Abstract
In neurodegenerative diseases, it remains unclear why certain brain regions are selectively vulnerable to protein aggregation. In transgenic mice expressing human A53T α-synuclein, the brainstem and spinal cord develop the most prominent α-synuclein inclusions which correlate with age-dependent motor dysfunction. Herein we present the novel finding that this selective aggregation is in part dependent on the inability of chaperone-mediated autophagy (CMA) to effectively degrade α-synuclein in these brain regions. Lysosomal assays revealed that CMA activity was significantly decreased in aggregation-prone regions compared to the remainder of the brain. Previously, CMA activity has been shown to be proportional to levels of the CMA receptor Lamp-2a. Using antibodies, brain tissue from Lamp-2a null mice, enzymatic deglycosylation, and mass spectrometry, we identified Lamp2a as a novel 72 kDa glycoprotein in the mouse brain. Examination of Lamp-2a levels revealed differences in expression across brain regions. The brainstem and the spinal cord had a more than three-fold greater levels of Lamp-2a as compared to regions less vulnerable to aggregation and exhibited a selective upregulation of Lamp-2a during development of α-synuclein inclusions. Despite this dynamic response of Lamp-2a, the levels of substrates bound to the brain lysosomes as well as the rates of substrate uptake and degradation were not proportional to the levels of Lamp-2a. These regional differences in CMA activity and Lamp-2a expression were found in both non-transgenic mice as well as A53T α-syn mice. Therefore, these are inherent variations and not a transgene-specific effect. However, differences in CMA activity may render select brain regions vulnerable to homeostatic dysfunction in the presence of stressors such as overexpression of human A53T α-syn. Collectively, the data provide a potential mechanism to explain the dichotomy of vulnerability or resistance that underlies brain regions during aggregate formation in neurodegenerative disease.
Keywords: Parkinson’s Disease, protein aggregation, Lamp-2a, lysosomes
INTRODUCTION
α-Synuclein (α-syn) has been identified as an integral component of the protein inclusions found in several related neurodegenerative disorders, including Parkinson’s disease (PD) (Spillantini et al., 1997, 1998a, 1998b). Although α-syn is expressed throughout the brain and in many other organs, inclusions are found predominantly in select brain regions (Uéda et al., 1993; Iwai et al., 1995; Duda et al., 2002). The aggregation of α-syn is in part facilitated by protein levels, evidenced by in vitro studies as well as cases of familial PD with increased gene dosage (Conway et al., 1998; Giasson et al., 1999; Chartier-Harlin et al., 2004). While much research has consequently focused on the protein degradation pathways that dictate α-syn homeostasis, these pathways are ubiquitously expressed. Therefore, the molecular mechanisms underlying the vulnerability of distinct brain regions to neurodegeneration remain unknown.
Recently, it has been shown in healthy mice that the ubiquitin proteasome system (UPS) is predominantly involved in clearance of α-syn. However, with increased age and α-syn expression, the UPS is impaired and the autophagy pathways are recruited (Ebrahimi-Fakhari et al., 2011). This highlights the interplay of various degradation systems and the relevance of autophagic degradation of α-syn in the ultimate development of disease. However, it remains unclear how dysfunction of protein degradation contributes to the regionally and temporally selective aggregation of α-syn.
α-Syn has been shown to be preferentially degraded by CMA in vitro (Cuervo et al., 2004). In CMA, cytosolic proteins containing a pentapeptide targeting motif are selectively recognized by the chaperone heat shock cognate protein 70kDa (hsc70). Hsc70 targets the substrate to the lysosomal receptor lysosome-associated membrane protein type 2A (Lamp-2a), through which the substrate passes into the lysosome where it is degraded (Cuervo and Dice, 1996). Levels of Lamp-2a have been shown to correlate directly with levels of CMA activity (Cuervo and Dice, 2000a, 2000b). Similar to macroautophagy, CMA is activated during conditions of cell stress (Cuervo et al., 1995; Cuervo et al., 1999; Kiffin et al., 2004). Decreased CMA function has been shown to leave neurons vulnerable to multiple insults (Massey et al., 2006) and has been shown to increase levels of high molecular-weight soluble and detergent-insoluble α-syn in cultured cells (Vogiatzi et al., 2008). A recent study found that Lamp-2a levels were decreased in α-syn inclusion-forming regions of the substantia nigra and amygdala of PD patients compared to control subjects and Alzheimer’s patients (Alvarez-Erviti et al., 2010).
In vitro, α-syn containing the A53T or A30P familial PD mutations or wild-type α-syn modified by oxidized dopamine were shown to bind strongly to the Lamp-2a receptor, blocking the uptake and degradation of other substrates by CMA (Cuervo et al., 2004; Martinez-Vicente et al., 2008). Overexpression of α-syn has been shown to impair CMA and increase cell toxicity in cultured cells (Xilouri et al., 2009). In mice, overexpression of wild-type mouse α-syn led to increased levels of Lamp-2a and Hsc70 (Mak et al., 2010). These data illuminate an important interplay between CMA and its substrate α-syn. However, it remains unclear how this interaction contributes to the regionally selective neurodegeneration observed in vivo.
To investigate this, we used both non-transgenic mice as well as a previously characterized transgenic mouse model expressing human A53T α-syn (Giasson et al., 2002). At approximately twelve months old, these transgenic mice develop age-dependent motor dysfunction and α-syn positive cytoplasmic inclusions that are most abundant in the brainstem and spinal cord (Giasson et al., 2002). We present the novel finding that levels of CMA activity are inherently decreased in select regions of the non-transgenic mouse brain. These same regions are the areas that are most vulnerable to α-syn aggregation in the presence of the α-syn transgene. Moreover, examination of the expression of lysosomal proteins, lysosomal substrate binding, and CMA degradation rates revealed that binding and degradation across different brain regions is not proportional to Lamp-2a or any other established CMA component, contrary to what has previously been shown in other organs. Ultimately, this suggests that pathways regulating α-synuclein clearance contribute to the selective vulnerability of particular brain regions to α-synuclein aggregation and neurodegeneration.
MATERIALS AND METHODS
Mouse breeding
The mice used in this study express human A53T α-syn (line M83) driven by the murine PrP promoter (Jackson Laboratory, Bar Harbor, Maine) and have been described previously (Giasson et al., 2002). To generate the A53T+/+ and nontransgenic (nonTg) mice used in experiments, heterozygous A53T+/− females were mated with A53T+/− males. Genotyping was performed by both end-point PCR using GeneAmp PCR system 9700 thermal cycler (Applied Biosystems, Carlsbad, CA) and quantitative PCR using Applied Biosystems 7500 real-time PCR System with the ABI MGB primer-probe set for human SNCA, the gene that encodes α-syn (Applied Biosystems assay ID Hs00240907_m1). SNCA values were normalized to mouse β-actin (Applied Biosystems assay ID 4352341E). The Lamp-2 knockout mice used in this study have been described previously (Tanaka et al., 2000).
Brain dissection for biochemical analysis
For analysis of brain regions, symptomatic A53T+/+ α-syn transgenic mice (12 to 17 months old) were euthanized 1–2 days after initial motor symptom onset as identified by spine stiffness, weight loss, and hindlimb paralysis as described previously (Giasson et al., 2002). Non-symptomatic A53T+/+ α-syn mice and non-transgenic mice were euthanized at an age matched within two weeks to the symptomatic mouse of that experimental group. Additionally, young A53T+/+ α-syn mice (2.5 months old) were euthanized for the experiments. Mice were anaesthetized with CO2 followed by cervical dislocation. The brain and spinal cord were dissected and rinsed thoroughly with ice-cold phosphate buffered saline (PBS). After dissection of the cerebellum, olfactory bulbs, and brainstem, the remainder of the brain was submerged in ice-cold PBS and sliced into 1 mm thick coronal sections using a vibratome (World Precision Instruments, Sarasota, FL). The brain regions of the hippocampus, cortex, striatum, and substantia nigra were dissected away from these sections. Dissected brain regions were weighed, rapidly frozen on dry ice, and stored at −80°C until analysis. For analysis of the hippocampus and spinal cord from mice of all four groups, tissue from the two brain regions was divided evenly between the RNA and western blot experiments so that the results were paired. The cortex and brainstem of these same mice were used for crude lysosome extractions so as to conserve the number of mice sacrificed.
Western Blotting
Tissue was homogenized with 10 volumes of lysis buffer [50mM Tris (pH7.5), 150mM NaCl, 5mM EDTA, 10% Glycerol, 1% TritonX-100, 0.5% Sodium Deoxycholate, 2% Sodium Dodecyl Sulfate (SDS), 8M Urea, 1mM PMSF, 1:50 Protease Inhibitor Cocktail (Sigma P8340)]. Similar results were seen with the use of an alternate lysis buffer [20mM HEPES (pH7.4), 150mM NaCl, 10% Glycerol, 1% TritonX-100, 1mM EGTA, 1.5mM MgCl2, 1mM PMSF, 1:50 Protease Inhibitor Cocktail (Sigma P8340)]. The tissues were homogenized with the aid of a mechanical homogenizer (Fisher Jumbo Stirrer 14-501) and then subjected to four consecutive freeze and thaw cycles of 2 min each (−80 °C ethanol bath/37 °C water bath). The sample was then centrifuged for 30 min at 18,000xg at 4 °C. The supernatant was retained and protein concentration was determined by the microBCA assay (Thermo Fisher Scientific, Rockford, IL). Unless otherwise indicated, 30 μg of material was loaded per lane for analysis by SDS-PAGE/western blot. The tissue was analyzed with the following primary antibodies: 1:300 Lamp-2a (Invitrogen 51-2200); 1:4000 NSE (Polysciences Inc. 16625), 1:1000 Hsc70 (Abcam ab2788), 1:1000 α-syn specific for human α-syn (Syn211, Sigma S5566), 1:10,000 GAPDH (Sigma G9545), 1:2000 β-tubulin (Sigma T-4026), 1:750 Cathepsin D (Abcam Ab6313), 1:300 Lamp-1 (BD Pharmagin 553792). Similar immunoreactivity for Lamp-2a was observed with 1:300 Lamp-2a (Abcam ab18528). Primary antibodies were detected with one of the following conjugated secondary antibodies: goat anti-rabbit IgG IRDye680 (1:5000, Rockland), donkey anti-mouse IgG IRDye800 (1:5000, Rockland), donkey anti-mouse IgG IRDye680 (1:5000, Rockland) and scanned with the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE). The protein bands from the western blots were quantified by densitometry using Odyssey infrared imaging system software (Odyssey version 2.1, Li-Cor Biosciences, Lincoln, NE).
Crude Lysosome Extraction for Western blots
Brain tissue was homogenized in Tris-buffered 0.25M Sucrose (pH 7.4) with 1:50 Protease Inhibitor Cocktail (SigmaP8340) and centrifuged at 6,800xg 15 min at 4°C. After washing the pellet with sucrose, the combined supernatants were centrifuged at 21,000xg 30 min at 4 °C. The supernatant was retained as the “cytoplasmic fraction” and the pellet, which contained the light mitochondria and lysosomes (light M+L fraction), was washed with sucrose and re-suspended in lysis buffer. For isolation of the lysosomal membrane fraction, the light M+L fraction was burst by hypotonic shock. The light pelleted M+L fraction was obtained as above and re-suspended in 25 μl cold 0.025M Sucrose. The samples were then centrifuged at 150,000xg for 30 min at 4 °C. The supernatant represents the lysosomal lumen, while the pellet, which was re-suspended in lysis buffer, is comprised of the lysosomal membrane.
Lysosomal Purification
In order to obtain a purified lysosomal fraction for use in the lysosomal activity assay, mitochondria were separated from lysosomes of the light M+L fraction as follows. The brain from a non-transgenic mouse was separated into the brainstem and the spinal cord (“pathogenic regions”) and the remainder of the brain (“non-pathogenic regions”). The light M+L fraction was obtained as described above, but without the addition of the protease inhibitor cocktail, and was re-suspended in 0.25 M Sucrose instead of lysis buffer. The mitochondria were then precipitated out of the M+L fraction by incubation with 115 μM CaCl2 for 30 min. The sample was then centrifuged 5,000xg for 10 min at 4 °C to pellet the mitochondria. The supernatant, which contains the lysosomes, was collected and centrifuged again at 5,000xg for 10 min at 4 °C to ensure purity. The supernatant was retained and centrifuged at 21,000xg for 30 min at 4 °C to pellet the lysosomes. The lysosomes were then re-suspended in 300 μl reaction buffer (10mM MOPS, pH 7.3, 0.25M Sucrose, 5.4μM cysteine, 1mM DTT). Protein concentration was measured by a micro-BCA protein assay, and lysosomal integrity was verified by an assay measuring the activity of the lysosomal enzyme β-Hexosaminidase with the flourogenic substrate 4-methylumbelliferyl-N-acetyl-β-glucosaminide dehydrate (Sigma M2133) in a fraction of lysosomes compared to the non-sediment medium. The enrichment of lysosomes and absence of mitochondria from this lysosomal fraction was verified by western blot with antibodies against Lamp-2a (Abcam ab18528) and cytochrome c oxidase subunit 1 (MTCO1) (Abcam ab14705).
Lysosomal Activity Assays with exogenous α-Synuclein
Human recombinant α-syn protein used for the assay was expressed and purified as described previously (Giasson et al., 1999). Twenty five micrograms of lysosomes purified as described above were incubated for 30 min at 37 °C with 0.2 μg of purified human α-syn, 10 μl of “6x Energy Regenerating System” (60mM MgCl2, 60mM ATP (Sigma A2383), 12mM phosphocreatine, 30μg creatine phosphokinase, in 0.25M Sucrose, pH 7.4), 0.6μg Hsc70 (Enzo Life Science, Farmingdale, NY), and brought up to 60 μl with reaction buffer (10mM MOPS, pH 7.3, 0.25M Sucrose, 5.4μM Cysteine, 1mM DTT). Control conditions were run in parallel with either no lysosomes or no 6x Energy Regenerating System, in which proteolysis buffer was substituted for the subtracted component, or with burst lysosomes. For the burst lysosomes, 25 μg of lysosomes were centrifuged at 21,000xg for 8 min at 4 °C. The supernatant was discarded and the pelleted lysosomes were subjected to hypotonic shock by re-suspension in 0.025M Sucrose (pH 7.4) for 30 min on ice followed by two cycles of freeze/thaw. These burst lysosomes were then added to the assay in place of the intact lysosomes. For the burst lysosomes, the neutral reaction buffer used for the intact lysosomes was replaced with an acidic reaction buffer (10mM citrate buffer, pH 5.0, 0.25M Sucrose, 5.4μM cysteine, 1mM DTT). At the end of the assay, the reaction was stopped by 1:30 Protease Inhibitor Cocktail (SigmaP8340) and 1 mM PMSF. Samples were subjected to SDS/PAGE alongside standards of purified human α-syn followed by western blot with a human specific α-syn antibody (Syn211, Sigma S5566).
Endogenous GAPDH Lysosomal Degradation Assays
Seventy five micrograms of lysosomes purified as described above were incubated at 37°C with 5 μl of “6x Energy Regenerating System” (60mM MgCl2, 60mM ATP (Sigma A2383), 12mM phosphocreatine, 30μg creatine phosphokinase, in 0.25M Sucrose, pH 7.4), and brought up to 30 μl with reaction buffer (10mM MOPS, pH 7.3, 0.25M Sucrose, 5.4μM Cysteine, 1mM DTT). As a control condition, one set of samples was incubated at 4 °C rather than 37 °C, and one set of samples received additional reaction buffer in place of the 6x energy regenerating system. At the end of the assay, the reaction was stopped by adding 1:30 Protease Inhibitor Cocktail (SigmaP8340) and 1 mM PMSF. Samples were subjected to SDS/PAGE followed by western blot with an antibody against GAPDH (Sigma G9545). To determine rates of GAPDH degradation, the intensity of the bands of GAPDH were compared to the intensity of known standards of purified GAPDH protein (Sigma G5262). The corresponding number of micrograms of GAPDH in the condition with no incubation and the 15hr time point were calculated. From those values the number of micrograms of GAPDH degraded per hour was determined. The resulting values were then divided by the relative levels of Lamp-2a in each region as determined by western blot.
Deglycosylation assay
Fifty micrograms of homogenate from the hippocampus and spinal cord of non-transgenic mice was denatured for 5 min at 100 °C, briefly cooled, and then incubated at 37 °C with a cocktail of enzymes that perform N- and O- linked deglycosylation; PNGaseF; α-2(3,6,8,9) Neuraminidase; O-Glycosidase; β (1–4) Galactosidase; β-N-Acetylglucosaminidase; Endoglycosidase H (3.33 μl each; Sigma E-DEGLY Kit, and Sigma A0810) for varying times. After incubation, the enzymes were heat inactivated, and the samples were analyzed by SDS-PAGE/western blot.
RNA isolation and RT-PCR
RNA was extracted from the hippocampus and spinal cord using the TRIzol reagent (Invitrogen, 15596-026), and cDNA was synthesized using the Superscript II First Strand Synthesis System for RT-PCR (Invitrogen 11904-018). Quantitative PCR was performed on this cDNA using Applied Biosystems 7500 real-time PCR System with the ABI MGB primer-probe set for Lamp-2a (Applied Biosystems assay ID mM00495274_m1). Lamp-2a values were normalized to neuron specific enolase (NSE) (Applied Biosystems assay ID mM00469062_m1)
Statistical Analysis
Data were analyzed using the Graphpad Prism Software (version 5.02) and were expressed as the mean ±SEM. One-way analysis of variance (ANOVA) with a Tukey’s post-hoc test was used to determine whether groups with multiple conditions were significantly different. A Paired t-test was used to determine whether paired groups were significantly different. p values <0.05 were considered statistically significant.
RESULTS
Brain regions vulnerable to α-synuclein aggregation display deficiencies in CMA
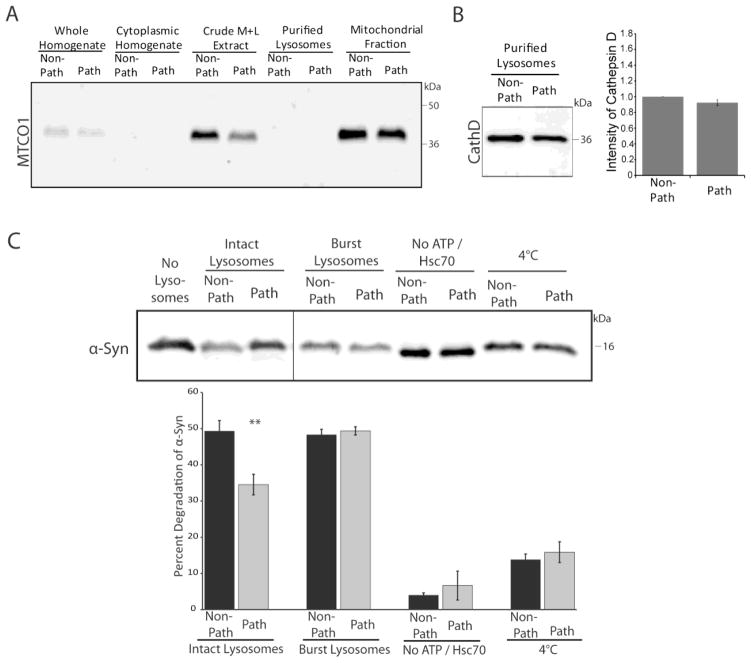
In A53T α-syn transgenic mice, α-syn aggregation occurs most prominently in the brainstem and the spinal cord (Giasson et al., 2002, Tsika et al., 2010). Consequently, we sought to determine whether the vulnerability of select brain regions to α-syn pathology is related to inherent differences in α-syn degradation. We first confirmed this previously established regional selectivity of α-syn aggregation using immunohistochemistry. Evaluation of the hippocampus and the brainstem of non-transgenic, non-symptomatic, and symptomatic mice with an antibody against human α-syn verified the presence of α-syn aggregates selectively in the brainstem of the symptomatic mice (Supplementary Figure 1). Since α-syn has been shown to be preferentially degraded by CMA (Cuervo et al., 2004), an activity assay measuring direct lysosomal uptake and degradation of α-syn was employed. From non-transgenic mice, purified lysosomes were extracted from the brainstem and spinal cord, regions that are vulnerable to developing α-syn aggregates, or from the remaining brain regions that are less prone to developing pathology. The use of non-transgenic mice prevented any confounding effects from existing associations between A53T α-syn and the lysosomes and provided insight as to regional discrepancies independent of transgene expression. The use of purified lysosomes precludes macroautophagy from influencing the results, as autophagosome formation is not able to occur. A β-hexosaminidase activity assay was used to verify the enrichment and intactness of purified lysosomes, as well as to validate that lysosomes across brain regions had similar levels of membrane permeability. Additionally, the absence of mitochondrial proteins in these lysosomal extracts was verified (Figure 1A). Purified lysosomes from the two groups of brain regions were then divided into twenty-five microgram aliquots and incubated at 37°C with a mixture that included an energy-regenerating system containing ATP, the Hsc70 chaperone, and exogenous purified human α-syn. Equal lysosomal loading for the two regions was verified by western blot for the lysosomal protease Cathepsin D (Figure 1B). Following the incubation of exogenous α-syn with the lysosomes, the amount of α-syn remaining was then determined by quantitative western blot with an antibody specific to human α-syn. A condition of burst lysosomes from each of the two regional groups was included as a control for the protease ability of the lysosomes. The lysosomes from the brainstem and spinal cord (Path) had a significantly decreased rate of wild-type α-syn degradation, 15% lower than the degradation rate for the remainder of the brain (Non-Path) (Figure 1C). This effect was dependent upon the addition of ATP and Hsc70, as has been characterized for CMA substrates (Chiang et al., 1989, Cuervo et al., 1994). Additionally, incubation of the lysosomes at 4 °C, which prevents lysosomal uptake, negated this difference in α-syn degradation (Figure 1C). Therefore, the regions that are most vulnerable to developing α-syn inclusions display decreased levels of CMA activity. This observation suggests that regional differences in substrate clearance may underlie the selective development of α-syn pathology. Therefore, we next sought to elucidate factors contributing to this impairment in CMA lysosomal degradation.
FIGURE 1.
Reduced CMA activity in brain regions that are most vulnerable to α-syn inclusions. A, Purified lysosomes were extracted from the pathogenic regions of the brainstem and spinal cord (“Path”) or the remainder of the brain (“Non-Path”) of non-transgenic mice. The purified lysosomal fractions were verified to be free of mitochondria by SDS-PAGE/western blot with the mitochondrial marker cytochrome c oxidase I (MTCO1). B, Equal lysosomal loading and protease capacity across the two regions was verified by SDS-PAGE/western blot for Cathepsin D and quantified by densitometry. The resulting values were adjusted proportionally to the value of the non-path region to normalize intensity across experiments. C, 25 μg of purified lysosomes were incubated with 0.2 μg of purified human α-syn with Hsc70 and an energy regenerating system at 37°C. After the incubation, the samples were analyzed by SDS-PAGE/western blot with a human specific α-syn antibody and quantified by densitometry. A condition of lysosomes burst by hypotonic shock was included as a control for the activity of lysosomal proteases in the two samples. As a control for CMA dependent uptake, a “No ATP/Hsc70” condition was included. An additional control condition involved incubation of the lysosomes at 4 °C during the reaction to prevent uptake. All values were held relative to a final condition in which the 0.2 μg of α-syn was incubated under the same conditions only without any lysosomes present. The brainstem and spinal cord have a decreased ability to degrade α-syn by CMA, a difference that is not due to any deficiencies in the degradation ability of the lysosomal proteases and is dependent on ATP and lysosomal uptake (**p<0.01 path versus non-path regions, t-test, intact lysosomes (n=10), all other conditions, (n=3))
Increased levels of Lamp-2a in the mouse brainstem and the spinal cord
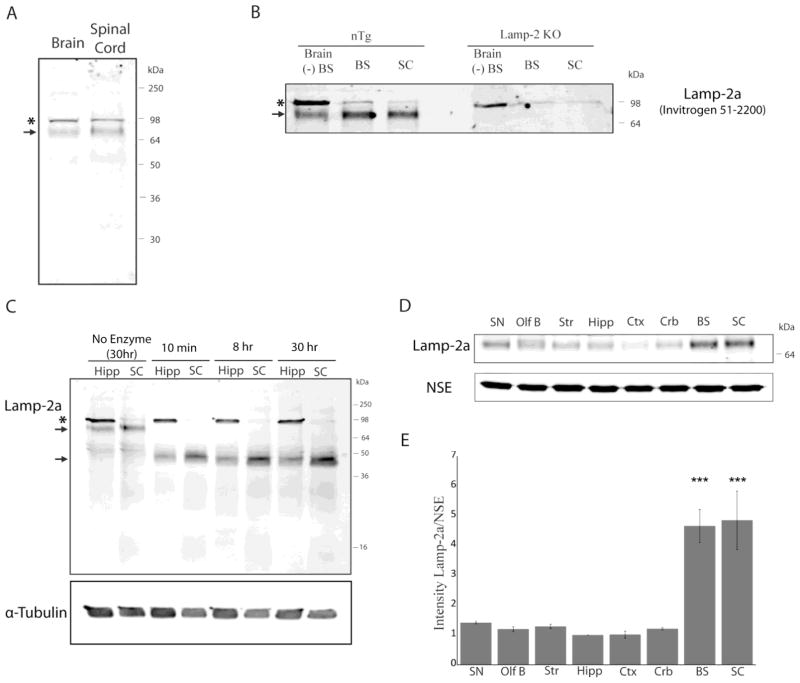
The expression of the lysosomal CMA receptor Lamp-2a has been shown to directly correlate with CMA activity (Cuervo and Dice, 2000a, 2000b). Therefore, the levels of this protein were examined across different regions of the mouse brain using an antibody generated against the C-terminal region of this protein (Invitrogen 51-2200). This antibody has been previously employed for the characterization of this receptor in the rat liver (Cuervo and Dice, 1996). Mouse Lamp-2a is a 415 amino acid protein with a 45,647 Da backbone that is heavily glycosylated. The molecular weight of the glycosylated form of the protein in the rat liver has previously been described to be 96 kDa (Cuervo and Dice, 1996). Within the brain and spinal cord, two principle immunoreactive bands were identified, (Figure 2A). Based on calibration of the gels with standard proteins, the apparent molecular weight of the two bands was calculated to be 96 and 72 kDa.
FIGURE 2.
Lamp-2a characterization in the mouse brain. A, The brain and the spinal cord from non-transgenic littermates of the A53T α-syn mice were analyzed by SDS-PAGE/western blot for Lamp-2a (Invitrogen 51-2200). Two distinct bands were identified at 96kDa (asterisk) and 72kDa (arrow). B, The brainstem, spinal cord, and remainder of the brain excluding these regions “Brain (−)BS” were dissected from non-transgenic littermates of the A53T α-syn mice or from Lamp-2 knockout mice. This tissue was analyzed by SDS-PAGE/western blot for Lamp-2a using the Invitrogen #51-2200 Lamp-2a antibody. The 96kDa band recognized by the antibody was present in the Lamp-2 knockout mice (asterisk), while the 72kDa band is specific to Lamp-2a (arrow). C, The hippocampus (Hipp) and spinal cord (SC) of an non-transgenic mouse were incubated with a cocktail of N- and O- linked deglycosylating enzymes (PNGaseF; α-2(3,6,8,9) Neuraminidase; O-Glycosidase; β(1–4) Galactosidase; β-N-Acetylglucosaminidase; Endoglycosidase H). After incubation, the enzymes were heat inactivated, and the samples were analyzed by SDS-PAGE/western blot for Lamp-2a. α-Tubulin was used as a loading control. D, The brains of non-transgenic mice were dissected into eight specific regions and analyzed by SDS-PAGE/western blot for Lamp-2a. NSE served as a loading control. (SN - substantia nigra; OlfB - olfactory bulbs; Str - striatum; Hipp - hippocampus; Ctx - cortex; Crb - cerebellum; BS - brainstem; SC - spinal cord). E, The intensity of the immunoreactive band of Lamp-2a was quantified by densitometry relative to the intensity of NSE. The resulting values were adjusted proportionally to the value of the hippocampus to normalize intensity across experiments. Lamp-2a is expressed most prominently in the brainstem and the spinal cord (n=3) (***p<0.001, SC or BS vs SN, OlfB, Str, Hipp, Ctx, or Crb, one way ANOVA with Tukey’s post-hoc test).
In order to validate the identity of these two immunoreactive bands, the antibody used in this study was reacted with brain homogenates from Lamp-2 knockout mice that are deficient in all three isoforms of the Lamp-2 protein (Tanaka et al., 2000). This revealed that the 96kDa band recognized by the Lamp-2a antibody is present in the Lamp-2 knockout mice whereas the band with an apparent molecular weight of 72kDa is absent from Lamp-2 knockout mice (Figure 2B). Use of a second polyclonal anti-Lamp-2a antibody (Abcam 18528) produced similar results, with a very strong non-specific signal at 96kDa (Supplementary Figure 2A). Together, these antibodies represent the only two Lamp-2a antibodies currently commercially available. Due to the very high non-specific signal of this Abcam18528 antibody, that antibody was not used further in the present study. Analysis of the expression pattern generated by the Invitrogen Lamp-2a antibody across 7 different tissues revealed a pattern of immunoreactivity similar to what has been previously characterized, with a single band of approximately 96kDa present in the liver (Supplementary Figure 2B).
The identity of the 72kDa band as Lamp-2a was reinforced by mass spectrometry. A purified lysosomal fraction and a lysosomal membrane fraction from the brainstem and spinal cord were separated on SDS-PAGE gels. From both fractions bands corresponding to 96kDa and 72kDa were excised and digested with trypsin. The corresponding tryptic peptides were analyzed by mass spectrometry. Four unique peptides corresponding to sequences in Lamp-2 were identified in the 72kDa band from both the enriched lysosomal fraction and the lysosomal membrane fraction (Supplemental Table 1). However, no peptides corresponding to Lamp-2 were identified in the 96kDa band. Therefore, the 72kDa band represents Lamp-2a in the mouse brain and was the focus of future analysis. This 72kDa band of Lamp-2a was found across multiple neural cell types, including both neurons and glia (Supplementary Figure 2C).
Lamp-2a is a heavily glycosylated protein with 16 N-linked and 4 O-linked glycans that serve to protect the protein from the proteases in the lysosome (Carlsson et al., 1988, 1990, 1993). The 72kDa isoform was readily deglycosylated within 10 minutes, resulting in immunoreactivity of a band of approximately 45kDa corresponding to the deglycosylated backbone of Lamp-2a (Figure 2C). This provides further support for the identity of this 72kDa band as Lamp-2a and indicates that the discrepancy in size from previous characterization of this protein is due to differences in glycosylation, not improper cleavage of the protein. Unfortunately, the ability of available Lamp-2a antibodies to recognize non-specific species in addition to Lamp-2a precluded the reliable use of this antibody for immunohistochemical analysis.
To determine whether differences in Lamp-2a expression exist across brain regions, the levels of the 72kDa Lamp-2a band were compared across eight different brain regions (Figure 2D). The brains of non-transgenic mice were used for this investigation in order to decipher inherent regional differences independent of transgene effects. Lamp-2a showed increased expression in the brainstem and spinal cord relative to other brain regions (Figure 2E).
Lamp-2a expression is upregulated selectively in the inclusion-forming regions upon the onset of motor symptoms in A53T α-synuclein transgenic mice
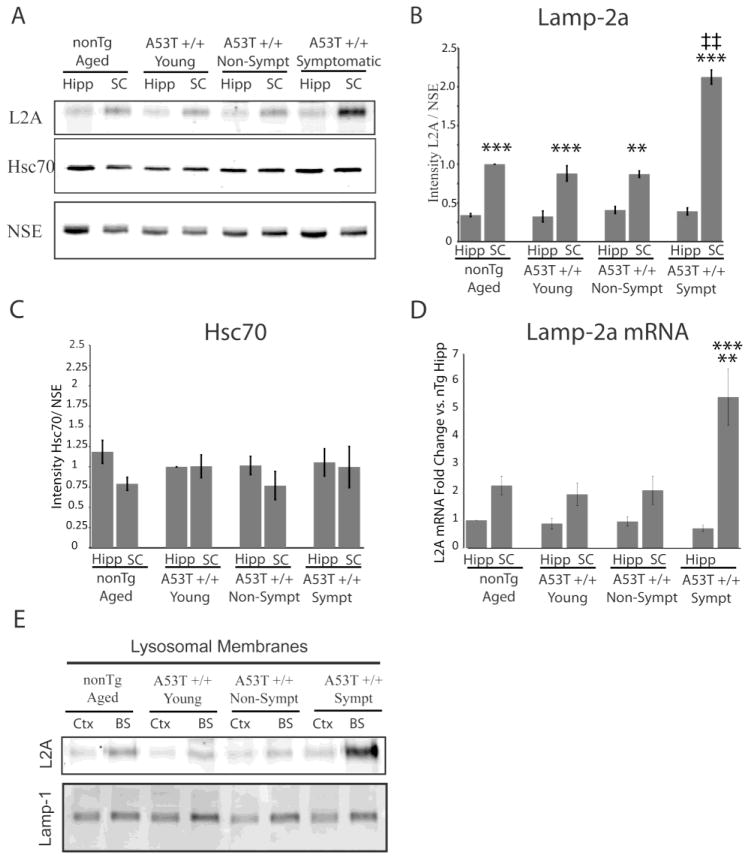
Increased expression of Lamp-2a has been shown previously to correlate with increased CMA activity (Cuervo and Dice, 2000a, 2000b). Therefore, the enhanced expression of Lamp-2a in the brainstem and spinal cord was surprising, as these regions showed decreased CMA-dependent capacity to remove α-synuclein (Figure 1C). Additionally, the brainstem and the spinal cord exhibit the most prominent α-syn aggregation in A53T α-syn transgenic mice (Giasson et al., 2002; Tsika et al., 2010). Consequently, it was important to examine the effects of both A53T α-syn transgene expression and disease onset on the expression of Lamp-2a. Four groups of mice were studied; A53T α-syn mice that had developed motor symptoms, age-matched A53T α-syn mice that had not yet developed symptoms, age-matched non-transgenic mice, and young A53T α-syn mice (2.5 months of age). For ease of comparison, two representative regions were chosen to reflect the prominence of α-syn inclusion formation. The hippocampus was chosen as a region that develops minimal α-syn inclusions, while the spinal cord is a region that develops robust α-syn inclusions in A53T α-syn mice (Giasson et al., 2002). Neither age nor transgene expression affected the overall levels of Lamp-2a within each respective region. However, upon the onset of motor symptoms, there was an increase in Lamp-2a selectively in the spinal cord (Figure 3A, B). Levels of Hsc70, the CMA chaperone, did not significantly change across regions or upon symptom development, indicating that these regional and symptom-based differences are specific to Lamp-2a and not generalized to the entire CMA system (Figure 3A, C).
FIGURE 3.
Increased levels of Lamp-2a in the spinal cord during the onset of symptoms in transgenic A53T α-syn mice. A, The hippocampus (Hipp) and the spinal cord (SC) from symptomatic A53T α-syn transgenic mice (sympt), age matched non-symptomatic A53T α-syn transgenic mice (non-sympt), age matched non-transgenic mice (nonTg), and young A53T α-syn transgenic mice (2.5mo) (young) were analyzed by SDS-PAGE/western blot for the CMA components Lamp-2a and Hsc70. NSE was used as a loading control. B, The intensity of the immunoreactive band of Lamp-2a was quantified by densitometry relative to the intensity of NSE. The resulting values were adjusted proportionally to the value of the non-transgenic spinal cord to normalize intensity across experiments. This reinforced the increased levels of lamp-2a in the spinal cord relative to the hippocampus but revealed no effect of age or transgene expression on expression. Upon the onset of symptom development there was a selective increase in Lamp-2a in the spinal cord (n=3) (**p<0.01, ***p<0.001, Levels of Lamp-2a in SC versus Hipp within a particular condition of mice; ‡‡ p<0.001, Levels of Lamp-2a in the sympt SC versus non-Tg SC, young SC, and non-sympt SC, one-way ANOVA with Tukey’s post-hoc test). C, The intensity of the immunoreactive band of Hsc70 was quantified by densitometry relative to the intensity of NSE. The resulting values were adjusted proportionally to the value of the young hippocampus to normalize intensity across experiments. No significant differences in Hsc70 expression were found across conditions. D, Quantitative PCR with a primer against Lamp-2a and a primer against neuron specific enolase (NSE) as a control was performed on cDNA synthesized from RNA extracted from same conditions described above. Levels of Lamp-2a mRNA were held relative to values for the non-transgenic hippocampus. Comparison across the groups of mice revealed no change in mRNA levels in the hippocampus, but in the spinal cord there was a significant increase in Lamp-2a mRNA in the symptomatic A53T α-syn transgenic mice relative to the other conditions (n=3) (**p<0.01, sympt SC versus non-Tg SC, young SC, and non-sympt SC, *** p<0.001, sympt SC versus Hipp, one-way ANOVA with Tukey’s post-hoc test). E, Lamp-2a is localized in lysosomal membranes. Crude lysosomal extract (light M+L fraction) was obtained from the cortex or brainstem of symptomatic A53T α-syn transgenic mice, age matched non-symptomatic A53T α-syn transgenic mice, age matched non-transgenic mice, and 2.5 months of age (young) A53T α-syn transgenic mice. Lysosomes were burst by hypotonic shock and the membranes were separated from the lumen by ultracentrifugation. The lysosomal membranes were analyzed by SDS-PAGE/western blot for Lamp-2a and Lamp-1.
To determine whether this increase in levels of the Lamp-2a protein was due to differences in mRNA, levels of Lamp-2a mRNA were quantified for the same conditions of mice. For this analysis, a primer against the C-terminal region of Lamp-2a was used that is specific to Lamp-2a and not the other Lamp-2 isoforms. Lamp-2a mRNA levels were held relative to levels of NSE mRNA as a control. Similar to the protein expression, levels of Lamp-2a mRNA were not affected by transgene expression or age within a particular region (Figure 3D). However, in the spinal cord there was a greater than two-fold increase in the levels of Lamp-2a mRNA in the symptomatic mice compared to the other conditions (Figure 3D). When the levels of Lamp-2a mRNA in the spinal cord were compared relative to the levels in the hippocampus of the same mouse for each of the four groups, the spinal cord of the symptomatic mouse had a greater than five-fold increase in the levels of Lamp-2a mRNA compared to the hippocampus (Figure 3D). These changes in mRNA and protein levels indicate a distinct recognition and response of the CMA system to the stress of α-syn aggregation.
The established relationship between Lamp-2a levels and CMA activity is dependent upon proper targeting of the Lamp-2a receptor to the lysosomal membrane (Cuervo and Dice, 2000a, 2000b). Therefore, the expression of Lamp-2a protein in the lysosomal membrane was examined. To conserve the number of mice sacrificed, for the lysosome extractions the regions of the cortex (another region with minimal α-syn pathology) and brainstem (another region that develops prominent inclusions) were selected as representative regions from the various conditions of mice. Following extraction of the lysosomes, the lysosome membranes were then separated from the lysosomal lumen through hypotonic shock. Western blot for Lamp-2a revealed that Lamp-2a was associated with the lysosomal membrane with a similar expression pattern to that of the whole cell homogenate (Figure 3E). Another lysosomal membrane protein, Lamp-1 was used as a control and did not exhibit the same expression pattern as Lamp-2a (Figure 3E). This establishes that Lamp-2a is indeed localized in the lysosomal membrane.
Lysosomal association of CMA substrates is not proportional to levels of Lamp-2a
While this data reinforces the relationship between α-syn and CMA, it is unclear how the regions that have both the highest level of endogenous Lamp-2a expression as well as the ability to dynamically upregulate these levels in response to altered substrate homeostasis have impaired lysosomal degradation and are ultimately the most vulnerable to protein aggregation. Therefore, the next series of investigations examined the functional dynamics of lysosomes within these different brain regions to reveal deficiencies not apparent from lysosomal protein levels.
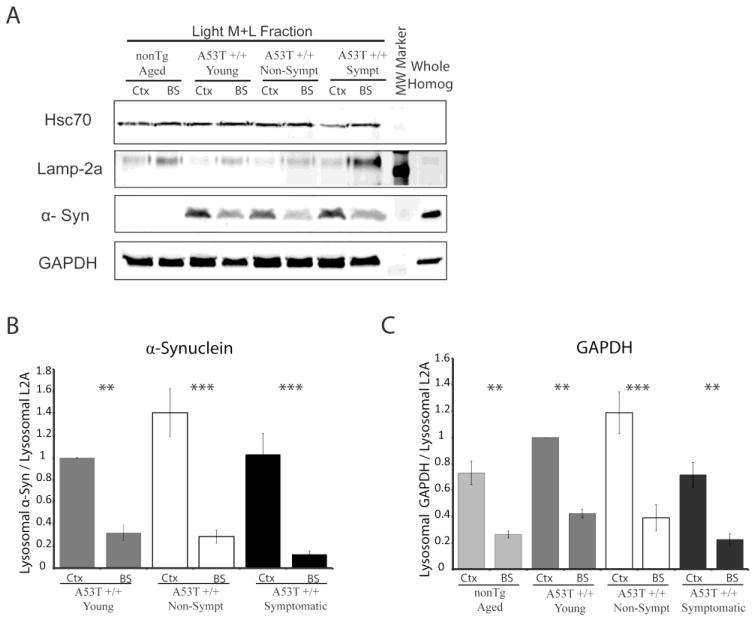
In order for substrates to be effectively taken up into the lysosomes for degradation, they must first efficiently associate with the lysosomes. Therefore, we compared the levels of CMA substrates associated with lysosomes in the cortex and brainstem of the different conditions of mice. Lysosomes were extracted from the cortex and brainstem of symptomatic, non-symptomatic, young, and non-transgenic mice. The levels of Hsc70, Lamp-2a, α-syn, and the established CMA substrate GAPDH associated with the lysosomes were then evaluated (Figure 4A). Lysosomal enrichment was verified by comparison to whole non-fractionated homogenate (Figure 4A). The number of micrograms of lysosomes in each condition was held constant, preventing the effect of any possible discrepancy in lysosomal quantity across various regions. Similar to what was documented in whole cell homogenate, levels of the CMA chaperone Hsc70 did not change across the different conditions (Figure 4A). This was verified by quantification and statistical analysis that revealed no significant difference in Hsc70 expression across conditions (data not shown). Therefore, any observed differences in substrate association and degradation are not due to impaired delivery of the substrate to the lysosomes by the CMA chaperone.
FIGURE 4.
Association of substrates with lysosomes is not proportional to the levels of Lamp-2a at the lysosomal membrane. A, Crude lysosomal extracts (light M+L fraction) were obtained from the cortex (Ctx) and the brainstem (BS) of symptomatic A53T α-syn transgenic mice, age matched non-symptomatic A53T α-syn transgenic mice, age matched non-transgenic mice, and 2.5 months of age (young) A53T α-syn transgenic mice. The extracted lysosomes were analyzed by SDS-PAGE/western Blot alongside non-fractionated homogenate from the whole brains of non-transgenic mice with an antibodies against Hsc70, Lamp-2a, human α-syn, and GAPDH. B, Intensity of α-syn and Lamp-2a immunoreactive bands were quantified by densitometry. The intensity of α-syn was then held relative to the intensity of Lamp-2a from the same sample. No band for α-syn was present in the non-transgenic mice, showing that the band is specific for transgenic A53T human α-syn, and was excluded from the report of densitometric analysis. α-Syn showed decreased association with lysosomes relative to the levels of Lamp-2a in the brainstem compared to the cortex across all conditions (n=4) (**p<0.01, ***p<0.001, BS versus Ctx, one-way ANOVA with Tukey’s post-hoc test). C, Immunoreactive bands of lysosomal extracts were analyzed as in (C) for GAPDH. GAPDH showed decreased association with lysosomes relative to levels of Lamp-2a in the brainstem compared to the cortex across all conditions (n=4) (**p<0.01, ***p<0.001, BS versus Ctx, one-way ANOVA with Tukey’s post-hoc test).
Levels of Lamp-2a at the lysosomal membrane have been previously described as the rate-limiting step in the lysosomal binding, uptake, and degradation of CMA substrates (Cuervo and Dice, 2000a, 2000b). Therefore, the levels of CMA substrates associated with the lysosomes were evaluated relative to levels of lysosomal Lamp-2a to determine if this correlation exists in the mouse brain. While greater levels of Lamp-2a were present in lysosomes from the brainstem relative to the cortex, transgenic α-syn showed decreased association with lysosomes in the brainstem compared to the cortex (Figure 4A, B). Additionally, this pattern of α-syn expression did not change upon symptom development, when the levels of Lamp-2a in the lysosomes of the brainstem selectively increased. The CMA substrate GAPDH also did not associate with lysosomes proportionally to Lamp-2a expression as the same amount of GAPDH was detected across all the conditions (Figure 4A, C). Therefore the higher levels of Lamp2a in lysosomal membrane do not correspond to increased substrate binding.
Rates of CMA substrate degradation across brain regions are not proportional to levels of Lamp-2a
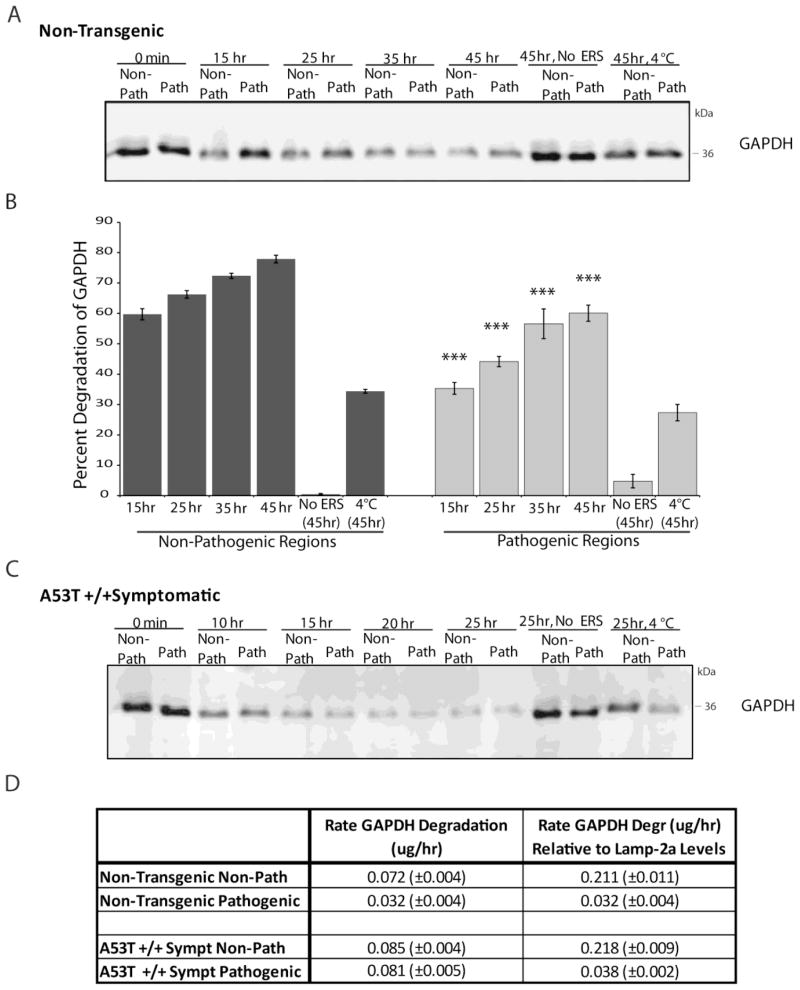
The incongruence between Lamp-2a levels and lysosomal substrate binding prompted further investigation of the rates CMA degradation across different regions and symptom conditions. To test this, a lysosomal activity assay was performed examining the degradation of the endogenously present CMA substrate GAPDH. Following extraction of lysosomes, bound CMA substrates remain associated with the lysosomes. The rate of uptake and degradation of these substrates can then be determined by monitoring the amount of remaining protein in the isolated system at varying lengths of time. Purified lysosomes were extracted from the inclusion-forming regions of the brainstem and the spinal cord or from the remaining brain regions that are less prone to developing pathology as previously described. The purified lysosomes were divided into equal fractions, and each fraction was used for different time points of incubation at 37 °C with an energy regenerating system containing ATP. Levels of the CMA substrate GAPDH were monitored at the different time points by quantitative western blot and compared with a corresponding lysosomal fraction that did not undergo incubation at 37 °C. GAPDH was selected for analysis due to its role as an established CMA substrate, as well as its equal levels of expression and equivalent lysosomal binding across brain regions and across transgenic and non-transgenic mice. The number of micrograms of lysosomes in each condition was held constant, preventing the effect of any possible discrepancy in lysosomal quantity across various regions. Similar to the assay involving exogenous addition of α-syn (Figure 1), endogenous GAPDH showed impaired uptake and degradation in the pathogenic regions of non-transgenic mice (Figure 5A,B). This effect was dependent on the presence of ATP and on lysosomal uptake, as determined by conditions lacking the energy regenerating system or incubated at 4 °C (Figure 5A,B). However, examination of symptomatic A53T α-syn mice revealed that the levels of GAPDH degradation were similar in the pathogenic and non-pathogenic regions (Figure 5C).
FIGURE 5.
Rates of CMA substrate degradation across brain regions are not proportional to levels of Lamp-2a. A, Purified lysosomes were extracted from the pathogenic regions of the brainstem and spinal cord (“Path”) or the remainder of the brain (“Non-Path”) of non-transgenic mice. To determine the degradation of GAPDH endogenously associated with lysosomes, 75ug lysosomes were purified from non-transgenic mice. The lysosomes were then incubated for the indicated timepoints at 37°C with an energy regenerating system. After the incubation, the samples were analyzed by SDS-PAGE/western blot with a GAPDH antibody. As a control for CMA dependent uptake, a “No ERS” condition was included where the ATP energy regenerating system was not included. An additional control condition involved incubation of the lysosomes at 4°C during the reaction to prevent uptake. B, the immunoreactive bands in (A) were quantified by densitometry. All values were held relative to a condition in which the lysosomes from each region were immediately frozen (0 min). The pathogenic regions of non-transgenic mice have a decreased ability to degrade endogenous GAPDH by CMA, a difference that is dependent on ATP and lysosomal uptake (n=3, ***p<0.001, Non-Path vs Path for each timepoint, one-way ANOVA with Tukey’s post-hoc test). C, The endogenous lysosomal degradation of GAPDH was assessed for symptomatic A53T+/+ α-synuclein mice as in (A). The pathogenic regions of the A53T α-synuclein mice display comparable degradation of endogenous GAPDH compared to the non-pathogenic regions (n=3). D, The rate of GAPDH degradation was determined by comparing the intensity of the bands of the 0 hr and 15 hr time points in (C) and (D) with known standards of purified GAPDH. The resulting values were then divided by the relative amounts of Lamp-2a present in the different conditions, as determined by western blot (n=3).
To investigate the relationship between levels of Lamp-2a and substrate uptake and degradation, the rate of GAPDH degradation was calculated for the pathogenic and non-pathogenic regions of the non-transgenic and symptomatic A53T mice. This degradation rate was then normalized to the levels of Lamp-2a in these regions. Because the number of micrograms of lysosomes was held constant across the different conditions, the resulting values were not influenced by different numbers of lysosomes in the various regions. This data revealed that while the rate of GAPDH degradation increased in the pathogenic regions of the symptomatic mice, this increase was proportional to the increase in Lamp-2a expression (Figure 5D). Therefore, the rate of degradation relative to levels of Lamp-2a protein remained constant (Figure 5D). However, the non-pathogenic regions had a much greater rate of protein degradation relative to the levels of Lamp-2a expression compared with the pathogenic regions (Figure 5D). Therefore, it appears that the rates of CMA substrates degradation across brain regions are not proportional to the levels of the CMA receptor Lamp-2a.
To further characterize the lack of correlation between Lamp-2a levels and CMA degradation across brain regions, a lysosomal activity assay for exogenously added α-syn was performed in the presence of an antibody against Lamp-2a. The antibody binds to residues in the c-terminal tail of Lamp-2a that are responsible for substrate recognition. Addition of the Lamp-2a antibody was able to significantly decrease the uptake and degradation of α-syn in the pathogenic regions but not the non-pathogenic regions of non-transgenic mice (Supplementary Figure 3). Therefore, without uptake through Lamp-2a receptor, substrate degradation in the pathogenic regions is largely impaired, while degradation in the non-pathogenic regions is still able to occur. This reinforces the finding that in addition to the previously characterized components of CMA, novel mechanisms may contribute to direct lysosomal uptake and degradation within the brain. Furthermore, these unique Lamp-2a-independent factors allow for inherent discrepancies in the functional dynamics of CMA substrate degradation across brain regions.
DISCUSSION
Three different autosomal dominant mutations in the gene encoding α-syn as well as duplication or triplication of the α-syn gene have been linked with familial forms of PD (Chartier-Harlin et al., 2004; Polymeropoulos et al., 1997; Krüger et al., 1998; Zarranz et al., 2004). Additionally, wild type α-syn is a component of the Lewy Body inclusions found in the degenerating brain regions of patients with sporadic PD, supporting a role for this protein in disease (Spillantini et al., 1997). Despite considerable advances in understanding the physiological and pathological roles of α-syn, it is unclear why α-syn forms inclusions only in select brain regions despite relatively uniform expression. While catastrophic failure of protein clearance permits proteins with amyloid forming capacity to aggregate and form inclusions, it is not known how these degradation systems may contribute to regionally selective aggregation. Ultimately, understanding the selective vulnerability of different neural populations is crucial to understanding the molecular mechanisms underlying disease.
Previous studies in vitro or in cell models have indicated that α-syn modified with the A53T mutation or modified by oxidized dopamine can impair CMA uptake and degradation by binding strongly to Lamp-2a and blocking the receptor (Cuervo et al., 2004; Martinez-Vicente et al., 2008). While it is possible that this process also occurs within the brain, it does not explain why certain brain regions are vulnerable to α-syn inclusion formation and cellular dysfunction while others are spared. Therefore, the present study employed a mouse model to identify regional differences in the CMA pathway that may explain selective α-syn aggregation. Investigations first focused on examining endogenous differences in CMA activity and the expression of CMA components across brain regions of non-transgenic mice. Subsequently, A53T α-syn mice were examined to determine the effects of a known stressor of α-syn homeostasis on CMA components.
The brainstem and spinal cord are the two brain regions that develop the most prominent inclusions in A53T α-syn transgenic mice. Examining the brains of non-transgenic mice for inherent regional differences that could explain this selective vulnerability revealed decreased levels of CMA activity in these regions that are vulnerable to forming α-syn inclusions compared to the non-pathogenic regions. This provides a potential mechanism by which the brainstem and spinal cord are more susceptible to protein aggregation when α-syn homeostasis is altered, such as with α-syn transgene expression. While PD in humans is most directly associated with pathology in the substantia nigra pars compacta, it has been proposed that Lewy body inclusions originate in the brainstem and anterior olfactory areas before later progressing to include other areas such as the locus coeruleus, raphe nucleus, substantia nigra, amygdala, and neocortex (Braak et al., 2006). Within the context of sporadic PD, this concept of increased regional vulnerability during times of homeostatic stress remains applicable. However, further investigations are required to elucidate the factors that may serve as tipping points in sporadic cases. Additionally, while evidence from PD patients as well as several mouse models highlights the brainstem as a region of prominent pathology (Van der Putten et al., 2000; Lee et al., 2002; Braak et al., 2006), variability exists between different α-syn mouse models (Kahle et al., 2000; Masliah et al., 2000). Overall, the present study provides evidence documenting regional differences in CMA activity that can provide a potential mechanism for the initiation of regionally selective α-synuclein aggregation in disease.
Moreover, the data does not support that the A53T mutation itself is the determining factor influencing the selective vulnerability of particular brain regions. The purpose of employing mice overexpressing human A53T α-syn was to model the effect of an established stressor known to shift α-syn protein homeostasis and promote aggregation. The regional differences in both lysosomal activity as well as Lamp-2a levels were observed in non-transgenic mice as well as A53T α-syn mice. Therefore, inherent differences in CMA are present across brain regions independent of transgene effects. Additionally, the deficiencies in α-syn uptake and degradation were seen with exogenously applied WT human α-syn as well as the endogenous A53T human α-syn. In the presence of human A53T α-syn, α-syn homeostasis is challenged and the regions that have deficient CMA activity are the most vulnerable to protein aggregation and cellular dysfunction, resulting in the observed pathology. Alternately, the regions that have higher levels of CMA activity are better equipped to deal with the insult.
CMA activity has previously shown to correlate with levels of the CMA receptor Lamp-2a (Cuervo and Dice, 2000a, 2000b). Therefore, the expression of Lamp-2a was investigated to determine whether regional differences in Lamp-2a expression account for the variations in CMA activity. In the mouse brain, Lamp-2a has an apparent molecular weight of 72kDa as determined by the absence of this band in western blots with Lamp-2 knockout mice and confirmed by mass spectrometry. This novel 72kDa molecular weight appears to be due to differential glycosylation of the protein.
Examination of Lamp-2a levels across brain regions revealed increased levels of Lamp-2a in the brainstem and the spinal cord compared to other regions. Given the unique composition of neural cell types in each brain region, further investigations are needed to determine whether this discrepancy in expression levels is due to the specific contributions of particular types of cells. Interestingly, a dynamic increase in the levels of Lamp-2a in response to α-syn aggregation but not to transgene expression is observed. Upon the onset of motor symptoms in the A53T transgenic mice, a dramatic increase in the levels of Lamp-2a mRNA and protein occurs. This response reinforces the critical role of CMA in the regulation of α-syn homeostasis. This upregulation is not present in the non-symptomatic A53T mice which are age matched to the symptomatic mice and have the same levels of transgene expression, and also does not occur in brain regions less vulnerable to aggregate formation in the symptomatic mouse, such as the hippocampus, which also expresses the A53T α-syn transgene. Therefore, the stressor for Lamp-2a upregulation is protein aggregation, not A53T α-syn transgene expression. While Lamp-2a acts as the modulator of CMA activity during a variety of cell stress conditions, the method by which the levels Lamp-2a in the lysosomal membrane are increased varies depending on the insult. Starvation does not induce the synthesis of new Lamp-2a but rather recruits already formed Lamp-2a from the lysosomal lumen to the lysosomal membrane (Cuervo and Dice, 2000a). Alternately, oxidative stress stimulates transcriptional upregulation of Lamp-2a (Kiffin et al., 2004; Mak et al., 2010). The present study reports a novel response of Lamp-2a that is sensitive to dynamic changes in cellular protein homoestasis. However, both the pattern of Lamp-2a expression as well as the apparent responsiveness of the system does not predict a deficiency in CMA activity or vulnerability to α-syn aggregation within the brainstem and spinal cord.
In addition to chaperone-mediated autophagy, dynamic contributions of multiple protein degradation systems likely contribute to the clearance of α-syn. The increased levels of Lamp-2a seen in the spinal cord in basal conditions may represent an attempt to compensate for the other lysosomal deficiencies, as CMA activity has been shown to be upregulated upon the inhibition of macroautophagy (Kaushik et al., 2008). Therefore, it is possible that additional lysosomal deficiencies independent of CMA exist in the regions that develop pathology and contribute to aggregate formation. Since α-syn is a CMA substrate, the clearance of substrate through this pathway remained the focus of subsequent experiments.
Expression of the Lamp-2a receptor, localization of Lamp-2a to the lysosomal membrane, expression of the CMA chaperone Hsc70, and expression of Cathepsin D within lysosomes were all found to not be deficient in the pathogenic regions. Therefore, other potential factors contributing to the decreased clearance of substrates in aggregate-prone regions were investigated, specifically the functional aspects of substrate biding, uptake, and degradation. This revealed that the levels of substrates associated with lysosomes were not proportional to Lamp-2a levels, as had been previously established. Consequently, Lamp-2a upregulation is unable to effectively increase association of CMA substrates with lysosomes.
Examination of the rates of substrate degradation relative to the levels of Lamp-2a revealed the most striking regional discrepancies. Isolated lysosomes from non-transgenic mice and symptomatic A53T α-syn mice were assayed for CMA activity by measuring the degradation of endogenous GAPDH associated with the extracted lysosomes. The lysosomes from the pathogenic regions displayed rates of GAPDH degradation that were proportional to the levels of Lamp-2a expression. However, these degradation rates were significantly lower than the rates of GAPDH degradation adjusted for Lamp-2a levels in the non-pathogenic regions. While substrate degradation within the pathogenic regions is dependent on Lamp-2a, degradation across brain regions is not proportional to Lamp-2a expression. Therefore, we report the novel finding that CMA activity, as determined by binding, uptake, and degradation, is regulated in a different manner in the mouse brain than what has previously been described, as the activity across brain regions is not proportional to levels of Lamp-2a.
One possible explanation for this discrepancy is that the lysosomal membrane composition is different across brain regions. Lipid microdomains have been shown to play a role in functional multimerization of Lamp-2a and regulation of CMA activity (Kaushik et al., 2006). Alternately, an additional chaperone may exist that regulates CMA activity in a manner that is not currently appreciated. For example, Hsp90, GFAP, EF1α, have been recently implicated as additional regulators of CMA activity (Bandyopadhyay et al., 2008, 2010). Future studies are in order to examine these possibilities. Another potential factor contributing to this lack of correlation between Lamp-2a levels and CMA activity is that in addition to Lamp-2a, another receptor may promote increased degradation in the regions that are less vulnerable to developing inclusions. Currently, Lamp-2a is the only receptor that has been characterized to participate in the direct lysosomal uptake and degradation of substrates in CMA. The twelve amino-acid cytoplasmic C-terminus of Lamp-2a is responsible for CMA substrate recognition. Specifically, site directed mutagenesis of the cytosolic tail of Lamp-2a revealed that a series of four positive residues (KHHH) is crucial for this role (Cuervo and Dice, 2000b). In the mouse, this series of four amino acids is slightly modified to KRHH but retains its positive charge. Therefore, if an additional substrate protein is able to participate in this function, it is likely to have a high level of conservation of these residues. The Lamp-2a protein results from the alternative splicing of Lamp-2 mRNA into three different isoforms. However, it is unlikely that Lamp-2b and Lamp-2c serve as additional lysosomal receptors since of the twelve amino acids located in this cytosolic C-terminal region, Lamp-2b and Lamp-2c differ in eight and nine of the amino acids, respectively. Additionally, immunoprecipitation studies have demonstrated that the CMA substrate GAPDH is able to associate with Lamp-2a but not the other isoforms of Lamp-2 (Cuervo and Dice, 2000b). However, the abundant lysosomal membrane protein Lamp-1 is also structurally homologous to Lamp-2a and contains a C-terminal cytosolic tail with the amino acid sequence of 397KRSH400, conserving three of the four residues from Lamp-2a’s 406KRHH409. Interestingly, double knockout mice for Lamp-1/Lamp-2 are embryonic lethal, while mice with a knockout of only one of these two proteins are viable, suggesting that there may be an overlapping function of these two proteins (Tanaka et al., 2000; Andrejewski et al., 1999; Eskelinen et al., 2004). Further studies must be performed to decipher these various possibilities.
While the upregulation of Lamp-2a expression in the pathogenic regions upon the onset of motor symptoms is able to increase lysosomal degradation to a rate comparable to the non-pathogenic regions, this is unlikely to reverse the onset of disease. At the onset of motor symptoms, the presence of α-syn aggregates is already apparent. Because substrates must be unfolded to pass through the Lamp-2a receptor, CMA is unable to degrade oligomers or aggregates (Martinez-Vicente et al., 2008; Salvador et al., 2000). This reinforces the importance of basal levels of substrate turnover in determining the vulnerability of a particular brain region to protein aggregation.
Previous studies in vitro and in cell models have demonstrated that A53T α-synuclein impairs CMA degradation. It was found that A53T α-syn is targeted to the lysosome as a CMA substrate. However, A53T α-syn bound more strongly to the Lamp-2a receptor and consequently blocked the uptake and degradation of itself and other CMA substrates (Cuervo et al., 2004). Similarly, Alvarez-Erviti et al. (2010) found in cultured cells that overexpression of A53T α-syn decreased the rate of GAPDH degradation. However, the present data indicates that in vivo, the rate of CMA degradation is not impaired by expression of A53T α-synuclein, even in the presence of α-synuclein aggregates. Uptake and degradation of the established CMA substrate GAPDH occurred at similar rates in A53T α-syn transgenic mice compared with non-transgenic mice. Additionally, examination of the levels of α-syn associated with lysosomes revealed that α-syn did not exhibit increased association with the lysosomes in regions that display lower CMA activity. The levels of α-syn associated with the lysosomes also did not increase with age, symptom development, or with regional formation of aggregates. Therefore, in the mouse brain, the interaction of A53T α-syn with CMA components differs from what has been found in vitro, and specifically does not appear to bind more strongly to Lamp-2a and block CMA activity.
CONCLUSION
In summary, this study provides insight into the role of CMA in disease in an in vivo context. We report for the first time inherent lysosomal differences across brain regions of non-transgenic mice and in mice expressing human A53T α-syn. Contrary to what has previously been established for the liver, we propose that within the brain, CMA activity across brain regions is independent of the Lamp-2a levels. Ultimately, regional differences in direct lysosomal degradation result in altered capacity for substrate clearance. In regions with increased levels of CMA activity, this serves a protective role in the presence of a stressor, such as α-syn expression, and minimizes the formation of α-syn inclusions in these areas. While Lamp-2a expression is greater in the brainstem and spinal cord than in the rest of the brain and is dynamically upregulated upon the onset of disease, CMA activity in these regions remains impaired, resulting in decreased capacity for α-syn clearance. Therefore, this data reveals that novel factors modulate direct lysosomal uptake and degradation within the brain and vary across brain regions. These unique differences in CMA activity provide a potential mechanism for the regional selectivity of α-syn inclusion formation and neurodegeneration.
Supplementary Material
Highlights.
Defects in CMA activity explain the regional selectivity of α-synuclein aggregation
Decreased lysosomal CMA clearance of α-synuclein in aggregate prone brain regions.
In the brain Lamp2a is a 72Kda glycoprotein.
Levels of the Lamp-2a are higher in aggregate prone brain regions.
Levels of Lamp-2a do not correlate with substrate CMA binding or degradation.
Acknowledgments
We thank Judith Blanz and Paul Saftig for the brain tissue from the Lamp-2 knockout mice, Richard Lightfoot for the purified human α-synuclein, Lynn Spruce and Steve H. Seeholzer for mass spectrometry, Dr. Virginia Lee for the primary cortical and hippocampal neurons, Dr. Serge Przedborski for the primary spinal cord neurons and purified motor neurons, Dr. Judith Grinspan for the precursor and mature oligodendrocytes, and Dr. Christie Bruno for the astrocytes,. This work was supported by NIH grants AG13966 and ES013508 NIEHS Center of Excellence in Environmental Toxicology. HI is the Gisela and Dennis Alter Chair in Pediatric Neonatology at the Children’s Hospital of Philadelphia.
ABBREVIATIONS
- α-syn
alpha-synuclein
- CMA
chaperone-mediated autophagy
- Lamp-2a
lysosome-associated membrane protein type 2A
- Hsc70
Heat-shock cognate protein of 70kDa
- PD
Parkinson’s disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67(12):1464–72. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- Andrejewski N, Punnonen EL, Guhde G, Tanaka Y, Lüllmann-Rauch R, Hartmann D, von Figura K, Saftig P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J Biol Chem. 1999;274(18):12692–701. doi: 10.1074/jbc.274.18.12692. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol. 2008;28(18):5747–63. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay U, Sridhar S, Kaushik S, Kiffin R, Cuervo AM. Identification of regulators of chaperone-mediated autophagy. Mol Cell. 2010;27;39(4):535–47. doi: 10.1016/j.molcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Bohl JR, Müller CM, Rüb U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21(12):2042–51. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- Carlsson SR, Roth J, Piller F, Fukuda M. Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem. 1988;263(35):18911–9. [PubMed] [Google Scholar]
- Carlsson SR, Fukuda M. The polylactosaminoglycans of human lysosomal membrane glycoproteins lamp-1 and lamp-2. Localization on the peptide backbones. J Biol Chem. 1990;265(33):20488–95. [PubMed] [Google Scholar]
- Carlsson SR, Lycksell PO, Fukuda M. Assignment of O-glycan attachment sites to the hinge-like regions of human lysosomal membrane glycoproteins lamp-1 and lamp-2. Arch Biochem Biophys. 1993;304(1):65–73. doi: 10.1006/abbi.1993.1322. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364(9440):1167–9. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246(4928):382–5. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4(11):1318–20. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Terlecky SR, Dice JF, Knecht E. Selective binding and uptake of ribonuclease A and glyceraldehyde-3-phosphate dehydrogenase by isolated rat liver lysosomes. J Biol Chem. 1994;269(42):26374–80. [PubMed] [Google Scholar]
- Cuervo AM, Knecht E, Terlecky SR, Dice JF. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol. 1995;269(5 Pt 1):C1200–8. doi: 10.1152/ajpcell.1995.269.5.C1200. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273(5274):501–3. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Hildebrand H, Bomhard EM, Dice JF. Direct lysosomal uptake of alpha 2-microglobulin contributes to chemically induced nephropathy. Kidney Int. 1999;55(2):529–45. doi: 10.1046/j.1523-1755.1999.00268.x. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. Regulation of lamp2a levels in the lysosomal membrane. Traffic. 2000a;1(7):570–83. doi: 10.1034/j.1600-0854.2000.010707.x. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci. 2000b;113(24):4441–50. doi: 10.1242/jcs.113.24.4441. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52(2):205–10. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. Distinct Roles In Vivo for the Ubiquitin-Proteasome System and the Autophagy-Lysosomal Pathway in the Degradation of {alpha}-Synuclein. J Neurosci. 2011;31(41):14508–20. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen EL, Schmidt CK, Neu S, Willenborg M, Fuertes G, Salvador N, Tanaka Y, Lüllmann-Rauch R, Hartmann D, Heeren J, von Figura K, Knecht E, Saftig P. Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol Biol Cell. 2004;15(7):3132–45. doi: 10.1091/mbc.E04-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274(12):7619–22. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34(4):521–33. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–75. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;1;20(17):6365–73. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Massey AC, Cuervo AM. Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J. 2006;6;25(17):3921–33. doi: 10.1038/sj.emboj.7601283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell. 2008;19(5):2179–92. doi: 10.1091/mbc.E07-11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15(11):4829–40. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18(2):106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;25;99(13):8968–73. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA. Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem. 2010;285(18):13621–9. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118(2):777–88. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;18;287(5456):1265–9. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci. 2006;103(15):5805–10. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rochet JC, Conway KA, Lansbury PT., Jr Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse alpha-synuclein. Biochemistry. 2000;39(35):10619–26. doi: 10.1021/bi001315u. [DOI] [PubMed] [Google Scholar]
- Salvador N, Aguado C, Horst M, Knecht E. Import of a cytosolic protein into lysosomes by chaperone-mediated autophagy depends on its folding state. J Biol Chem. 2000;275(35):27447–56. doi: 10.1074/jbc.M001394200. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci. 1998a;95(11):6469–73. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-3. synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998b;251(3):205–8. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lüllmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406 (6798):902–6. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- Tsika E, Moysidou M, Guo J, Cushman M, Gannon P, Sandaltzopoulos R, Giasson BI, Krainc D, Ischiropoulos H, Mazzulli JR. Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J Neurosci. 2010;30(9):3409–18. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uéda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci. 1993;90(23):11282–6. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20(16):6021–9. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem. 2008;283(35):23542–56. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. Aberrant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One. 2009;4(5):e5515. doi: 10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Muñoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.