Abstract
Many biologic functions follow circadian rhythms driven by internal and external cues that synchronize and coordinate organ physiology to diurnal changes in the environment and behavior. Urinary acid-base parameters follow diurnal patterns and it is thought these changes are due to periodic surges in gastric acid secretion. Abnormal urine pH is a risk factor for specific types of nephrolithiasis and uric acid stones are typical of excessively low urine pH. Here we placed 9 healthy volunteers and 10 uric acid stone formers on fixed metabolic diets to study the diurnal pattern of urinary acidification. All showed clear diurnal trends in urinary acidification but none of the patterns were affected by inhibitors of the gastric proton pump. Uric acid stone formers had similar patterns of change through the day but their urine pH was always lower compared to healthy volunteers. Uric acid stone formers excreted more acid (normalized to acid ingestion) with the excess excreted primarily as titratable acid rather than ammonium. Urine base excretion was also lower in uric acid stone formers (normalized to base ingestion) along with lower plasma bicarbonate concentrations during part of the day. Thus, increased net acid presentation to the kidney and the preferential use of buffers, other than ammonium, result in much higher concentrations of un-dissociated uric acid throughout the day and consequently an increased risk of uric acid stones.
Introduction
A property intrinsic to living organisms is a biologic circadian clock which is organized and oscillates at multiple hierarchies at both cellular 1,2 and multi-cellular levels 3,4 in both the animal and plant kingdoms 5,6. In mammalian biology, many aspects of behavior and physiology follow cyclic rhythms which are generally presumed to confer adaptive advantage by coordinating behavior and organ physiology to ambient day-and-night cycles 4. In the kidney, significant circadian rhythms exist for multiple renal hemodynamic, glomerular and tubular parameters 7,8. These renal circadian rhythms are influenced by external cues such as feeding, ambient light, and activity, as well as inherently by intrinsic clocks 9,10.
In 1845, Henry Bence Jones, who is considered the pioneer of urinary chemistry for his studies of urinary light chains, glucose, and cystine in disease states 11, noted diurnal variation in urine pH in normal individuals 12. Subsequent studies also demonstrated morning alkaline and evening acidic trends of urine although this finding was not always uniformly observed 13-18. However, the precise circadian profile of urine acidification remains incompletely defined, and the factors responsible for hour-to-hour fluctuations in pH are not known. Gastric acid secretion with the concomitant alkalization of plasma has been proposed to be the origin of postprandial changes in plasma and urine pH 19,20. In addition, it is unclear whether renal disorders affect circadian patterns of urinary chemistry and whether such derangements in rhythmic changes in urinary acidification contribute to pathophysiology.
Although the kidney is capable of elaborating urine at an enormously wide range of hydrogen ion concentrations when stressed (pH from <5 to >8; [H+] from < 10nM to > 10 μM), normal day-to-day urine pH is poised within a much narrower span in humans somewhere between pH 5.5 and 6.5. Acidification of urine is of critical importance for prevention of calcium phosphate crystallization in the urinary space 21-23. However, urinary pH cannot be lowered too much because of another constraint in higher primates who maintain relatively high plasma and urinary uric acid 22,24. Urinary acidification to below pH 5.5, while protective against calcium phosphate precipitation, poses a substantial risk of uric acid precipitation 22. Precipitation of calcium phosphate and uric acid thus set the upper and lower limits of urinary pH respectively and calcium phosphate and uric acid nephrolithiasis in fact represent quintessential clinical disorders of urinary pH.
In humans with uric acid nephrolithiasis, excessively acidic urine causes titration of urate to the highly insoluble uric acid despite normal or even low total uric acid content in urine. The pathogenesis of low urine pH has been ascribed to both increased acid load to the kidney and defective utilization of ammonia in urinary buffering 25-31. It is not clear whether the unduly acidic urine in uric acid stone formers occurs at specific intervals or persists throughout the day. Current standard clinical practice, clinical investigations, and clinical trials, all use 24-hr urine collections to assess risk for uric acid stones and adequacy of response to therapy. We reported that during treatment of uric acid nephrolithiasis with alkali, excessive nocturnal and early morning urinary acidity can linger despite apparent alkalinization of pooled 24-hr urine 32. This can potentially result in a false sense of security for the clinician that uric acid stone risk is eliminated but elevated propensity for uric acid precipitation still persist in the patient during specific periods of the day. While most patients likely respond to day time alkali therapy, the possibility remains in some individuals where persistent early morning aciduria can sustain the elevated stone risk despite alkali therapy.
The study of 24-hr urine profiles in uric acid stone formers will enhance our understanding of its pathophysiology and the origin of the excessive aciduria. It will also guide us in designing better clinical tests for diagnosis and monitoring of therapy in uric acid nephrolithiasis. We embarked to detail the circadian pattern of urinary acidification parameters in normal volunteers to delineate normal circadian physiology and uric acid stone formers to identity pathophysiologic defects. In addition, we tested the longstanding belief that gastric acid secretion contributes to changes in urinary pH by blocking gastric acid production in the two groups of subjects.
Results
Demographic Characteristics
The demographic characteristics of the study population are depicted in Table 1. Ten UASF and nine HV participated in the study. Most of the subjects in both groups were male and non-Hispanic Caucasians which is typical of UASF’s. The mean age did not differ significantly between the two groups. The UASF’s weighed more and had a higher BMI than the HV’s.
Table 1.
Patient Demographic Data
| Healthy Volunteers (HV) |
Uric Acid Stone Formers (UASF) |
|
|---|---|---|
| Gender: M/F | 6/3 | 9/1 |
| Race: (white / black) | 7/2 | 9/1 |
| Ethnicity: (non-Hispanic / Hispanic) | 9/0 | 9/1 |
| Age (years) | 52.8 ± 13.1 | 57.0 ± 8.2 |
| Weight (kg) | 85 ± 19 | 109 ± 19 * |
| Height (cm) | 171 ± 10 | 172 ± 6 |
| Body Mass Index (kg/m2) | 28.5 ± 4.3 | 36.9 ± 6.8 * |
p<0.05 t-test
Serum and Urine Chemistry
Table 2 shows the fasting serum chemistry for both groups in each study phase. In both phases, UASF had a slightly higher serum creatinine due to higher creatinine production rate but creatinine production rate per body mass was not different between the two groups. Most importantly, creatinine clearance was not different between the two groups. Serum uric acid was persistently higher in UASF’s in both phases of the study. Serum bicarbonate, chloride, and venous pH did not differ between the two groups. UASF’s taking placebo demonstrated a very slight but statistically significantly higher serum potassium than in the PPI phase and higher than HV’s in both phases.
Table 2.
Fasting Serum Profile
| Placebo | PPI | |||
|---|---|---|---|---|
| HV | UASF | HV | UASF | |
| Creatinine (mg/dL) (μM) |
0.84 ± 0.13 74 ± 11 |
1.06 ± 0.02 a 94 ± 1.7 |
0.88 ± 0.15 78 ± 13 |
1.12 ± 0.21 c 99 ± 18 |
| Creatinine Clearance (ml/min) (ml/min/1.73m2) |
139 ± 35 120 ± 20 |
131 ± 39 100 ± 26 |
129 ± 35 112 ± 17 |
126 ± 36 95 ± 23 |
| Glucose (mg/dL) (mM) |
97 ± 10 5.4 ± 0.6 |
103 ± 25 5.6 ± 1.4 |
96 ± 6 5.3 ± 0.3 |
106 ± 26 5.0 ± 1.4 |
| Uric Acid (mg/dL) (μM) |
6.0 ± 1.6 357 ± 95 |
8.0 ± 1.5 a 476 ± 89 |
6.3 ± 1.6 375 ± 95 |
8.1 ± 1.5 c 482 ± 89 |
| Sodium (mEq/L) | 139 ± 3 | 138 ± 3 | 138 ± 2 | 138 ± 2 |
| Potassium (mEq/L) | 4.0 ± 0.3 | 4.5 ± 0.7 a | 3.9 ± 0.2 | 4.0 ± 0.3 b |
| Chloride (mEq/L) | 106 ± 3 | 107 ± 2 | 107 ± 2 | 107 ± 3 |
| Bicarbonate (mEq/L) | 27.0 ± 1.3 | 26.1 ± 3.3 | 26.7 ± 1.5 | 25.9 ± 1.1 |
| Venous pH | 7.41 ± 0.02 | 7.40 ± 0.02 | 7.41 ± 0.01 | 7.40 ± 0.01 |
HV: Healthy volunteers, UASF: Uric acid stone formers
p < 0.05 UASF vs HV; on placebo
p < 0.05 UASF placebo vs UASF on PPI
p< 0.05 UASF vs HV; on PPI
Comparisons made with mixed-model repeated measures analysis.
Urine was collected for 24 hours prior to each diurnal study and results are shown in Table 3. In both study phases, urine pH in UASF was significantly lower than in HV (UASF vs. HV in placebo phase: 5.42 ± 0.36 vs. 5.86 ± 0.28; p = 0.01; UASF vs. HV in PPI phase: 5.32 ± 0.36 vs. 5.93 ± 0.23; p < 0.001). Urinary sodium, potassium, sulfate, and phosphate did not differ among the groups indicting equivalence of dietary intake. In both phases, citrate excretion was numerically lower in UASF but the difference was not statistically significant. Twenty-four hour Titrable acidity and NAE was higher, and urinary ammonium was lower numerically in UASF compared with HV although none of the differences reached a p value of <0.05 in this small cohort. However, the proportion of NAE as ammonium (NH4/NAE) was significantly lower in UASF (UASF vs. HV in placebo phase: 0.55 ± 0.08 vs. 0.75 ± 0.07; p = 0.003; UASF vs. HV in PPI phase: 0.55 ± 0.15 vs. 0.78 ± 0.16, p = 0.001). Twenty-four hour urinary uric acid excretion did not differ between the groups. There was no difference between the placebo and PPI phase.
Table 3.
Baseline 24 hour Urine Chemistry
| Placebo | PPI | |||
|---|---|---|---|---|
| HV | UASF | HV | UASF | |
| Total Volume (L/d) | 2.77 ± 0.91 | 2.07 ± 0.67 | 2.69 ± 1.02 | 2.33 ± 0.86 |
| pH | 5.86 ± 0.28 | 5.42 ± 0.36 a | 5.93 ± 0.23 | 5.32 ± 0.36 b |
| Creatinine (gm/d) (mmoles/d) |
1.7 ± 0.6 15.0 ± 5.3 |
1.9 ± 0.5 a 18.8 ± 4.4 |
1.6 ± 0.5 14.2 ± 4.4 |
2.0 ± 0.5 17.7 ± 0.5 |
| Potassium (mEq/d) | 43 ± 13 | 45 ± 12 | 45 ± 14 | 42 ± 13 |
| Sulfate (mEq/d) | 41.2 ± 9.6 | 41.0 ± 9.0 | 39.6 ± 10 | 37.4 ± 12 |
| Phosphate (mg/dl) (mM) |
704 ± 291 22.7 ± 9.4 |
807 ± 304 26.0 ± 9.8 |
683 ± 307 22.0 ± 9.9 |
789 ± 220 22.5 ± 7.1 |
| Ammonium (NH4) (mEq/d) | 39 ± 15 | 32 ± 16 | 39 ± 19 | 33 ± 12 |
| Titratable Acid (mEq/d) | 24 ± 9 | 33 ± 9 c | 25 ± 11 | 34 ± 9 e |
| Citrate (mEq/d) | 9.9 ± 3.7 | 7.3 ± 4.8 d | 9.1 ± 2.2 | 6.2 ± 3.8 b |
| Bicarbonate (mEq/d) Median [range] |
1.1 ± 2.1 0 [0 – 5.6] |
0.3 ± 0.9 0 [0 – 2.72] |
1.6 ± 2.1 0 [0 – 5.9] |
0.4 ± 1.1 b 0 [0 – 3.3] |
| Uric Acid (mg/d) | 657 ± 337 | 540 ± 322 | 570 ± 123 | 472 ± 273 |
| Net Acid Excretion (NAE) (mEq/d) |
53 ± 20 | 58 ± 22 | 53 ± 29 | 60 ± 12 |
| NAE/Sulfate (mEq/mEq) | 0.75 ± 0.07 | 0.55 ± 0.08a | 0.78 ± 0.16 | 0.55 ± 0.15 b |
| NH4/NAE | 0.75 ± 0.07 | 0.55 ± 0.08a | 0.78 ± 0.16 | 0.55 ± 0.15 b |
| Urinary Unmeasured Anions (mEq/d) |
4.5 ± 12.9 | 10.3 ± 8.7 | 7.8 ± 9.9 | 6.9 ± 11.1 |
| Fractional Excretion of Uric Acid (%) |
5.9 ± 2.6 | 4.0 ± 2.6 a | 5.4 ± 2.1 | 3.5 ± 2.0 b |
p < 0.05 UASF vs HV; placebo
p< 0.05 UASF vs HV; on PPI
p = 0.08 UASF vs HV; placebo
p = 0.07 UASF vs HV; placebo
p = 0.06 UASF vs HV; on PPI
Comparisons made with mixed-model repeated measures analysis.
Diurnal Study
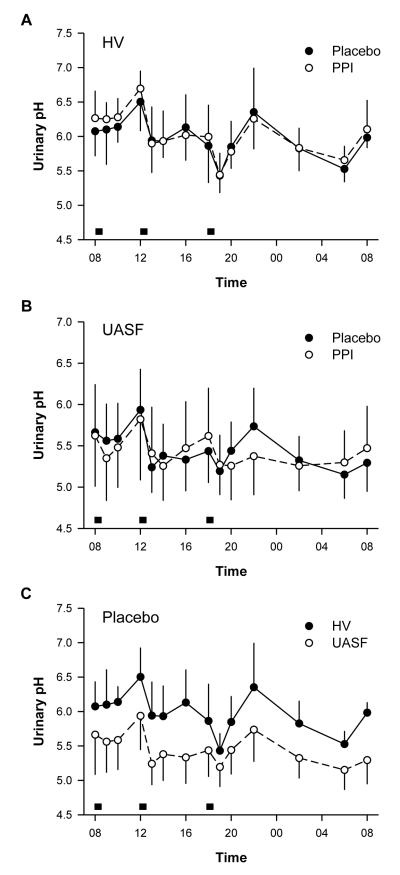
Figures 1A and B depict the diurnal urine pH in the HV and UASF group under each study phase. Throughout the day, both HV and UASF demonstrated marked variations in urine pH. In both groups, urine pH increased in the morning, peaked at noon, fell following lunch, rose again during the afternoon, and fell again after the evening meal. A nocturnal peak in urine pH was usually seen around 22:00. Throughout the night, UpH decreased to a nadir around 06:00 before rising again in the morning. Changes in urine pH over time are statistically significant within HV and UASF group in either the placebo or PPI phases (HV-placebo HV-PPI, UASF-placebo, UASF-PPI all p<0.001). The shape of the curves did not differ between the placebo and PPI phases for both HV and UASF (p=0.95). PPI treatment has no effect on the value or profile of urine pH at all (p=0.60 for HV; p=0.72 for UASF)
Figure 1.
Urine pH (UpH) in uric acid stone formers (UASF) and normal volunteers (HV) on placebo vs. proton pump inhibitors (PPI). Meals are indicated by grey boxes.
Throughout the day, urine pH was significantly lower in UASF than in HV in either the placebo (Figure 1C) or PPI phase (similar to placebo; not shown) (p<0.001). However, the time profile of urine pH was not statistically different between the two groups (HV vs. UASF; p=0.14) in placebo phase. In contrast to HV on PPI, the UASF receiving PPI demonstrated a slightly blunted curve in the evening and overnight (p=0.05).
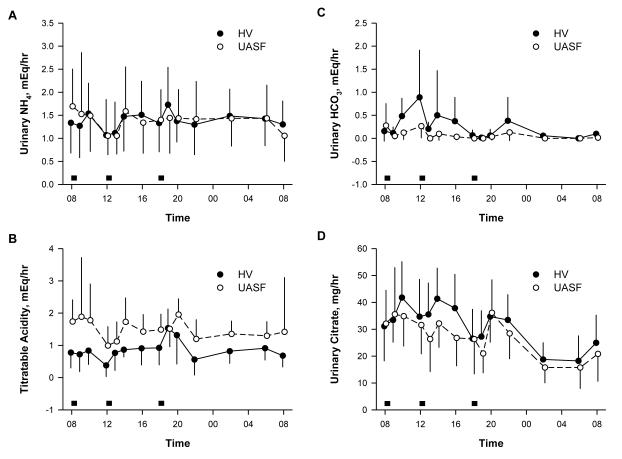
Figure 2 compares urinary excretion rates of principal acid-base components in HV and UASF during the placebo phase. Since there was no difference between PPI and placebo, only the placebo phase is shown. In both HV and UASF, excretion of these solutes varied significantly over time (all p<0.001). Acid excretion in general is higher in the late afternoon and evening likely reflecting the fact that the protein for breakfast is less than that of lunch and dinner (gms of protein: breakfast 7, lunch 24, dinner 30). Urinary ammonium excretion rate (Figure 2A) did not differ between HV and UASF in regards to both amount (p=0.11) and pattern (p=0.40). The pattern of TA excretion also did not differ between the groups (p=0.27). However, the amount of TA was significantly higher in the UASF group (p=0.005) (Figure 2B). The amount and pattern of the two urinary bases differed between the groups. USAF excreted less bicarbonate (p=0.01) and had a flatter bicarbonate profile (p=0.05) compared HV (Figure 2C). In addition, UASF excreted significantly less citrate (p=0.012) and tended to have a different citrate pattern (p=0.056) with blunted citrate excretion in the UASF occurring mostly in the morning.
Figure 2.
Excretion of acid-base parameters in healthy volunteers (HV) and uric acid stone formers (UASF). Grey boxes represent meals.
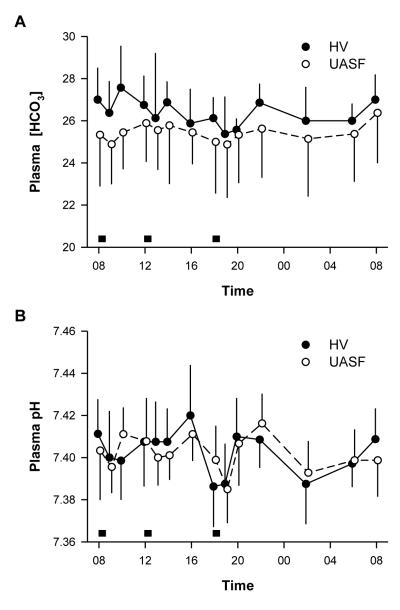
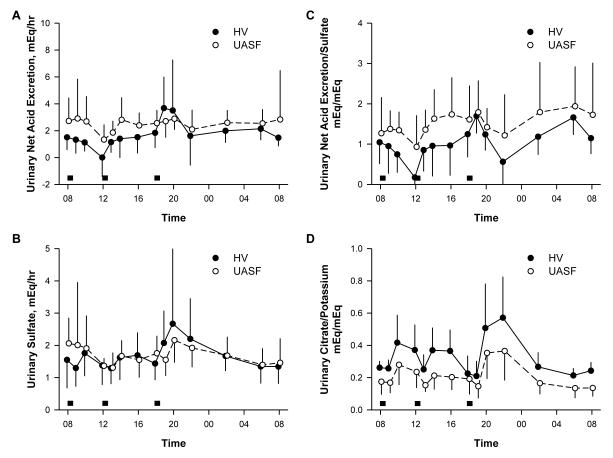
Plasma bicarbonate concentration changed significantly during the 24 hour period (p<0.001 for both groups) but the pattern was similar in HV and UASF (p=0.94) (Figure 3A). However, serum bicarbonate was higher in HV compared to UASF throughout the day (p=0.003). There were no differences between the PPI and placebo phase in either NV or UASF for both pH and bicarbonate so only the placebo data is shown in Figure 3. Interestingly, despite the differences in bicarbonate concentrations, changes in venous pH over time did not reach statistical significance within either group during the two treatment periods (p=0.21) and did not differ between the groups (p=0.45) (Figure 3B). UASF have higher NAE than HV even though they are on identical metabolic diets (Figure 4A). The equivalence of dietary acid load is proven by the same amount and pattern of sulfate excretion (Figure 4B). Although exogenous dietary acid load was equivalent, UASF have consistently higher acid excretion throughout the day with the exception of perhaps the period around 18:00-20:00 in the evening. This is best illustrated by the consistently higher NAE/sulfate ratio throughout the day in UASF (Figure 4C) (p= 0.02). The higher acid load is also compatible with the lower citrate excretion (Figure 2D). Potassium excretion can be used as a surrogate for dietary alkali intake. UASF have the same potassium excretion as HV’s but lower citrate excretion per K ingested (p=0.001) (Figure 4D).
Figure 3.
Plasma acid-base parameters in uric acid stone formers (UASF) and normal volunteers (HV). Grey boxes represent meals.
Figure 4.
Urinary acid-base parameters normalized to dietary surrogates of acid (sulfate) or base (potassium) ingestion. Grey boxes represent meals.
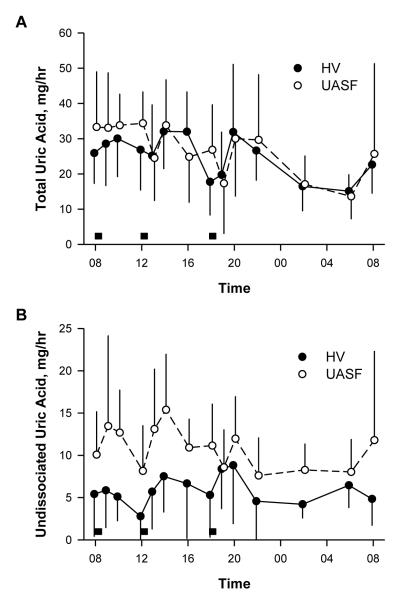
Figure 5 shows the most important determinant of uric acid stone risk in the UASF. Absolute rates or patterns of total uric acid excretion did not differ between the two groups (p=0.45) and there was no difference in the time profile in excretion between the two groups (p=0.7) (Figure 5A). However, UASF had persistently about twice the undissociated uric acid content in urine as HV (Figure 5B) (p<0.001).
Figure 5.
Total (urate + undissociated uric acid) and undissociated uric acid excretion in urine. Grey boxes represent meals.
Discussion
Instead of examining pooled 24 hr samples, we tried to define circadian variation of urine pH and acid-base parameters in healthy subjects as well as in uric acid stone formers. We also examined the contribution of gastric acid secretion on circadian variation in both groups and evaluated whether differences in gastric acid secretion are responsible for a lower urine pH in UASF vs. HV.
Both HV’s and UASF’s were placed on a strict metabolic diet thereby eliminating exogenous factors on acid-base balance. Both groups demonstrated significant variations in urine pH throughout the day, and these changes were temporally related to meals. Although there was little change immediately following breakfast, urine pH declined after the noon and evening meals. Urine pH subsequently rose and peaked approximately four hours after each meal. Overnight urine pH fell steadily, before it began to rise in the early morning hours. While they confirm the previously described “morning alkaline tide”, our data did not demonstrate a true post-prandial alkaline tide in either blood or urine. A rise in serum pH after the ingestion of a meal was described previously 19,20 while there is conflicting evidence for whether there is a consistent post-prandial alkaline tide in the urine 33,34. This difference may, in part, be due to variances in diet, timing of urine collections 35, and use of pH paper to define urinary pH in some studies 33,34. In our study, the higher frequency of urine collections allowed for a higher resolution and the instant measurement using a pH electrode permitted more accurate assessment of circadian changes. The decline in urine pH following lunch and dinner may not have been appreciated previously, because of longer post-prandial urine collection times.
The post-prandial alkaline tide described in serum raises expectations that a similar phenomenon might occur in the urine due to increased filtered bicarbonate load. However, there are multiple factors that contribute to urine pH. The increased filtered load of HCO3− may or may not exceed tubular reabsorption and the production and excretion of non-bicarbonate buffers will also affect UpH significantly. The effects of variations in diet are also unclear. The urine pH may reflect the combined effects of gastric acid and pancreatic bicarbonate secretion, and the extent of each process may alter urine pH. Others have shown that in patients after vagotomy, receiving H2-blockers or PPI, have varying degrees of blunted post-prandial alkaline tide in the blood 19,20,33,34,36. If gastrointestinal acid or base secretion was responsible for diurnal urine pH changes, then administration of a proton pump inhibitor would completely block those effects. Our study did not demonstrate any effect of the PPI medication, thus raising into question the role of gastric acid secretion in affecting urine pH. Alternative mechanisms that may account for these variations include circadian clock genes which have been found to control acid-base transporters in the kidney 37,38. Further investigation of such genes may identify mechanisms responsible for circadian variation in urine pH.
Several features are noteworthy in the UASF’s. Despite the more acidic urine, the circadian pattern is well preserved in UASF’s. Unlike some previous studies reporting lack of diurnal changes in urine pH in UASF, 39,40 we found that UASF exhibited the same peaks and troughs overnight and in relation to meals, as in HV. Previous studies were performed in a smaller number of UASF, without using strict metabolic diets, and collected urines in wider (>6-hour) intervals. However, at each time point, UASF urine pH was significantly more acidic than HV. With the exception of two time points, UASF maintained a mean urine pH of 5.6 or less throughout all time points of the day which significantly contributes to uric acid stone development 24. The undissociated UA excretion rate and concentration is much higher in UASF throughout the entire 24 hr period.
Differences in the diurnal excretion of ammonium, TA, citrate and bicarbonate reflect findings previously described in pooled 24 hour urine collections 30 but a higher time resolution is presented in this diurnal study. UASF demonstrated lower citrate, and bicarbonate excretion and higher TA, which persisted throughout the entire day. Each urinary component exhibited a circadian pattern of excretion. The citrate and bicarbonate plots were slightly blunted in the UASF indicating possibly a different pattern of excretion. Both groups excreted ammonium and TA in a similar pattern. It has been suggested that increased acid production in UASF may account for lower urine pH 26,31,41. This mechanism may also account for consumption of base and the differences observed in the diurnal excretion of alkali.
In addition to a lower urine pH, UASF demonstrated persistently lower serum bicarbonate. This is the first demonstration that a systemic acid-base disturbance is actually present in UASF’s. Serum bicarbonate measured at 8:00 am were not statistically different between the groups when analyzed as a single time point, thereby explaining why this difference may not have been previously appreciated in all published studies. However, when the sequential bicarbonate values were compared, UASF maintained significantly lower serum bicarbonate than HV throughout most of the day. These results further support the notion that in addition to a renal mechanism, systemic factor(s) such as possible increase in acid generation contribute(s) to the development of acidic urine in these patients. The origin of the increased acid load is not know yet but can be due to increased endogenous acid production, increase alkali loss in the intestine, and finally increased absorption of exogenous organic acid from perhaps colonic bacterial sources.
One weakness in this study was the discrepancy between the weights of the subject in the two groups. UASF are typically obese and because of this characteristic, finding a healthy matching group is in fact difficult. The HV included in this study were generally overweight, but not obese. It is important to note that none of our subjects showed any evidence of starvation ketosis during the study. In an attempt to improve matching, we simply analyzed the 5 HV (mean ± SD: 31.4 ± 2.2) with highest BMI against 5 subjects with lowest BMI in UASF (mean ± SD: 33.0 ± 4.3, NS), the urinary pH (HV 5.86 ± 0.20 vs. UASF 5.29 ± 0.13, p<0.05) and the fraction of net acid excreted as ammonium (HV 0.68 ± 0.12 vs. UASF 0.54 ± 0.08, p<0.05) are both still lower in UASF’s indicating that body weight and BMI per se cannot account for the difference. Another drawback is that the overnight sampling did not have the same time resolution as the daytime sampling. We aimed to maximize the number of study samples without causing excessive interruption of sleep.
In conclusion, these data indicate that circadian variation in urine pH exists in both HV and UASF. Although UASF demonstrate variability in urine pH, their urine remains acidic throughout the day, leaving them unprotected from the development of uric acid stones. The etiology of the unduly acidic urine is due to a combination of a higher acid load to the kidney and the underutilization of ammonia as a buffer. One might speculate that circadian variation occurs to protect us from persistently acidic or alkaline urine pH that predisposes to stone development. Although gastric acid secretion contributes to variations in serum pH, it does not appear to affect the diurnal changes in urine pH. These results provide a foundation for future studies to determine what factors contribute to variations in urine pH, whether these persist irrespective of diet, and why UASF maintain the circadian variation but at a lower urine pH.
Methods
Study Participants
The study was conducted at the General Clinical Research Center at the University of Texas Southwestern Medical Center (Dallas, TX) with approval by the Institutional Review Board (IRB). All participants provided informed consent. Study subjects included nine healthy volunteers (HV) who had no history of kidney stones and ten stone formers with proven uric acid stones (UASF) recruited from the Mineral Metabolism Clinic at the University of Texas Southwestern Medical Center. HV’s were recruited via IRB-approved advertisements. All subjects were older than 18 years of age. Excluded from both groups were subjects with creatinine clearance <70ml/min, proteinuria, liver disease, chronic diarrhea, peptic ulcer, or chronic use of any medications that affect gastric acid secretion or renal function. UASF’s were instructed to hold all medications that affect acid or base excretion for 1 week prior to initiation of the study.
Study Protocol
This double-blind crossover study included two 14-day phases. In the first phase, subjects were randomized to receive the long-acting proton pump inhibitor (PPI), pantoprazole, 40 mg/day, or placebo. The dose was chosen based on previous studies showing adequate suppression of gastric acid production 42-44. The capsules were consumed each morning with breakfast for 14 days. Participants subsequently underwent a 7 day wash out period, during which study medications were not administered, and then received the alternate study medication for 14 days in the second phase. During each phase, subjects were advised to hold blood pressure medications that might affect renal sodium excretion.
On days 8-10 of each phase, subjects were directed to consume a diet low in acid ash and sodium (~100 mEq/day). On days 11-13 of each phase, a frozen metabolic diet was provided by the dietary services at the GCRC and consisted of 30% fat, 55% carbohydrate, 15% protein, 300 mg cholesterol, 400 mg calcium, 800 mg phosphorus, 100 mEq sodium, 40 mEq potassium, and 3000 cc distilled water per day, which subjects ingested as outpatients. From day 13 to 14, urine was collected for 24 hours under mineral oil and was refrigerated. Subjects were then admitted to the inpatient unit at the GCRC after dinner on day 13 and remained as inpatients until the morning of day 15. During the inpatient stay, subjects were maintained on the metabolic diet, although the volume of water was decreased to 2.7 L. Body weight and height was obtained at the time of admission. We monitor serum and urine ketones and detected no evidence of ketosis throughout the study.
The diurnal study was conducted on days 14 to 15. On day 14, an intravenous catheter was placed in the hand for collection of free flow blood samples. The hand was warmed in a 37°C box for 30 minutes prior to each blood draw to obtain arterialized samples. Starting at 8:00 am, urine and blood were collected every two hours throughout the day, except for the two hours following meals when samples were collected hourly. To allow subjects to have reasonable sleep overnight, samples were obtained every four hours from 22:00 until 6:00 on day 15. Thus collections were obtained at: 8:00, 9:00, 10:00, 12:00, 13:00, 14:00, 16:00, 18:00, 19:00, 20:00, 22:00, 2:00, 6:00, and 8:00. Samples were obtained prior to ingestion of meals which were provided at 8:00, 12:00 and 18:00 immediately after the blood draw at those designated times.
Timed urine samples were analyzed for total volume, pH, creatinine, electrolytes, uric acid, citrate, sulfate, ammonium, titratable acidity (TA), and bicarbonate. Blood sample measurements included serum electrolytes, creatinine, uric acid, glucose, and venous pH.
Analytical Procedures
A systemic multichannel analysis was performed to provide measurements of serum creatinine, sodium, potassium, chloride, total carbon dioxide, glucose, and uric acid (Beckman CX9ALX; Beckman Coulter, Fullerton, CA). Venous blood gas measurements, including serum pH and PCO2, were made using the Radiometer ABL 5 (Radiometer America Inc, Westlake, OH).
Urine pH was measured with a pH electrode and urine creatinine was obtained via the picric acid method. Urine and fasting serum creatinine measurements were then used to calculate endogenous creatinine clearance. An ion-specific electrode was used to measure urinary potassium (Beckman Coulter) and urine sulfate was assessed by ion chromatography. Urine ammonium was measured by the glutamate dehydrogenase method while citrate was assessed enzymatically with reagents from Boehringer-Mannheim Biochemicals (Indianapolis, IN). Urinary bicarbonate was calculated from measurements of urine pH and PCO2, while titratable acid was assessed by the automated burette titration system (Radiometer, Copenhagen, Denmark). Uric acid was determined via the uricase method using alkalinized samples to prevent precipitation. Net acid excretion (NAE) was calculated using the sum of ammonium and TA minus the sum of citrate and bicarbonate in mEq. The urinary anion gap (UAG) was calculated as the sum of all anions (chloride, sulfate, phosphate, urate, creatinate, oxalate, citrate, bicarbonate) subtracted from the sum of all cations (sodium, potassium, calcium, magnesium, ammonium), all in milliequivalents.
Statistical Analyses
Data are presented as mean ± standard deviation (SD). Demographic variables were compared between UASF and HV using the two sample t-test. Twenty four urine results and serum biochemical data were compared using mixed model repeated measures analysis followed by least square means contrasts to compare the two groups or to compare the two treatment phases.
Mixed model repeated measures analysis was also used for the diurnal studies. The models consisted of group, phase, and time main effects and interactions, with subject modeled as a random effect. An autoregressive covariance structure was used to model the repeated time effect and account for the correlation within individuals. 45 The interaction terms from these models were used to test for differences in diurnal pattern. The difference in diurnal pattern between the UASF and HV groups was assessed using the group-by-time interaction factor. The difference in diurnal pattern between the placebo and PPI phases was assessed using the phase-by-time interaction factor. A significant interaction (p<0.05) indicates that the groups do not have the same pattern over time. Urine bicarbonate was rank-transformed prior to analysis. Statistical analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
Acknowledgements
The authors were supported by the National Institutes of Health (M01-RR00633, P01-DK-20543, R01 DK081423, UL1-RR024982, K23-RR21710, O’Brien Kidney Center P30-DK079328) and Seed Funds from the Charles and Jane Pak Center. The authors wish to thank the capable nursing staff of the General Clinical Research Center and the technical staff at the Mineral Metabolism Laboratory at UTSouthwestern. Dr. Charles Y.C. Pak was extremely helpful in his insightful comments and suggestions.
References
- 1.Bray MS, Young ME. The role of cell-specific circadian clocks in metabolism and disease. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2009;10(Suppl 2):6–13. doi: 10.1111/j.1467-789X.2009.00684.x. doi:10.1111/j.1467-789X.2009.00684.x. [DOI] [PubMed] [Google Scholar]
- 2.Mehra A, Baker CL, Loros JJ, Dunlap JC. Post-translational modifications in circadian rhythms. Trends in biochemical sciences. 2009;34:483–490. doi: 10.1016/j.tibs.2009.06.006. doi:10.1016/j.tibs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cermakian N, Boivin DB. The regulation of central and peripheral circadian clocks in humans. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2009;10(Suppl 2):25–36. doi: 10.1111/j.1467-789X.2009.00660.x. doi:10.1111/j.1467-789X.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- 4.Mills JN. Human circadian rhythms. Physiol Rev. 1966;46:128–171. doi: 10.1152/physrev.1966.46.1.128. [DOI] [PubMed] [Google Scholar]
- 5.Harmer SL. The circadian system in higher plants. Annual review of plant biology. 2009;60:357–377. doi: 10.1146/annurev.arplant.043008.092054. doi:10.1146/annurev.arplant.043008.092054. [DOI] [PubMed] [Google Scholar]
- 6.Mas P, Yanovsky MJ. Time for circadian rhythms: plants get synchronized. Current opinion in plant biology. 2009;12:574–579. doi: 10.1016/j.pbi.2009.07.010. doi:10.1016/j.pbi.2009.07010. [DOI] [PubMed] [Google Scholar]
- 7.Bonny O, Firsov D. Circadian clock and the concept of homeostasis. Cell Cycle. 2009;8:4015–4016. doi: 10.4161/cc.8.24.10224. [DOI] [PubMed] [Google Scholar]
- 8.Firsov D, Bonny O. Circadian regulation of renal function. Kidney Int. 2010;78:640–645. doi: 10.1038/ki.2010.227. doi:10.1038/ki.2010.227. [DOI] [PubMed] [Google Scholar]
- 9.Moore-Ede MC. Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol. 1986;250:R737–752. doi: 10.1152/ajpregu.1986.250.5.R737. [DOI] [PubMed] [Google Scholar]
- 10.Moore-Ede MC, Herd JA. Renal electrolyte circadian rhythms: independence from feeding and activity patterns. Am J Physiol. 1977;232:F128–135. doi: 10.1152/ajprenal.1977.232.2.F128. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld L. Henry Bence Jones (1813-1873): the best “chemical doctor” in London. Clin Chem. 1987;33:1687–1692. [PubMed] [Google Scholar]
- 12.Jones HB. On the variations of the acidity of the urine in the state of health. Philos Trans R Soc. 1845;135 [Google Scholar]
- 13.Ayres JW, Weidler DJ, MacKichan J, Wagner JG. Circadian rhythm of urinary pH in man with and without chronic antacid administration. Eur J Clin Pharmacol. 1977;12:415–420. doi: 10.1007/BF00561060. [DOI] [PubMed] [Google Scholar]
- 14.Kanabrocki EL, et al. Circadian characteristics of dialyzable and non-dialyzable human urinary electrolytes, trace elements and total solids. Chronobiol Int. 1988;5:175–184. doi: 10.3109/07420528809079558. [DOI] [PubMed] [Google Scholar]
- 15.Mills JN, Stanbury SW. Intrinsic diurnal rhythm in urinary electrolyte output. J Physiol. 1951;115:18p–19p. [PubMed] [Google Scholar]
- 16.Elliot JS, Sharp RF, Lewis L. Urinary pH. J Urol. 1959;81:339–343. doi: 10.1016/S0022-5347(17)66022-1. [DOI] [PubMed] [Google Scholar]
- 17.Barnett GD, Blume FE. Alkaline Tides. J Clin Invest. 1938;17:159–165. doi: 10.1172/JCI100939. doi:10.1172/JCI100939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanbury SW, Thomson AE. Diurnal variation in electrolyte excretion. Clin Sci (Lond) 1951;10:267–293. [PubMed] [Google Scholar]
- 19.Niv Y. Pentagastrin-induced urinary alkaline tide--a repeatable phenomenon that is abolished after vagotomy. Israel journal of medical sciences. 1992;28:97–98. [PubMed] [Google Scholar]
- 20.Niv Y, Asaf V. Abolition of postprandial alkaline tide in arterialized venous blood of duodenal ulcer patients with cimetidine and after vagotomy. Am J Gastroenterol. 1995;90:1135–1137. [PubMed] [Google Scholar]
- 21.Davids MR, Edoute Y, Jungas RL, Cheema-Dhadli S, Halperin ML. Facilitating an understanding of integrative physiology: emphasis on the composition of body fluid compartments. Can J Physiol Pharmacol. 2002;80:835–850. doi: 10.1139/y02-114. [DOI] [PubMed] [Google Scholar]
- 22.Moe OW. Uric acid nephrolithiasis: proton titration of an essential molecule? Curr Opin Nephrol Hypertens. 2006;15:366–373. doi: 10.1097/01.mnh.0000232876.04975.33. doi:10.1097/01.mnh.0000232876.04975.33. [DOI] [PubMed] [Google Scholar]
- 23.Moe OW, Preisig PA. Dual role of citrate in mammalian urine. Curr Opin Nephrol Hypertens. 2006;15:419–424. doi: 10.1097/01.mnh.0000232882.35469.72. [DOI] [PubMed] [Google Scholar]
- 24.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004;13:181–189. doi: 10.1097/00041552-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Abate N, Chandalia M, Cabo-Chan AV, Jr., Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. doi:10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 26.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol. 2010;5:1277–1281. doi: 10.2215/CJN.08331109. doi:10.2215/CJN.08331109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maalouf NM, et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 28.Pak CY, Poindexter JR, Peterson RD, Koska J, Sakhaee K. Biochemical distinction between hyperuricosuric calcium urolithiasis and gouty diathesis. Urology. 2002;60:789–794. doi: 10.1016/s0090-4295(02)01908-8. [DOI] [PubMed] [Google Scholar]
- 29.Pak CY, et al. Biochemical profile of stone-forming patients with diabetes mellitus. Urology. 2003;61:523–527. doi: 10.1016/s0090-4295(02)02421-4. [DOI] [PubMed] [Google Scholar]
- 30.Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 31.Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–1428. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 32.Cameron MA, Baker LA, Maalouf NM, Moe OW, Sakhaee K. Circadian variation in urine pH and uric acid nephrolithiasis risk. Nephrol Dial Transplant. 2007;22:2375–2378. doi: 10.1093/ndt/gfm250. [DOI] [PubMed] [Google Scholar]
- 33.Johnson CD, Harris PA, Wastell C. Quantitative relation between gastric acid secretion and changes in urinary acid excretion. Gut. 1990;31:862–866. doi: 10.1136/gut.31.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson CD, Rai AS. Urine acid output as a test of completeness of vagotomy. Br J Surg. 1990;77:417–420. doi: 10.1002/bjs.1800770419. [DOI] [PubMed] [Google Scholar]
- 35.Vaziri ND, Byrne C, Ryan G, Wilson A. Preservation of urinary postprandial alkaline tide despite inhibition of gastric acid secretion. Am J Gastroenterol. 1980;74:328–331. [PubMed] [Google Scholar]
- 36.Longkumer T, Parthasarathy G, Kate V, Ananthakrishnan N, Koner BC. Assessment of vagotomy status with postprandial urinary alkaline tide. Tropical gastroenterology : official journal of the Digestive Diseases Foundation. 2009;30:91–94. [PubMed] [Google Scholar]
- 37.Rohman M. Saifur, et al. Circadian clock genes directly regulate expression of the Na(+)/H(+) exchanger NHE3 in the kidney. Kidney Int. 2005;67:1410–1419. doi: 10.1111/j.1523-1755.2005.00218.x. doi:10.1111/j.1523-1755.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 38.Nishinaga H, et al. Circadian expression of the Na+/H+ exchanger NHE3 in the mouse renal medulla. Biomed Res. 2009;30:87–93. doi: 10.2220/biomedres.30.87. [DOI] [PubMed] [Google Scholar]
- 39.Bilobrov VM, Chugaj AV, Bessarabov VI. Urine pH variation dynamics in healthy individuals and stone formers. Urol Int. 1990;45:326–331. doi: 10.1159/000281730. [DOI] [PubMed] [Google Scholar]
- 40.Murayama T, Taguchi H. The role of the diurnal variation of urinary pH in determining stone compositions. J Urol. 1993;150:1437–1439. doi: 10.1016/s0022-5347(17)35801-9. [DOI] [PubMed] [Google Scholar]
- 41.Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 42.Howden CW, Ballard ED, Koch FK, Gautille TC, Bagin RG. Control of 24-hour intragastric acidity with morning dosing of immediate-release and delayed-release proton pump inhibitors in patients with GERD. J Clin Gastroenterol. 2009;43:323–326. doi: 10.1097/MCG.0b013e31818a386e. doi:10.1097/MCG.0b013e31818a386e. [DOI] [PubMed] [Google Scholar]
- 43.Kirchheiner J, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19–31. doi: 10.1007/s00228-008-0576-5. doi:10.1007/s00228-008-0576-5. [DOI] [PubMed] [Google Scholar]
- 44.Miner P, Delemos B, Xiang J, Lococo J, Ieni J. Effects of a single dose of rabeprazole 20 mg and pantoprazole 40 mg on 24-h intragastric acidity and oesophageal acid exposure: a randomized study in gastro-oesophageal reflux disease patients with a history of nocturnal heartburn. Alimentary pharmacology & therapeutics. 2010;31:991–1000. doi: 10.1111/j.1365-2036.2010.04255.x. doi:10.1111/j.1365-2036.2010.04255.x. [DOI] [PubMed] [Google Scholar]
- 45.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd edn SAS Institute; 2006. [Google Scholar]