219995_s_at | 55.76 | <0.01 | ZNF750 | zinc finger protein 750 |
| 204469_at | 50.94 | 0.01 | PTPRZ1 | protein tyrosine phosphatase, receptor-type, Z polypeptide 1 |
| 204653_at | 49.93 | 0.01 | TFAP2A | transcription factor AP-2 α |
| 217528_at | 49.02 | 0.08 | CLCA2 | chloride channel accessory 2 |
| 217744_s_at | 47.24 | 0.03 | PERP | PERP, TP53 apoptosis effector |
| 201820_at | 46.26 | 0.03 | KRT5 | keratin 5 |
| b. Top 25 Down-regulated genes in psoriasis non-lesional EPI vs. non-lesional RD | ||||
|---|---|---|---|---|
| Probe Set ID | FCH | FDR | Symbol | Description |
| 209687_at | -250.00 | <0.01 | CXCL12 | chemokine (C-X-C motif) ligand 12 |
| 217022_s_at | -250.00 | <0.01 | IGHA1 | immunoglobulin heavy constant alpha 1 |
| 200795_at | -200.00 | <0.01 | SPARCL1 | SPARC-like 1 (hevin) |
| 215388_s_at | -166.67 | <0.01 | CFH / CFHR1 | complement factor H /complement factor H-related 1 |
| 213975_s_at | -166.67 | <0.01 | LYZ | lysozyme (renal amyloidosis) |
| 217757_at | -142.86 | <0.01 | A2M | alpha-2-macroglobulin |
| 215076_s_at | -125.00 | 0.01 | COL3A1 | collagen, type III, α 1 |
| 208982_at | -125.00 | <0.01 | PECAM1 | platelet/endothelial cell adhesion molecule |
| 209613_s_at | -111.11 | <0.01 | ADH1B | alcohol dehydrogenase 1B(class I), β polypeptide |
| 213800_at | -111.11 | <0.01 | CFH | complement factor H |
| 206201_s_at | -111.11 | <0.01 | MEOX2 | mesenchyme homeobox 2 |
| 208335_s_at | -90.91 | <0.01 | DARC | Duffy blood group, chemokine receptor |
| 222043_at | -83.33 | <0.01 | CLU | clusterin |
| 202291_s_at | -83.33 | <0.01 | MGP | matrix Gla protein |
| 219777_at | -76.92 | <0.01 | GIMAP6 | GTPase, IMAP family member 6 |
| 201744_s_at | -76.92 | 0.01 | LUM | lumican |
| 212950_at | -71.43 | <0.01 | GPR116 | G protein-coupled receptor 116 |
| 204115_at | -62.50 | <0.01 | GNG11 | guanine nucleotide binding protein (G protein), γ 11 |
| 218353_at | -62.50 | <0.01 | RGS5 | regulator of G-protein signaling 5 |
| 203868_s_at | -62.50 | <0.01 | VCAM1 | vascular cell adhesion molecule 1 |
| 221731_x_at | -62.50 | <0.01 | VCAN | versican |
| 201438_at | -58.82 | <0.01 | COL6A3 | collagen, type VI, α 3 |
| 217028_at | -58.82 | <0.01 | CXCR4 | chemokine (C-X-C motif) receptor 4 |
| 202404_s_at | -55.56 | 0.01 | COL1A2 | collagen, type I, α 2 |
| 202404_s_at | -55.56 | 0.01 | NR2F2 | nuclear receptor subfamily 2, group F, member 2 |
The Probe Set ID whose fold change was largest was listed when multiple probe sets were available for same gene. Un-annotated genes were excluded from the top 25 gene list.
LCM localizes disease-related DEGs in psoriasis epidermis
Gene expression profiles between lesional and non-lesional EPI samples were compared. Table 2a-b present the top 25 up- and down-regulated genes in the EPI-related psoriasis transcriptome, respectively. Up-regulated genes include those related to hyperproliferation (KRT6A, KRT6B, and KRT16), epidermal differentiation complex (S100 family proteins and small proline-rich proteins), and proteolysis regulation molecules (SERPINs and PI3). These results are consistent with previous works using bulk-tissue samples, but earlier studies were not able to specify the cells with altered gene expression. Among down-regulated genes, we examined the protein expression of CRIP-1 and CCL27 (Table 2b) using immunohistochemistry (IHC). Both proteins were detected in non-lesional epidermis but were decreased in lesional epidermis, confirming our microarray results (Figure 1a-b).
Table 2. Short lists of DEGs in psoriasis lesional EPI vs. non-lesional EPI (LCM)*.
| a. Top 25 Up-regulated genes in psoriasis lesional EPI vs. non-lesional EPI | ||||||
|---|---|---|---|---|---|---|
| Probe Set ID | Symbol | EPI (LCM) | Bulk | Description | ||
| FCH | FDR | FCH | FDR | |||
| 211906_s_at | SERPINB4 | 470.72 | <0.01 | 419.60 | <0.01 | serpin peptidase inhibitor, clade B member 4 |
| 209720_s_at | SERPINB3 | 366.10 | <0.01 | 171.61 | <0.01 | serpin peptidase inhibitor, clade B member 3 |
| 203535_at | S100A9 | 136.52 | <0.01 | 106.56 | <0.01 | S100 calcium binding protein A9 |
| 203691_at | PI3 | 128.03 | <0.01 | 67.67 | <0.01 | peptidase inhibitor 3, skin-derived |
| 207356_at | DEFB4 | 114.24 | <0.01 | 69.88 | <0.01 | defensin, beta 4 |
| 205916_at | S100A7 | 84.37 | <0.01 | 10.67 | 0.21 | S100 calcium binding protein A7 (psoriasin) |
| 213680_at | KRT6B | 67.99 | <0.01 | 3.61 | 0.54 | keratin 6B |
| 205863_at | S100A12 | 57.07 | <0.01 | 32.81 | <0.01 | S100 calcium binding protein A12 |
| 209125_at | KRT6A | 55.71 | <0.01 | 18.77 | 0.02 | keratin 6A |
| 217272_s_at | SERPINB13 | 55.50 | <0.01 | 8.13 | <0.01 | serpin peptidase inhibitor, clade B member 13 |
| 220322_at | IL1F9 | 55.34 | <0.01 | 19.15 | <0.01 | interleukin 1 family, member 9 (IL-36 gamma) |
| 206488_s_at | CD36 | 53.72 | <0.01 | 1.80 | 0.30 | CD36 molecule |
| 202917_s_at | S100A8 | 50.84 | 0.03 | 53.07 | 0.14 | S100 calcium binding protein A8 |
| 205513_at | TCN1 | 50.11 | <0.01 | 39.15 | <0.01 | transcobalamin I |
| 219795_at | SLC6A14 | 47.10 | <0.01 | 5.84 | 0.13 | solute carrier family 6 member 14 |
| 206177_s_at | ARG1 | 46.24 | <0.01 | 3.57 | 0.07 | arginase, liver |
| 220664_at | SPRR2C | 45.97 | <0.01 | 32.99 | <0.01 | small proline-rich protein 2C |
| 213796_at | SPRR1A | 35.17 | <0.01 | 6.03 | 0.24 | small proline-rich protein 1A |
| 208650_s_at | CD24 | 29.72 | <0.01 | 7.49 | <0.01 | CD24 molecule |
| 206643_at | HAL | 24.28 | <0.01 | 5.42 | <0.01 | histidine ammonia-lyase |
| 207381_at | ALOX12B | 22.66 | <0.01 | 4.59 | <0.01 | arachidonate 12-lipoxygenase, 12R type |
| 209800_at | KRT16 | 21.75 | <0.01 | 16.48 | 0.02 | keratin 16 |
| 201242_s_at | ATP1B1 | 21.33 | <0.01 | 2.46 | 0.57 | ATPase, Na+/K+ transporting, β 1 polypeptide |
| 206561_s_at | AKR1B10 | 20.04 | <0.01 | 20.14 | <0.01 | aldo-keto reductase family 1, member B10 |
| 213060_s_at | CHI3L2 | 19.97 | <0.01 | 5.46 | <0.01 | chitinase 3-like 2 |
| b. Top 25 Down-regulated genes in psoriasis lesional EPI vs. non-lesional EPI | ||||||
|---|---|---|---|---|---|---|
| Probe Set ID | Symbol | EPI (LCM) | Bulk | Description | ||
| FCH | FDR | FCH | FDR | |||
| 210809_s_at | POSTN | -62.50 | <0.01 | -1.44 | 0.35 | periostin, osteoblast specific factor |
| 205081_at | CRIP1 | -20.41 | <0.01 | -2.48 | 0.17 | cysteine-rich protein 1 (intestinal) |
| 214598_at | CLDN8 | -15.38 | <0.01 | -4.57 | 0.08 | claudin 8 |
| 201540_at | FHL1 | -9.80 | <0.01 | -1.88 | 0.35 | four and a half LIM domains 1 |
| 219087_at | ASPN | -9.52 | <0.01 | -1.79 | 0.50 | asporin |
| 215516_at | LAMB4 | -9.17 | <0.01 | -1.98 | <0.01 | laminin, beta 4 |
| 201843_s_at | EFEMP1 | -8.85 | <0.01 | -1.89 | 0.22 | EGFcontaining fibulin-like extracellular matrix protein1 |
| 213369_at | PCDH21 | -8.26 | <0.01 | -2.26 | 0.07 | protocadherin 21 |
| 202746_at | ITM2A | -7.87 | <0.01 | -2.35 | 0.03 | integral membrane protein 2A |
| 206170_at | ADRB2 | -7.52 | <0.01 | -2.95 | 0.01 | adrenergic, beta-2-, receptor, surface |
| 209292_at | ID4 | -7.41 | <0.01 | -3.06 | 0.06 | inhibitor of DNA binding 4 |
| 207955_at | CCL27 | -7.25 | 0.01 | -3.38 | 0.21 | chemokine (C-C motif) ligand 27 |
| 202973_x_at | FAM13A | -7.25 | <0.01 | -1.76 | 0.07 | family with sequence similarity 13, member A |
| 201506_at | TGFBI | -7.04 | <0.01 | -1.72 | 0.13 | transforming growth factor, β-induced, 68kDa |
| 215239_x_at | ZNF273 | -6.85 | <0.01 | -2.29 | 0.28 | zinc finger protein 273 |
| 218804_at | ANO1 | -6.80 | <0.01 | -1.58 | 0.57 | anoctamin 1 |
| 208096_s_at | COL21A1 | -6.71 | <0.01 | -1.85 | 0.14 | collagen, type XXI, α 1 |
| 218820_at | C14orf132 | -6.62 | <0.01 | -2.47 | 0.12 | chromosome 14 open reading frame 132 |
| 217627_at | ZNF573 | -6.25 | <0.01 | -2.41 | 0.13 | zinc finger protein 573 |
| 209335_at | DCN | -6.21 | <0.01 | -1.54 | 0.26 | decorin |
| 221645_s_at | ZNF83 | -5.65 | <0.01 | -1.78 | 0.12 | zinc finger protein 83 |
| 214723_x_at | ANKRD36 | -5.49 | <0.01 | -1.53 | 0.58 | ankyrin repeat domain 36 |
| 220940_at | ANKRD36B | -5.49 | <0.01 | -1.54 | 0.49 | ankyrin repeat domain 36B |
| 203881_s_at | DMD | -5.46 | 0.02 | -2.10 | 0.54 | dystrophin |
| 206030_at | ASPA | -5.41 | <0.01 | -1.51 | 0.74 | aspartoacylase |
The Probe Set ID whose fold change was largest was listed when multiple probe sets were available for same gene. Un-annotated genes were excluded from the top 25 gene list.
Figure 1. Immunohistochemical staining patterns were correlated with cDNA microarray data.
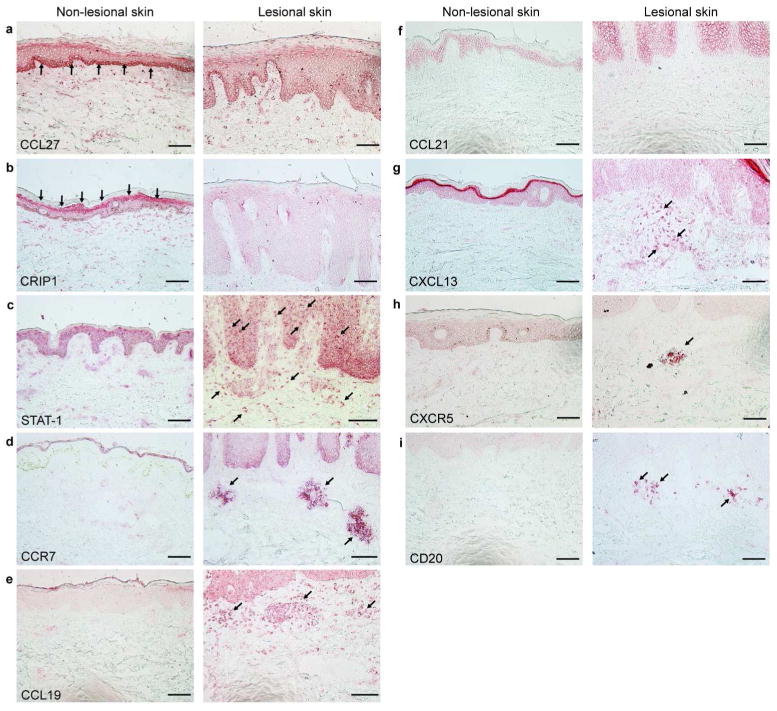
(a) CCL27 was expressed in the basal layer of non-lesional epidermis. (b) CRIP1 was expressed in the granular layer of non-lesional epidermis. (c) STAT-1 stained in the cytosol of cells in non-lesional epidermis, whereas it stained in the nuclei of cells in lesional epidermis and dermis. (d-f) CCR7 and CCL19 were positive in dermal aggregates in lesional skin, whereas CCL21 was detected in neither non-lesional nor lesional skin. (g-h) CXCL13 (3 out of 5 samples) and CXCR5 (6 out of 8 samples) were detected in lesional dermis. (i) CD20+ cells (3 out of 5 samples) were also found in lesional dermis. Arrows indicate the positive layers and cells. Bar=100μm.
We also focused our attention on transcription factors (TFs) identified in the EPI transcriptome. TFs that are considered to be involved in keratinocyte proliferation (NFE2L3, MAFF, and EHF) were up-regulated in lesional EPI, whereas those thought to be involved in keratinocyte differentiation (LASS6, TFAP2B, and GATA3) were down-regulated. The complete gene lists of EPI transcriptome and TFs are found in Tables S2 and S3.
LCM localizes genes selectively expressed in dermal inflammatory cell aggregates of psoriasis lesions
Psoriasis lesions contain dense aggregates of LAMP3/DC-LAMP+ DCs, marking mature DCs, that are intermixed with T cells in the dermis, but gene expression in this “lymphoid” area has not been investigated. Table 3 presents the top 25 up-regulated genes in RD/ICs. Interestingly, six of the 25 were genes encoding chemokines or chemokine receptors, including CCL19 and its receptor CCR7. Genes associated with immune response, such as LAMP3/DC-LAMP, CD28, GZMB, and CD83 are also on this list. The complete list is available in Table S4.
Table 3. A short list of DEGs in psoriasis lesional RD/ICs vs. non-lesional RD (LCM)*.
| Top 25 Up-regulated genes in psoriasis lesional RD/ICs vs. non-lesional RD | ||||||
|---|---|---|---|---|---|---|
| Probe Set ID | Symbol | RD/ICs (LCM) | Bulk | Description | ||
| FCH | FDR | FCH | FDR | |||
| 216834_at | RGS1 | 27.12 | 0.02 | 11.53 | 0.08 | regulator of G-protein signaling 1 |
| 201890_at | RRM2 | 10.66 | 0.04 | 4.29 | 0.23 | ribonucleotide reductase M2 |
| 205569_at | LAMP3 | 10.58 | 0.01 | 4.38 | 0.08 | lysosomal-associated membrane protein 3 (CD208) |
| 204470_at | CXCL1 | 8.02 | 0.08 | 3.88 | 0.36 | chemokine (C-X-C motif) ligand 1 |
| 203559_s_at | ABP1 | 7.70 | 0.02 | 1.45 | 0.82 | amiloride binding protein 1 |
| 209774_x_at | CXCL2 | 7.22 | <0.01 | 1.62 | 0.23 | chemokine (C-X-C motif) ligand 2 |
| 213241_at | PLXNC1 | 7.14 | <0.01 | -1.08 | 0.96 | plexin C1 |
| 220330_s_at | SAMSN1 | 7.14 | 0.02 | 2.82 | 0.21 | SAM domain, SH3 domain and nuclear localization signals 1 |
| 206337_at | CCR7 | 7.10 | 0.01 | 1.75 | 0.38 | chemokine (C-C motif) receptor 7 |
| 209969_s_at | STAT1 | 6.93 | 0.01 | 3.49 | 0.10 | signal transducer and activator of transcription 1 |
| 210072_at | CCL19 | 6.83 | <0.01 | 4.10 | 0.02 | chemokine (C-C motif) ligand 19 |
| 207165_at | HMMR | 6.63 | 0.02 | 2.59 | 0.26 | hyaluronan-mediated motility receptor |
| 206545_at | CD28 | 6.03 | 0.09 | 1.39 | 0.93 | CD28 molecule |
| 219648_at | MREG | 5.94 | 0.04 | 2.15 | 0.49 | melanoregulin |
| 210164_at | GZMB | 5.83 | 0.03 | 3.00 | 0.21 | granzyme B |
| 204440_at | CD83 | 5.58 | <0.01 | 2.10 | 0.03 | CD83 molecule |
| 207861_at | CCL22 | 5.41 | 0.04 | 2.36 | 0.35 | chemokine (C-C motif) ligand 22 |
| 206134_at | ADAMDEC1 | 5.33 | 0.01 | 3.54 | 0.04 | ADAM-like, decysin 1 |
| 204026_s_at | ZWINT | 5.05 | 0.03 | 2.37 | 0.26 | ZW10 interactor |
| 204714_s_at | F5 | 5.05 | 0.01 | -1.29 | 0.84 | coagulation factor V |
| 205242_at | CXCL13 | 4.99 | 0.01 | 2.40 | 0.14 | chemokine (C-X-C motif) ligand 13 |
| 214023_x_at | TUBB2B | 4.93 | 0.06 | -1.20 | 0.96 | tubulin, beta 2B |
| 208103_s_at | ANP32E | 4.57 | 0.02 | 1.33 | 0.79 | acidic nuclear phosphoprotein 32 family, member E |
| 207957_s_at | PRKCB | 4.53 | 0.04 | -1.34 | 0.86 | protein kinase C, β |
| 204932_at | TNFRSF11B | 4.46 | 0.02 | 1.06 | 0.98 | tumor necrosis factor receptor superfamily member 11b |
The Probe Set ID whose fold change was largest was listed when multiple probe sets were available for same gene. Un-annotated genes were excluded from the top 25 gene list.
Confirmation by quantitative RT-PCR (qRT-PCR) for EPI and RD transcriptomes were performed on six specific mRNAs as shown in Figure S3a. There is a high correlation between differential expressions detected by the double amplification based microarray and qRT-PCR (r=0.87, p=0.0002, Figure S3b).
Protein expression of detected genes in dermal aggregates was confirmed by IHC and immunofluorecent staining (IF)
To further confirm the differential expression of certain targets, tissue sections were examined by IHC.
STAT1 stained in the cytosol of cells in non-lesional epidermis and in the nucleus of cells in lesional epidermis. In addition, STAT1+ cells were increased in lesional dermis compared to non-lesional dermis (Figure 1c). CCR7 and CCL19 both stained in dermal aggregates of lesional skin (Figure 1d-e). CCL21, another ligand for CCR7, was not detected by IHC, consistent with our microarray data, where expression of CCL21 was quite low. CXCL13/BCA-1 was detected in lesional dermis (Figure 1g). CXCR5, a receptor for CXCL13/BCA-1, and CD20, B cell marker, were also positive in lesional dermis (Figure 1h-i). Collectively, differences in gene expression of these molecules obtained from microarray analysis using LCM samples correlated very well to differences in their protein expression in tissues.
To further explore the nature of chemokines and relevant receptors in skin tissues, we performed double-label IF. CCR7 and CCL19 were expressed in close proximity in lesional dermal aggregates (Figure 2a). CCR7 co-stained with LAMP3/DC-LAMP and the T cell marker CD3 (Figure 2b-c). CCL19 co-stained with myeloid DC marker CD11c, LAMP3/DC-LAMP, and CD3 (Figure 2d-f). CXCL13/BCA-1 and CXCR5 were also expressed in close proximity in lesional dermal aggregates (Figure 2g). CXCR5+ cells co-stained with CD20 (Figure 2h). These results demonstrate that dermal aggregates in psoriasis, mainly composed of DCs, T cells, and to a lesser degree B cells, were associated with the coordinated expression of lymph node organizing chemokines and their receptors.
Figure 2. Characterization of lymphoid tissue like structures in lesional skin.
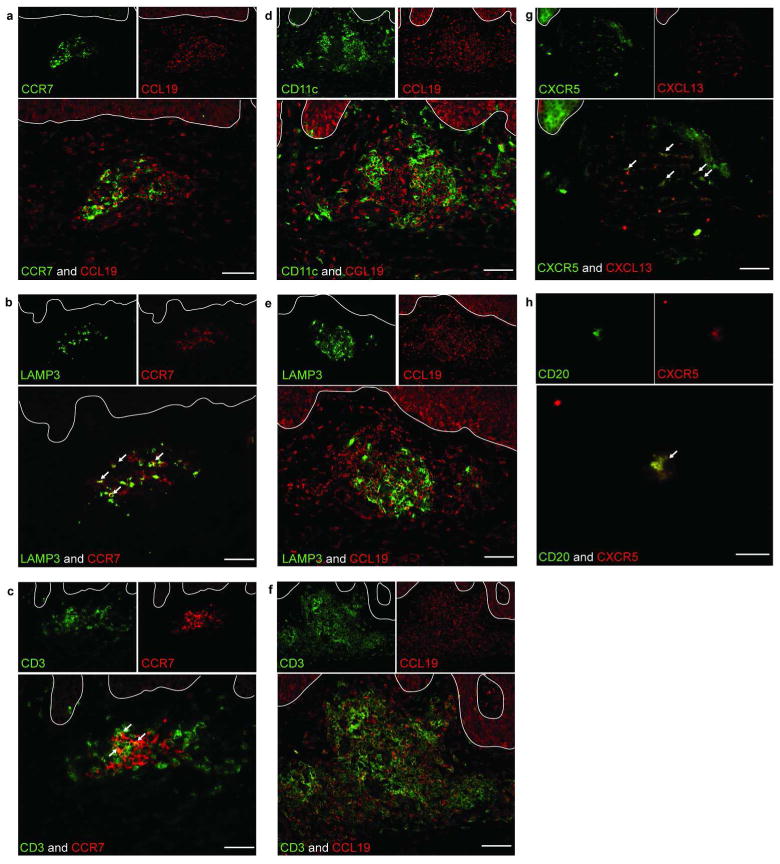
(a) Both CCR7 and CCL19 were localized in dermal aggregates of the lesional skin. (b) LAMP3/DC-LAMP was co-expressed with CCR7. (c) CD3 was also co-expressed with CCR7. (d-f) CCL19 expression was detected on CD11c+ (d) and LAMP3/DC-LAMP+ DCs (e) as well as CD3+ T cells (f). We also stained CXCR5 in combination with CXCL13 and CD20. (g-h) Both CXCL13 and CXCR5 stained in dermal aggregates of lesional skin (g) and CXCR5 co-stained with CD20 (h). White arrows indicate the double positive cells. White lines represent epidermal dermal junctions. Bar=100μm.
LCM detects more DEGs than bulk-tissue analysis
A more detailed discussion can be found in SRD section 2. The psoriasis transcriptome, defined as the DEGs between lesional and non-lesional samples using FCH>2.0 and FDR<0.1 cut-offs, was determined for bulk skin samples (psDEGs-Bulk), laser-captured EPI (psDEGs-EPI), and laser-captured RD (psDEGs-RD), which tends to include ICs in lesional samples. psDEGs-Bulk contained 155/58 (up-/down-regulated) probe-sets (Table S5). psDEGs-EPI contained 815/631 probe-sets (Table S2), and psDEGs-RD contained 309/181 probe-sets (Table S4). The psoriatic transcriptome for PD was not defined because of an insufficient amount of RNA obtained from non-lesional PD (Figure S1c). Scatter-plots show positive correlations between Log2FCH of Bulk and those of EPI and RD (Figure 3a-b). Correlation between Bulk and EPI was higher (r=0.68, p<1×10-16) than that between Bulk and RD (r=0.31, p<1×10-16), suggesting a greater contribution of EPI-related genes than RD-related genes in Bulk analysis. Importantly, trend lines (red lines in Figure 3a-b) were shifted towards larger FCH in LCM samples compared to Bulk samples. In fact, 76.47% of probe-sets with absolute FCH>2.0 in both EPI and Bulk showed higher FCH in EPI than Bulk, and for RD it was 58.61% (Figure 3c and S2d, see SRD section 2-i). These results may reflect the overall increase in DEGs detected via LCM compared to bulk-tissue analysis (Figure 3d-e). We acknowledge that, as we expected, many fewer genes were detected in our Bulk analysis compared to previously published studies with larger patient cohorts (n=26, Yao et al. 2008; n=15, Suárez-Fariñas et al. 2010; n=58, Gudjonsson et al. 2010, see a discussion of the effect of sample size in SRD section 2-ii). We further compared our uniquely detected genes in EPI (644 up- and 586 down-regulated) and RD (241 up- and 146 down-regulated) to the psoriasis transcriptomes published in the three studies listed above as well as that detected by next generation sequencing (NGS) technology (Jabbari et al, 2012). Approximately 50% of unique genes in EPI and 30% of unique genes in RD were confirmed in at least one out of the four transcriptomes (Figure 3f-g). Furthermore, we performed RT-PCR on the top eight unique down-regulated genes (ABAT, COL4A5, CXCR7, DDB2, ERAP1, NRTN, SRPX, and TCF4) that were not detected in the above four studies and we show that expression of each gene was clearly down-regulated in our bulk-tissue samples (Figure 3h). Our LCM-unique DEGs thus have the potential to provide many new genes that have not previously been associated with the psoriasis transcriptome. The complete list of LCM-unique probe-sets is provided in Table S6.
Figure 3. Unique detection of DEGs by LCM samples compared to Bulk skin sample.
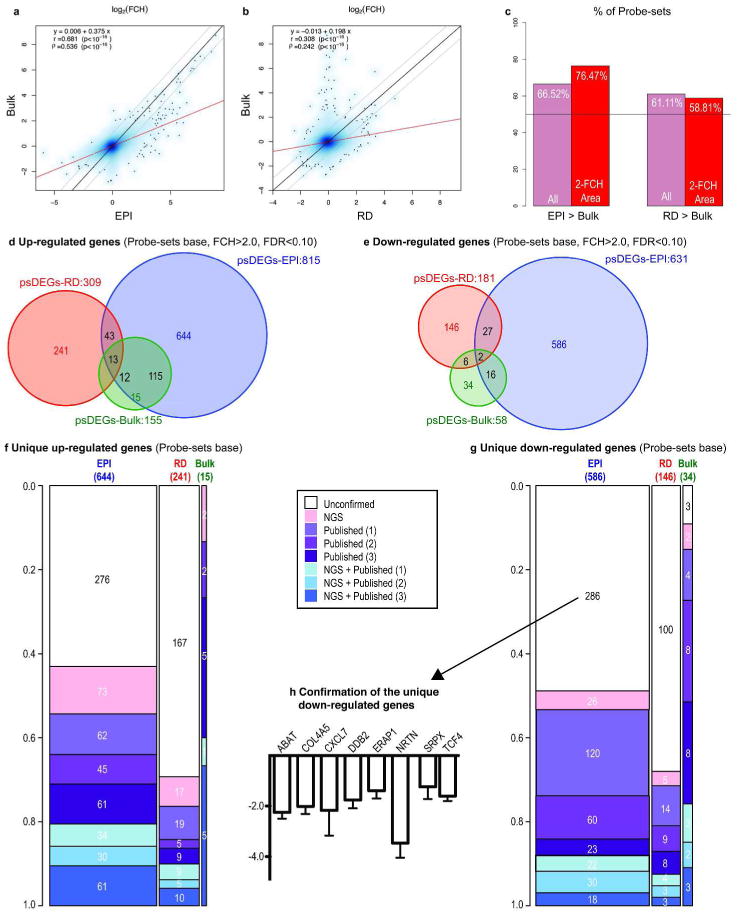
(a-b) Scatterplots of Log2FCH of Bulk vs. EPI and RD. Black lines: identity lines; gray lines: ±2-FCH; red lines: robust linear regression estimates. (c) Percentage of probe-sets with larger absolute FCH in LCM samples than in Bulk. Pink bars: all probe-sets; red bars: probe-sets considering |FCH|>2.0 (d-e) Venn-Diagrams of Bulk, EPI and RD psoriasis transcriptome. (f-g) Proportion of unique EPI-, RD-, and Bulk-related probe-sets that appear in 1, 2 or 3 of the published Affymetrix studies (considering HU133A2.0 probe-sets) or in the NGS-transcriptome. The width of each bar depicts the number in each category. (h) RT-PCR fold changes of the top eight unconfirmed down-regulated genes using bulk tissue samples (n=9).
Discussion
By combining LCM and cDNA microarray analysis, we sought to define gene expression within different regions of human skin, as altered by psoriasis vulgaris. We focused on the cellular aggregates found in psoriatic lesional dermis, where LAMP3/DC-LAMP+ DCs have been identified along with T cells (Zaba et al. 2009). The importance of this aggregate structure in the pathogenesis of psoriasis is suggested by the observations that it is not identified in non-lesional skin and that effective treatment leads to its disappearance (Zaba et al. 2007).
Psoriasis vulgaris contains at least two distinct subsets of myeloid DCs: BDCA1- and BDCA1+ DCs. The BDCA1- subset includes immature/inflammatory TNFα and iNOS-producing DCs and their distribution is rather scattered (Lowes et al. 2005). BDCA1+ DCs are immature and do not express LAMP3/DC-LAMP in normal skin. However, psoriasis lesions contain clusters of LAMP3/DC-LAMP+ DCs bearing BDCA1. We have previously compared the gene expression profiles of BDCA1- and BDCA1+ DCs sorted by FACS from psoriasis skin (Zaba et al. 2010). Although BDCA1+ DCs are phenotypically matured compared to BDCA1- DCs based on much greater expression of CD83, CD86, and HLA-DR by flow cytometric analysis (Zaba et al. 2009), these molecules were not recognized as DEGs in that study (Zaba et al. 2010). In contrast, we were able to identify CD83, CD86, and LAMP3/DC-LAMP as up-regulated DEGs in lesional RD/ICs. Because LCM permits us to identify mature DCs, we are thus able to study genes associated with the mature DC subsets of psoriasis.
Using LCM, we established the ectopic expression of CCL19 and its receptor CCR7 in the dermal cellular aggregates of lesional skin. Using bulk tissue extracts, Zhou et al. (2003) also detected the up-regulation of CCL19 and CCL21 mRNA in psoriasis skin. However, to date, the cells producing these key lymphoid-organizing chemokines have not been localized in vivo. We show that CCL19 production occurs selectively within perivascular T cell and DC aggregates and that it likely acts to recruit CCR7+ (self-antigen-specific) T cells and DCs into this focus. Approximately 80% skin resident T cells are thought to be CCR7- effector-memory T cells (TEM) (Clark et al, 2006) and there is a concept that CCR7+ central-memory T cells (TCM) circulate from blood to draining lymph node in order to be activated by DCs (Clark, 2010). Our results, however, propose that the organized aggregates in psoriatic skin contain a sizable number of CCR7+ T cells, which would be TCM that are normally circulating between blood and lymph nodes. Hence skin lesion may create the same environment for expansion of TEM from TCM. The concept of lymphoid organization in lesions is supported by a previous report showing in transgenic mice that ectopic expression of CCL19 alone could organize functional lymphoid structures within the pancreas (Luther et al. 2002). With regard to DC maturation, Gillet's group recently published that self-RNA-LL37 complexes could activate myeloid DCs through TLR8 in vitro. Moreover, these complexes were co-localized with LAMP3/DC-LAMP in psoriatic lesional dermis (Ganguly et al. 2009). Together with our data presented here, this suggests that DC maturation could reflect both chemoattraction of precursors from blood and local activation by self-RNA-LL37 complexes.
Many genes altered in the psoriatic epidermis have been studied previously with bulk gene-sets. We showed a heavy representation of epidermis-related genes in Bulk analysis. However, many DEGs detected in prior studies have not been localized to defined skin regions or specific cell types. With LCM, we were able to successfully localize to EPI or RD 935 probe-sets that were detected in at least one of the three cited studies analyzing bulk-tissues (Figure S5a-b, see SRD section 2-ii). For example, CRIP-1, which is the second largest down-regulated gene in lesional EPI, has not previously been localized to the epidermis, although the down-regulation of this gene is consistent across earlier bulk studies. We also explored major differences in the expression of transcription factors (TFs) between lesional and non-lesional EPI. NFE2L3 and MAFF were up-regulated in lesional EPI. NFE2L3 is a member of the “cap‘n’collar” family of TFs that dimerize with other leucine zipper proteins, such as small Maf proteins (Kobayashi et al. 1999). NFE2L2, another TF of this family, was shown to be involved in the proliferation and KRT6 expression of forestomach keratinocytes in mice when it dimerizes with MAFF (Motohashi et al. 2004). Although NFE2L3 but not NFE2L2 appeared on our EPI list, NFE2L3 is known to partially compensate for the role of NFE2L2 in mice keratinocytes during re-epithelialization in wound healing (Bauns et al. 2002). These results suggest the possible involvement of NFE2L3 together with MAFF in the proliferation of keratinocytes in psoriasis. In contrast, LASS6, TFAP2B, and GATA3 were down-regulated in lesional EPI. LASS6 is involved in ceramide synthesis in mice (Mizutani et al. 2005). TFAP2B increases cystatin A expression in normal human keratinocytes (Takahashi et al. 2000). GATA3 is a master TF for the differentiation of Th2 cells (Ho et al. 2009), but it also transactivates the lipid acyltransferase gene AGPAT5, leading to the formation of epidermal barrier (de Guzman Strong et al. 2006). These genes are therefore involved in terminal differentiation of keratinocytes. This TF gene list thus induces the key genes involved in keratinocyte proliferation and differentiation and reflects hyper-proliferation of keratinocytes and impaired differentiation in psoriatic epidermis.
Two concerns have been raised regarding applying LCM to global gene expression analysis. The first is that “extreme” gene amplification from small mRNA amounts obtained by LCM may bias the set of genes amplified such that it is not representative of gene pools amplified by conventional one-cycle method (Wang et al. 2007). The second, which comes from a variety of cancer analyses, is that it may be difficult to derive normal cells by LCM for comparison to pathological counterparts (Klee et al. 2009). Both issues have been addressed in this study, and relevant data and discussion are presented in SRD section 1. In contrast to prior work, we found an extremely high concordance between genes detected in psoriasis tissue using single and double amplification methods (r=0.93, p<1×10-16, Figure S4d). In this sense, our study was more comparable to that using high quality control mRNA pools (Singh et al. 2005) than to those doing gene detection in biopsies of human cancers (Luzzi et al. 2003; de Bruin et al. 2005; Klee et al. 2009). The second concern regarding the comparison of pathological to normal cell counterparts is well obviated by using skin, where tissue structure makes it possible to derive intrinsically valid comparisons. We therefore did not detect limitations of the LCM method compared to bulk-tissue analysis. We found it instead to be advantageous, providing us the ability to localize and increase disease-related gene products to specific cells/skin regions. Though our Bulk comparison identified a smaller DEG set than previously published studies, we established through statistical simulations that this was due mainly to our study's much smaller sample size (n=3) and consequent weaker statistical power (see SRD section 2-ii). Using LCM we identified several hundred new DEG products in psoriatic EPI+RD that were not detected in the three large studies, suggesting greater sensitivity for detection of some gene products. Still, the greatest advantage of this technique is the ability to study defined cellular regions of the epidermis or dermis that could not otherwise be separated from intact skin by enzymatic or other physical methods (Clemmensen et al. 2009).
In conclusion, we have established the validity of the combined use of LCM and cDNA microarray technologies. We demonstrated that this approach, while not replacing conventional bulk-tissue analysis, complements it through 1) localization of transcripts to specific cells or regions, and 2) more sensitive detection of transcripts within small regions of tissue, particularly where dilution of transcripts associated with “minority” cell populations may occur. It would be interesting to apply this method to other conditions in which focal cellular processes may differ; to explore, for example, cells specific to the invasive edge of a cancer or cells of certain appendages in the skin.
Materials and Methods
The detailed protocols and statistical analysis are described in supplemental materials and methods.
Patients and Samples
Institutional review board (The Rockefeller University) and written informed consent were obtained before enrolling patients to participate in this study. The study was performed in adherence with the Declaration of Helsinki Principals. Paired lesional and non-lesional samples from 7 psoriatic patients were used. Samples from the first four patients were used in the single vs. double amplification comparison. Samples from the remaining three patients were subjected to LCM.
LCM
LCM was performed according to the manufacturer's protocol for the CellCut system (Molecular Machines and Industries).
RNA extraction
Total RNA was extracted from bulk tissue homogenates, sliced frozen tissue sections, and microdissected samples using RNeasy Kit (QIAGEN).
cDNA microarray analysis
Target amplification and labeling was performed according to the Affymetrix protocols for one-cycle or two-cycle cDNA synthesis. Two-cycle cDNA synthesis was slightly modified according to a previous report (Kube et al, 2007). Affymetrix HGU133A2.0 arrays were used. The data has been deposited at the Gene Expression Omnibus repository (GSE26866).
qRT-PCR on LCM samples
First-strand cDNA synthesis was performed using SuperScriptIII and Random Primers (Invitrogen). The resulting cDNA was used for quantitative PCR reaction. All data were normalized to RPLP0/hARP (Wingens et al. 1998). Primers and probes used in this experiment are listed in Table S12.
IHC and IF
Frozen skin sections were prepared and standard procedures were used. Antibodies used in this experiment are listed in Table S13.
Statistical analysis
Microarray data was analyzed using GeneSpring GX version10.0 software (Agilent) and R/Bioconductor packages. The Harshlight package (Suárez-Fariñas et al. 2005) was used to scan Affymetrix chips for spatial artifacts. Expression values were obtained using the RMA procedure. Expression values were modeled using the mixed-effect framework of Bioconductor's limma package. Genes with FDR<0.1 and FCH>2.0 were considered as DEGs. Probe-set distance to the 3′ end of the transcriptome was compared between single and double amplified samples according to a previous report (Klee et al, 2009).
Supplementary Material
Acknowledgments
This research was supported by the Milstein Medical Program and in part by National Institutes of Health (NIH) grant UL1 RR024143. We appreciate the assistance and advice from the Genomic Resource Center (Zhang, W. and Zhao, C.) at The Rockefeller University. We thank Michelle A. Lowes for helpful comments and discussions on this manuscript.
Abbreviations used in this manuscript
- LCM
Laser capture microdissection
- DEGs
differentially expressed genes
- qRT-PCR
quantitative reverse-transcribe polymerase chain reaction
- FCH
fold change
- FDR
false discovery rate
- EPI
epidermis
- PD
papillary dermis
- RD
reticular dermis
- ICs
dermal inflammatory cell aggregates
- psDEGs
psoriatic transcriptome
- IHC
immunohistochemistry
- IF
immunofluorecent staining
- DC
dendritic cell
- TF
transcription factor
- NGS
next generation sequencing
- SRD
- TEM
effector-memory T cells
- TCM
central-memory T cells
Footnotes
Conflict of Interests: The authors declare no competing financial interests.
References
- Bowcock AM, Shannon W, Du F, et al. Insights into psoriasis and other inflammatory diseases from large-scale gene expression studies. Hum Mol Genet. 2001;10:1793–1805. doi: 10.1093/hmg/10.17.1793. [DOI] [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann MG, et al. Nrf2 Transcription Factor, a Novel Target of Keratinocyte Growth Factor Action Which Regulates Gene Expression and Inflammation in the Healing Skin Wound. Mol Cell Biol. 2002;22:5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dematol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmesen A, Thomassen M, Clemmesen O, et al. Extraction of high-quality epidermal RNA after ammonium thiocyanate-induced dermo-epidermal separation of 4 mm human skin biopsies. Exp Dermatol. 2009;18:979–984. doi: 10.1111/j.1600-0625.2009.00921.x. [DOI] [PubMed] [Google Scholar]
- Clément-Ziza M, Gentien D, Lyonnet S, et al. Evaluation of Methods for Amplification of Picogram Amounts of Total RNA for Whole Genome Expression Profiling. BMC Genomics. 2009;10:246–260. doi: 10.1186/1471-2164-10-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin EC, van de Pas S, Lips EH, et al. Macrodissection versus microdissection of rectal carcinoma: minor influence of stroma cells to tumor cell gene expression profiles. BMC Genomics. 2005;14:142. doi: 10.1186/1471-2164-6-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guzman Strong C, Wertz PW, Wang C, et al. Lipid Defect Underlies Selective Skin Barrier Impairment of an Epidermal-Specific Deletion of Gata-3. J Cell Biol. 2006;175:661–670. doi: 10.1083/jcb.200605057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espina V, Wulfkuhle JD, Calvert VS, et al. Laser-Capture Microdissection. Nat Protoc. 2006;1:586–603. doi: 10.1038/nprot.2006.85. [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Johnston A, et al. Assessment of the Psoriatic Transcriptome in a Large Sample: Additional Regulated Genes and Comparisons with In Vitro Models. J Invest Dermatol. 2010;130:1829–1840. doi: 10.1038/jid.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider AS, Duculan J, Whynot JA, et al. Increased JunB mRNA and protein expression in psoriasis vulgaris lesions. J Invest Dermatol. 2006;126:912–914. doi: 10.1038/sj.jid.5700183. [DOI] [PubMed] [Google Scholar]
- Haider AS, Lowes MA, Suárez-Fariñas M, et al. Cellular Genomic Maps Help Dissect Pathology in Human Skin Disease. J Invest Dermatol. 2008;128:606–615. doi: 10.1038/sj.jid.5701067. [DOI] [PubMed] [Google Scholar]
- Ho IC, Tai TS, Pai SY. GATA3 and the T-Cell Lineage: Essential Functions Before and After T-helper-2-cell Differentiation. Nat Rev Immunol. 2009;9:125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari A, Suárez-Fariñas M, Dewell S, et al. Transcriptional Profiling of Psoriasis Using RNA-seq Reveals Previously Unidentified Differentially Expressed Genes. J Invest Dermatol. 2012;132:246–249. doi: 10.1038/jid.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Crispin M, Billick E, Mitsui H, et al. Human keratinocytes have a response to injury that upregulates CCL20 and other genes linking innate and adaptive immunity. J Invest Dermatol. 2012;132:105–113. doi: 10.1038/jid.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee EW, Erdogan S, Tillmans L, et al. Impact of Sample Acquisition and Linear Amplification on Gene Expression Profiling of Lung Adenocarcinoma: Laser Capture Micro-Dissection Cell-Sampling versus Bulk Tissue-Sampling. BMC Med Genomics. 2009;2:13–23. doi: 10.1186/1755-8794-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Ito E, Toki T, et al. Molecular Cloning and Functional Characterization of a New Cap‘n’ Collar Family Transcription Factor Nrf3. J Biol Chem. 1999;274:6443–6452. doi: 10.1074/jbc.274.10.6443. [DOI] [PubMed] [Google Scholar]
- Kube DM, Savci-Heijink CD, Lamblin AF, et al. Optimization of Laser Capture Microdissection and RNA Amplification for Gene Expression Profiling of Prostate Cancer. BMC Mol Biol. 2007;8:25–38. doi: 10.1186/1471-2199-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Chamian F, Abello MV, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc Natl Acad Sci USA. 2005;102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Bidgol A, Hargreaves DC, et al. Differing Activities of Homeostatic Chemokines CCL19, CCL21, and CXCL12 in Lymphocyte and Dendritic Cell Recruitment and Lymphoid Neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- Luzzi V, Mahadevappa M, Raja R, et al. Accurate and reproducible gene expression profiles from laser capture microdissection, transcript amplification, and high density oligonucleotide microarray analysis. J Mol Diagn. 2003;5:9–14. doi: 10.1016/S1525-1578(10)60445-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbridge JN, Knapp AM. Changes in Keratinocyte Maturation During Wound Healing. J Invest Dermatol. 1987;89:253–263. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and Its Related Family Members Regulate Synthesis of Specific Ceramides. Biochem J. 2005;390:263–271. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, Katsuoka F, Engel JD, et al. Small Maf Proteins Serve as Transcriptional Cofactors for Keratinocyte Differentiation in the Keap1-Nrf2 Regulatory Pathway. Proc Natl Acad Sci U S A. 2004;101:6379–6384. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rømer J, Hasselager E, Nørby PL, et al. Epidermal overexpression of interleukin-19 and -20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine a or calcipotriol. J Invest Dermatol. 2003;121:1306–11. doi: 10.1111/j.1523-1747.2003.12626.x. [DOI] [PubMed] [Google Scholar]
- Singh R, Maganti RJ, Jabba SV, et al. Microarray-Based Comparison of Three Amplification Methods for Nanogram Amounts of Total RNA. Am J Physiol Cell Physiol. 2005;288:1179–1189. doi: 10.1152/ajpcell.00258.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Fariñas M, Pellegrino M, Wittkowski KM, et al. Harshlight: a “corrective make-up” program for microarray chips. BMC Bioinformatics. 2005;6:294–304. doi: 10.1186/1471-2105-6-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Fariñas M, Lowes MA, Zaba LC, et al. Evaluation of he psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA) Plos one. 2010;5:e10247. doi: 10.1371/journal.pone.0010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Oyama N, Itoh Y, et al. Transcriptional factor AP-2gamma increases human cystatin A gene transcription of keratinocytes. Biochem Biophys Res Commun. 2000;278:719–723. doi: 10.1006/bbrc.2000.3850. [DOI] [PubMed] [Google Scholar]
- Wang E, Panelli M, Marincola FM. Complementary techniques: RNA amplification for gene profiling analysis. Adv Exp Med Biol. 2007;593:39–53. doi: 10.1007/978-0-387-39978-2_5. [DOI] [PubMed] [Google Scholar]
- Wingens M, van Bergen BH, Hiemstra PS, et al. Induction of SLPI (ALP/HUSI-I) in Epidermal Keratinocytes. J Invest Dermatol. 1998;111:996–1002. doi: 10.1046/j.1523-1747.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- Yao Y, Richman L, Morehouse C, et al. Type I Interferon: Potential Therapeutic Target for Psoriasis? PLoS One. 2008;3:e2737. doi: 10.1371/journal.pone.0002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis Is Characterized by Accumulation of Immunostimulatory and Th1/Th17 Cell-Polarizing Myeloid Dendritic Cells. J Invest Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J Allergy Clin Immunol. 2010;125:1261–1268. doi: 10.1016/j.jaci.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Krueger JG, Kao MC, et al. Novel Mechanisms of T-Cell and Dendritic Cell Activation Revealed by Profiling of Psoriasis on the 63,100-element Oligonucleotide Array. Physiol Genomics. 2003;13:59–78. doi: 10.1152/physiolgenomics.00157.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


