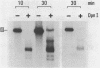
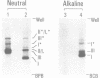
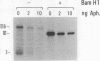
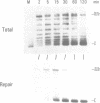
Abstract
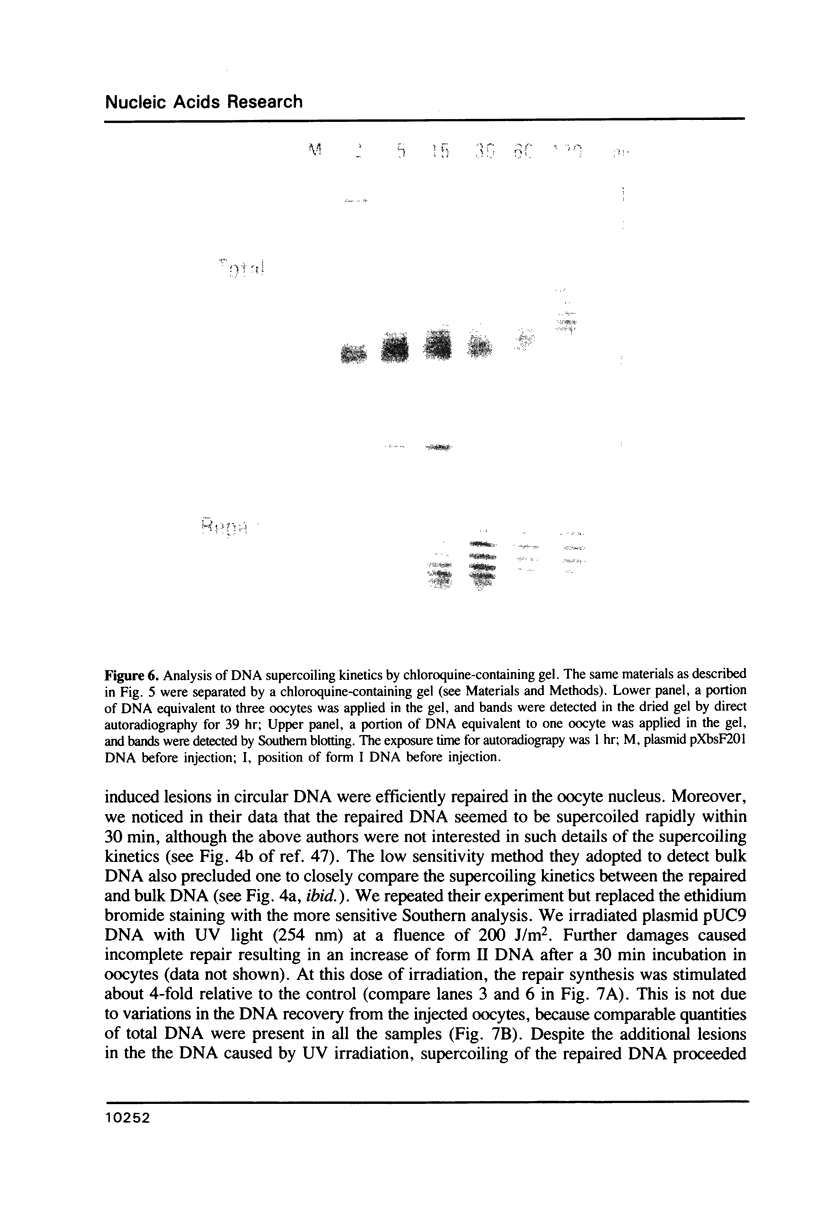
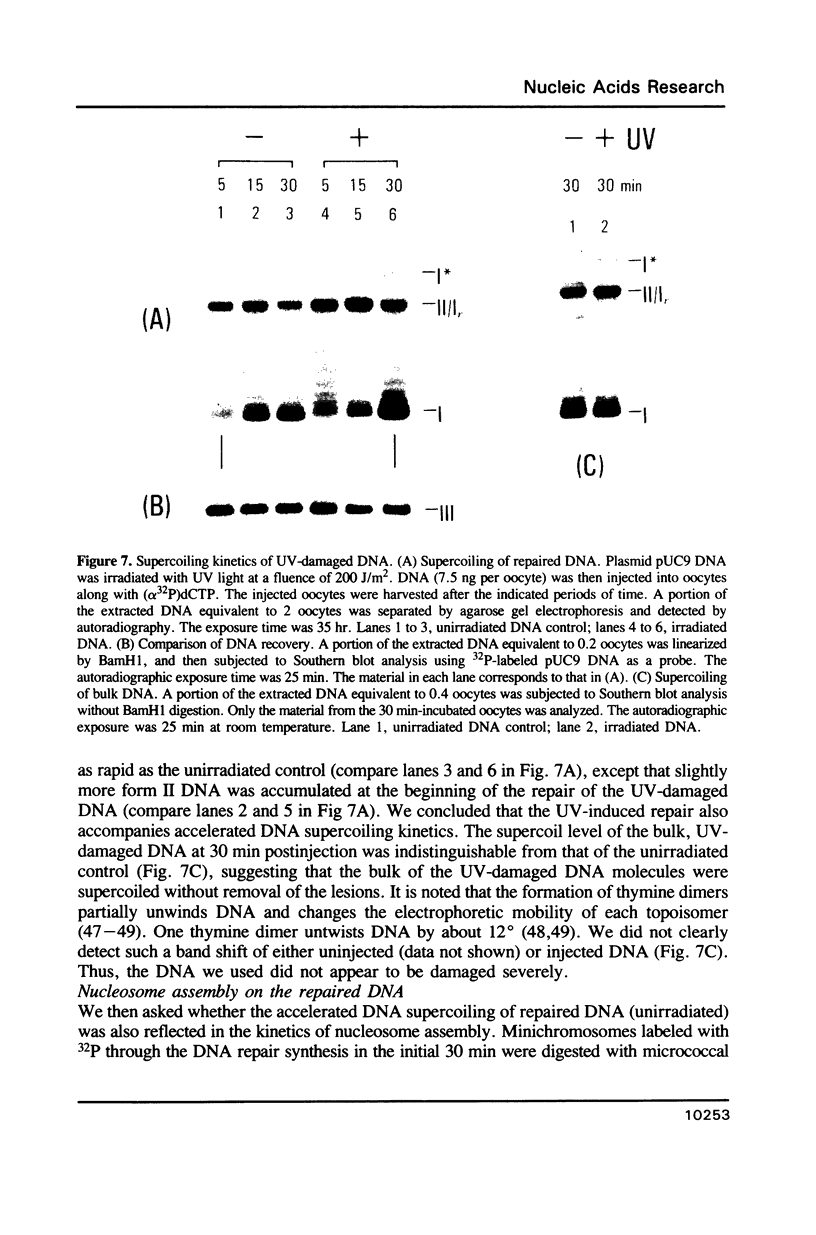
Minichromosomes were assembled by injection of circular DNA into the nucleus of Xenopus oocytes. We observed that, in the course of DNA supercoiling and chromatin assembly, a small percentage of the injected DNA molecules incorporated a radioactive precursor. This DNA synthesis was carried out by aphidicolin-sensitive DNA polymerase, and generated short repair-like patches covalently linked to the injected DNA. We found that the DNA thus repaired was rapidly supercoiled almost to completion within 15 to 30 min after injection, whereas 60 to 120 min were required to supercoil the intact, bulk DNA molecules. Such differential supercoiling kinetics was also observed when UV-damaged DNA was injected. Chromatin assembly, which was characterized by DNA fragment sizes protected from micrococcal nuclease digestion, was consistent with the rapid DNA supercoiling and proceeded more efficiently on the repaired DNA. These results indicate that there are at least two kinetically distinct ways of assembling minichromosomes in the oocyte nucleus, and that the repaired DNA molecules preferentially follow the faster pathway.
Full text
PDF








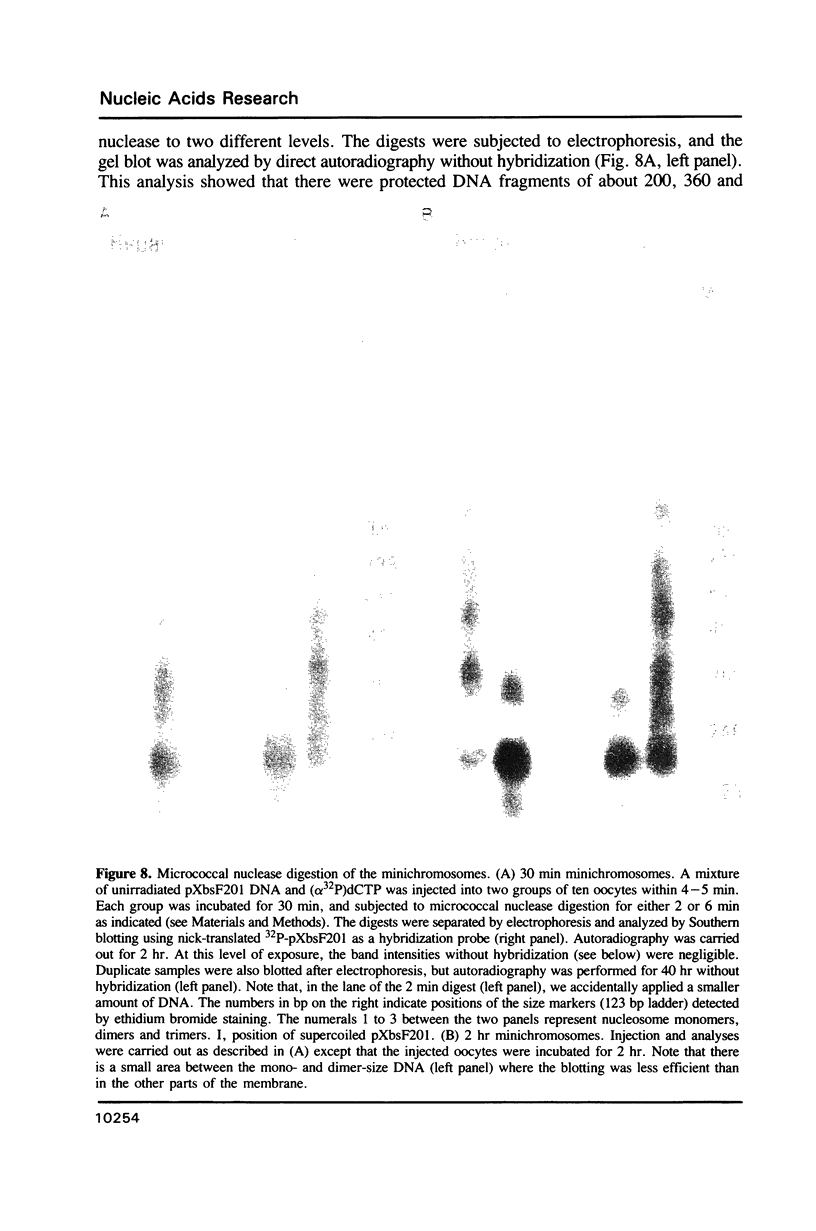




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almouzni G., Méchali M. Assembly of spaced chromatin involvement of ATP and DNA topoisomerase activity. EMBO J. 1988 Dec 20;7(13):4355–4365. doi: 10.1002/j.1460-2075.1988.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almouzni G., Méchali M. Assembly of spaced chromatin promoted by DNA synthesis in extracts from Xenopus eggs. EMBO J. 1988 Mar;7(3):665–672. doi: 10.1002/j.1460-2075.1988.tb02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D. F., Sakonju S., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3' border of the region. Cell. 1980 Jan;19(1):27–35. doi: 10.1016/0092-8674(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Cusick M. E., Herman T. M., DePamphilis M. L., Wassarman P. M. Structure of chromatin at deoxyribonucleic acid replication forks: prenucleosomal deoxyribonucleic acid is rapidly excised from replicating simian virus 40 chromosomes by micrococcal nuclease. Biochemistry. 1981 Nov 10;20(23):6648–6658. doi: 10.1021/bi00526a020. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Kato A. C. Comparison of the effect of ultraviolet radiation and ethidium bromide intercalation on the conformation of superhelical phiX174 replicative form DNA. J Mol Biol. 1973 Jul 15;77(4):479–494. doi: 10.1016/0022-2836(73)90217-9. [DOI] [PubMed] [Google Scholar]
- Dresler S. L., Gowans B. J., Robinson-Hill R. M., Hunting D. J. Involvement of DNA polymerase delta in DNA repair synthesis in human fibroblasts at late times after ultraviolet irradiation. Biochemistry. 1988 Aug 23;27(17):6379–6383. doi: 10.1021/bi00417a028. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Honda B. M., Laskey R. A., Thomas J. O. Assembly of nucleosomes: the reaction involving X. laevis nucleoplasmin. Cell. 1980 Sep;21(2):373–383. doi: 10.1016/0092-8674(80)90474-2. [DOI] [PubMed] [Google Scholar]
- Ford C. C., Woodland H. R. DNA synthesis in ocytes and eggs of Xenopus laevis injected with DNA. Dev Biol. 1975 Mar;43(1):189–199. doi: 10.1016/0012-1606(75)90140-2. [DOI] [PubMed] [Google Scholar]
- Gargiulo G., Worcel A. Analysis of the chromatin assembled in germinal vesicles of Xenopus oocytes. J Mol Biol. 1983 Nov 5;170(3):699–722. doi: 10.1016/s0022-2836(83)80128-4. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Hammond R. A., Byrnes J. J., Miller M. R. Identification of DNA polymerase delta in CV-1 cells: studies implicating both DNA polymerase delta and DNA polymerase alpha in DNA replication. Biochemistry. 1987 Oct 20;26(21):6817–6824. doi: 10.1021/bi00395a035. [DOI] [PubMed] [Google Scholar]
- Hanaoka F., Kato H., Ikegami S., Oashi M., Yamada M. Aphidicolin does inhibit repair replication in HeLa cells. Biochem Biophys Res Commun. 1979 Mar 30;87(2):575–580. doi: 10.1016/0006-291x(79)91833-3. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Hunting D. J., Dresler S. L., Lieberman M. W. Multiple conformational states of repair patches in chromatin during DNA excision repair. Biochemistry. 1985 Jun 18;24(13):3219–3226. doi: 10.1021/bi00334a022. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Kaiserman H. B., Benbow R. M. Characterization of a stable, major DNA polymerase alpha species devoid of DNA primase activity. Nucleic Acids Res. 1987 Dec 23;15(24):10249–10265. doi: 10.1093/nar/15.24.10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A. DNA replication. J Biol Chem. 1988 Jan 5;263(1):1–4. [PubMed] [Google Scholar]
- Lacks S., Greenberg B. A deoxyribonuclease of Diplococcus pneumoniae specific for methylated DNA. J Biol Chem. 1975 Jun 10;250(11):4060–4066. [PubMed] [Google Scholar]
- Laskey R. A., Honda B. M., Mills A. D., Finch J. T. Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature. 1978 Oct 5;275(5679):416–420. doi: 10.1038/275416a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Morris N. R. Assembly of SV40 chromatin in a cell-free system from Xenopus eggs. Cell. 1977 Feb;10(2):237–243. doi: 10.1016/0092-8674(77)90217-3. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Toomey N. L. Differential effects of dimethylsulfoxide on the activities of human DNA polymerases alpha and delta. Nucleic Acids Res. 1986 Feb 25;14(4):1719–1726. doi: 10.1093/nar/14.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Gray H. B., Jr, Robberson D. L. A sensitive endonuclease probe for lesions in deoxyribonucleic acid helix structure produced by carcinogenic or mutagenic agents. J Biol Chem. 1977 Dec 10;252(23):8740–8746. [PubMed] [Google Scholar]
- Legerski R. J., Penkala J. E., Peterson C. A., Wright D. A. Repair of UV-induced lesions in Xenopus laevis oocytes. Mol Cell Biol. 1987 Dec;7(12):4317–4323. doi: 10.1128/mcb.7.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mertz J. E. Linear DNA does not form chromatin containing regularly spaced nucleosomes. Mol Cell Biol. 1982 Dec;2(12):1608–1618. doi: 10.1128/mcb.2.12.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullinger A. M., Johnson R. T. Scanning electron microscope analysis of structural changes and aberrations in human chromosomes associated with the inhibition and reversal of inhibition of ultraviolet light induced DNA repair. Chromosoma. 1987;96(1):39–44. doi: 10.1007/BF00285881. [DOI] [PubMed] [Google Scholar]
- Nishida C., Reinhard P., Linn S. DNA repair synthesis in human fibroblasts requires DNA polymerase delta. J Biol Chem. 1988 Jan 5;263(1):501–510. [PubMed] [Google Scholar]
- Noll M., Zimmer S., Engel A., Dubochet J. Self-assembly of single and closely spaced nucleosome core particles. Nucleic Acids Res. 1980 Jan 11;8(1):21–42. doi: 10.1093/nar/8.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Razvi F., Gargiulo G., Worcel A. A simple procedure for parallel sequence analysis of both strands of 5'-labeled DNA. Gene. 1983 Aug;23(2):175–183. doi: 10.1016/0378-1119(83)90049-5. [DOI] [PubMed] [Google Scholar]
- Ruberti I., Worcel A. Mechanism of chromatin assembly in Xenopus oocytes. J Mol Biol. 1986 Jun 5;189(3):457–476. doi: 10.1016/0022-2836(86)90317-7. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Jorcano J. L., Eder G., Lurz R. In vitro core particle and nucleosome assembly at physiological ionic strength. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3284–3288. doi: 10.1073/pnas.76.7.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Chromatin assembly in Xenopus oocytes: in vivo studies. Cell. 1984 May;37(1):21–32. doi: 10.1016/0092-8674(84)90297-6. [DOI] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Structure of the two distinct types of minichromosomes that are assembled on DNA injected in Xenopus oocytes. Cell. 1985 Apr;40(4):923–932. doi: 10.1016/0092-8674(85)90352-6. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Tremethick D., Worcel A. Characterization of the repressed 5S DNA minichromosomes assembled in vitro with a high-speed supernatant of Xenopus laevis oocytes. Mol Cell Biol. 1988 Oct;8(10):4257–4269. doi: 10.1128/mcb.8.10.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989 Jul 14;58(1):15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- So A. G., Downey K. M. Mammalian DNA polymerases alpha and delta: current status in DNA replication. Biochemistry. 1988 Jun 28;27(13):4591–4595. doi: 10.1021/bi00413a001. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Streeck R. E., Zachau H. G. Closely spaced nucleosome cores in reconstituted histone.DNA complexes and histone-H1-depleted chromatin. Eur J Biochem. 1978 Feb;83(2):615–628. doi: 10.1111/j.1432-1033.1978.tb12131.x. [DOI] [PubMed] [Google Scholar]
- Stillman B. Chromatin assembly during SV40 DNA replication in vitro. Cell. 1986 May 23;45(4):555–565. doi: 10.1016/0092-8674(86)90287-4. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Watkins J. F., Smerdon M. J. Nucleosome rearrangement in vitro. 2. Formation of nucleosomes in newly repaired regions of DNA. Biochemistry. 1985 Dec 3;24(25):7288–7295. doi: 10.1021/bi00346a040. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Dean F., Weissbach L., Hurwitz J. In vitro replication of duplex circular DNA containing the simian virus 40 DNA origin site. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5710–5714. doi: 10.1073/pnas.82.17.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Han S., Wong M. L. Assembly of newly replicated chromatin. Cell. 1978 Nov;15(3):969–977. doi: 10.1016/0092-8674(78)90280-5. [DOI] [PubMed] [Google Scholar]
- Yasui W., Ryoji M. Characterization of early DNA synthesis in Xenopus eggs after injection of circular plasmid DNA. Nucleic Acids Res. 1989 May 25;17(10):3709–3723. doi: 10.1093/nar/17.10.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler M. K., Marini N. J., Stowers D. J., Benbow R. M. Stockpiling of DNA polymerases during oogenesis and embryogenesis in the frog, Xenopus laevis. J Biol Chem. 1985 Jan 25;260(2):974–981. [PubMed] [Google Scholar]
- Zuber M., Tan E. M., Ryoji M. Involvement of proliferating cell nuclear antigen (cyclin) in DNA replication in living cells. Mol Cell Biol. 1989 Jan;9(1):57–66. doi: 10.1128/mcb.9.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]