Abstract
Podocytes are highly differentiated cells that play an important role in maintaining glomerular filtration barrier integrity; a function regulated by small GTPase proteins of the Rho family. To investigate the role of Rho A in podocyte biology, we created transgenic mice expressing doxycycline-inducible constitutively active (V14Rho) or dominant-negative Rho A (N19Rho) in podocytes. Specific induction of either Rho A construct in podocytes caused albuminuria and foot process effacement along with disruption of the actin cytoskeleton as evidenced by decreased expression of the actin associated protein synaptopodin. The mechanisms of these adverse effects, however, appeared to be different. Active V14Rho enhanced actin polymerization, caused a reduction in nephrin mRNA and protein levels, promoted podocyte apoptosis, and decreased endogenous Rho A levels. In contrast, the dominant-negative N19Rho caused a loss of podocyte stress fibers, did not alter the expression of either nephrin or Rho A, and did not cause podocyte apoptosis. Thus, our findings suggest that Rho A plays an important role in maintaining the integrity of the glomerular filtration barrier under basal conditions, but enhancement of Rho A activity above basal levels promotes podocyte injury.
Introduction
Podocytes are highly differentiated cells that play an important role in maintaining the integrity of the glomerular filtration barrier (1–3). Their function is regulated by small GTPases belonging to the Rho GTPase family (4–6). These small GTPases act as molecular switches controlling activation of multiple downstream effector molecules (7–10). Among their pleiotropic actions, Rho-dependent signaling cascades modulate cellular morphology and actin polymerization, adhesion, cell migration, proliferation and apoptosis as well as participate in contractile responses (7–10). While these actions likely serve homeostatic functions under normal physiologic conditions, Rho-dependent signaling cascades are highly activated during inflammatory states, which, in turn, may have pathological consequences (11–19). In this regard, a large body of data implicates Rho GTPases in the pathogenesis of disease processes in the kidney including glomerular diseases (11–19). Moreover, a growing literature suggests that Rho A may also play an important homeostatic function by promoting a podocyte phenotype that stabilizes the glomerular architecture (4–6). In this scenario, some basal level of RhoA activity would be beneficial. In contrast, high levels of Rho A activity induced by inflammatory processes may cause podocyte injury (11–19). Indeed, recent studies provide strong evidence that enhanced Rho A activity in podocytes has adverse effects on glomerular filtration barrier function (20). The mechanisms of altered glomerular permselectivity after Rho A activation, however, have not been extensively characterized. Moreover, there is little information on role of basal Rho A activity in regulating glomerular filtration barrier integrity.
In the present studies, we investigated the effect of modulating Rho A activity in glomerular podocytes by creating transgenic (TG) mice that expressed either a constitutively active Rho A (V14Rho) or a dominant-negative Rho A (N19Rho) specifically in podocytes using a doxycycline inducible system. Using these TG mice, we found that either activation or inhibition of Rho A in podocytes in vivo had adverse effects on podocyte function.
Results
Creation of V14Rho and N19Rho TG mice
For the experiments, we utilized the Tet-On system (21), which requires two TG mice for podocyte specific expression. The first TG animal expresses the reverse tetracycline-controlled transcriptional activator (rtTA) under the control of the human podocin (NPHS2) promoter (22). The second TG mouse expresses either V14Rho or N19Rho under the control of tet operator sequence (tetO) and a minimal CMV promoter (PminCMV) (21). By breeding the two TG mice, animals are obtained that express both transgenes. In these “double” TG mice, treatment with doxycycline induces transgene expression.
For the experiments, two independent TG lines were established for each transgene. Figure 1A and Figure 1D show expression of the HA-tagged transgenes after 1 week of doxycycline treatment by immunoblotting for the HA epitope using glomerular preparations from “double” TG mice (rtTA and either the V14Rho or N19Rho transgenes) as well as “single” TG mice (rtTA, v14Rho or N19Rho transgenes) and non-TG mice. Expression of either the V14Rho or N19Rho protein was detectable by immunoblotting in “double” TG mice but not in “single” TG or non-TG mice (Figure 1A and Figure 1D). In the absence of doxycycline, the Rho proteins were not detected in either non-TG, “single” TG or “double” TG mice (not shown).
Figure 1.
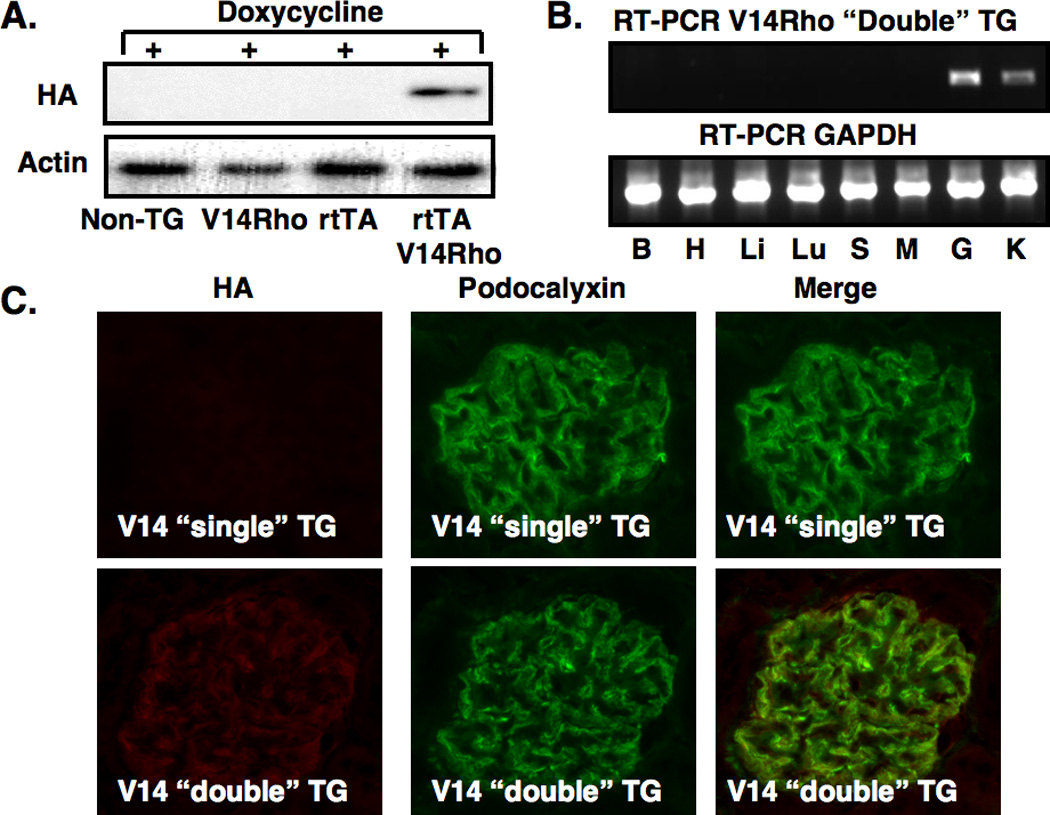
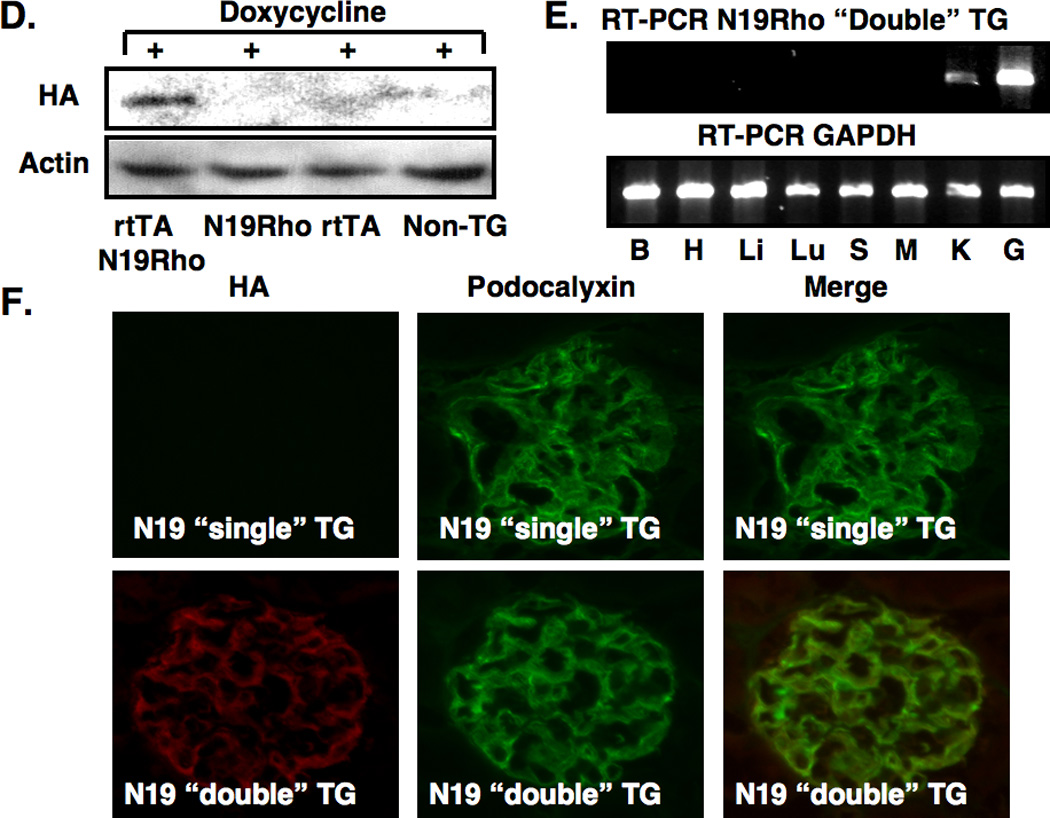
Creation of TG mice and induction of the transgene. In panels A and D, induction of the N19Rho and V14Rho transgenes, respectively, was investigated by immunoblotting for the HA epitope in non-TG mice, “single” TG mice and “double” TG mice after doxycycline treatment. Both transgenes were induced by doxycycline in “double” TG mice but not in control mice. Transgene expression was not detected by immunoblotting in the absence of doxycycline (data not shown). In panels B and E, tissue specific expression of the transgenes was investigated by RT-PCR in “double” TG mice after treatment with doxycycline using transgene specific primers. A RT-PCR product was detected in both kidney cortex and isolated glomerular preparations from N19Rho “double” TG mice and V14Rho “double” TG mice as indicated. No RT-PCR products were detected in other tissues from the “double” TG mice. The GAPDH control confirmed that the RT reaction was successful in the tissues examined. No RT-PCR products were obtained in any tissues from “double” TG mice in either the absence of doxycycline treatment or in the absence of an RT reaction (not shown). Similarly, no RT-PCR products were obtained in any tissues from doxycycline treated “single” TG or non-TG mice (not shown). B, H, Li, Lu S, M, K, G are brain, heart, liver, lung, spleen, muscle (skeletal), kidney cortex and glomeruli, respectively. In panels C and F, tissue sections were stained for expression of the HA tagged transgenes and the podocyte marker podocalyxin as indicated. As shown in the upper panels, podocalyxin, but not the HA epitope, was detected in “single” TG mice treated with doxycycline. In contrast, the lower panel shows that both the HA epitope and podocalyxin were detected in “double” TG mice treated with doxycycline. When the two images are merged the HA epitope and podocalyxin had a similar cellular distribution in the “double” TG mice.
Figures 1B and 1E show tissue specific expression of the V14Rho and N19Rho by RT-PCR using mRNA prepared from mice treated with doxycycline. As shown in the top panel of Figures 1B and 1E, a RT-PCR product of the appropriate size was detected in kidney cortex and glomerular preparations from “double” TG mice. No RT-PCR products were detected in other tissues from the “double” TG mice. The GAPDH RT-PCR reaction confirmed that the reverse transcriptase reaction was successful in the tissues examined (lower panel). In data not shown, expression of the V14Rho or N19Rho transgenes were: 1. Not detected by RT-PCR in any of the tissues from other doxycycline treated ”single” TG mice and non-TG controls, and 2. Not detected by RT-PCR in “double” TG mice in either the absence of doxycycline treatment or in the absence of a RT reaction.
We next determined cell specific expression of the transgene. For these studies, tissue sections were stained for expression of the HA tagged V14Rho or N19Rho transgenes (rhodamine) and the podocyte marker podocalyxin (fluorescein). As shown in Figure 1C and 1F, only podocalyxin was detected in “single” TG mice treated with doxycycline. In contrast, the lower panels show that both the HA epitope and podocalyxin were detected in “double” TG mice treated with doxycycline. Merging the two images suggested that the HA epitope and podocalyxin shared a similar cellular distribution. In data not shown, the HA epitope was not detected in rtTA “single” TG mice and non-TG controls in the presence of doxycycline or in “double” TG mice in the absence of doxycycline.
Effect of transgenes on albuminuria
For these studies, “double” TG mice and controls (“single” TG and non-TG mice) were treated with doxycycline or vehicle for 2 weeks and prior to measuring albuminuria. Experimental results were similar using the progeny from the independent lines and these data were combined for the analyses. Induction of either the V14Rho transgene (Figure 2A) or the N19Rho transgene (Figure 2B) caused a significant increase in albuminuria in “double” TG mice compared to controls. As shown in Table 1, similar results were seen when albuminuria data was expressed as micrograms albumin per milligram creatinine. Moreover, the increase in albuminuria after transgene induction was reversible 2 to 4 weeks after discontinuing doxycycline treatment (Table 2).
Figure 2.
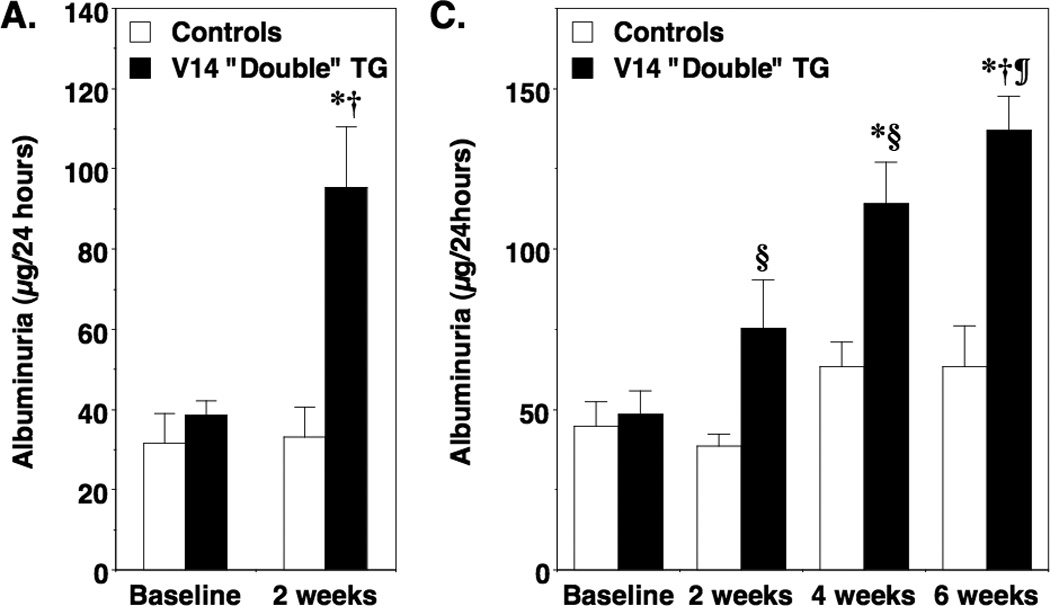
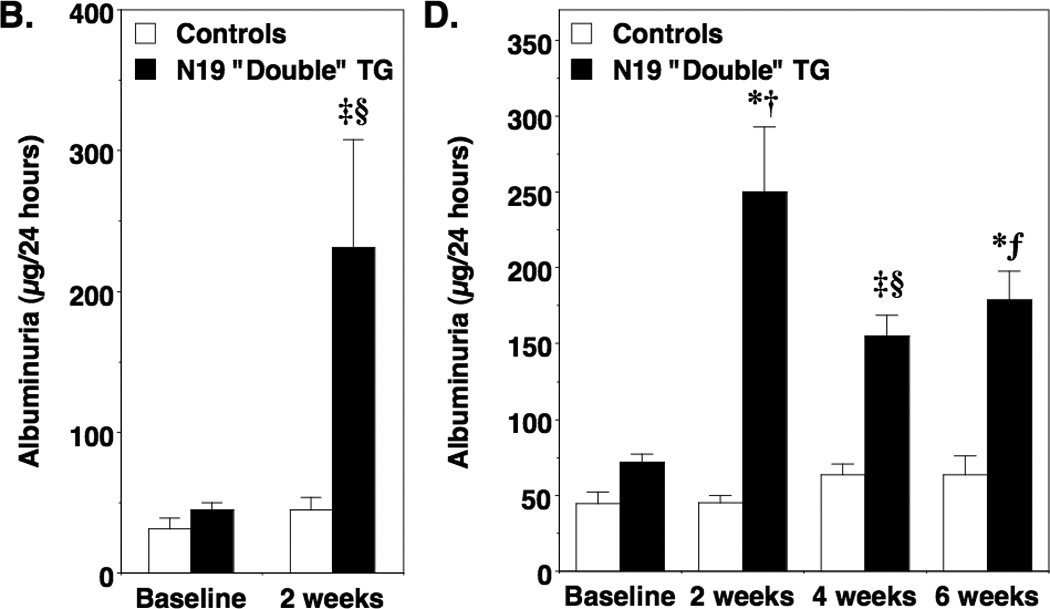
Effect of transgene induction on albuminuria. In panel A and B, albuminuria was measured after 2 weeks of treatment in both N19Rho “double” TG mice and V14Rho “double” TG mice as well as controls (“single” TG and non-TG mice). Treatment with doxycycline significantly increased albuminuria in V14Rho (panel A) and N19Rho (panel B) “double” TG mice compared to “double” TG mice at baseline or compared to controls at either baseline or after treatment with doxycycline. In panels C and D, mice were treated for 6 weeks with doxycycline and albuminuria was measured at the 2, 4 and 6-week time points. In V14Rho “double” TG mice (panel C), albuminuria was increased during doxycycline treatment compared to controls and tended to increase over time. At the 6-week time point, albuminuria was significantly increased compared to the 2-week time point in V14Rho “double” TG animals. Albuminuria was also increased in N19Rho “double” TG mice compared to controls after doxycycline treatment at all time points examined, but tended to remain relatively stable over the treatment period. Eight to 16 mice were studied per group. ‡P<0.05 vs “double” TG at baseline, *P<0.01 vs “double” TG at baseline, §P<0.05 vs controls after treatment with doxycycline, fP<0.01 vs controls after treatment with doxycycline, †P<0.001 vs controls after treatment with doxycycline, ¶P<0.01 vs “double” TG at 2 weeks
Table 1.
Albuminuria (micrograms albumin/milligram creatinine)
| V14Rho studies |
N19Rho studies |
|||
|---|---|---|---|---|
| Controls | “Double” TG | Controls | “Double TG | |
| Baseline | 44 ± 19 | 25 ± 13 | 37 ± 11 | 25 ± 4 |
| Doxycycline | 36 ± 8 | 108 ± 36*† | 36 ± 5 | 178 ± 54**† |
P<0.025 or
P<0.05 vs doxycycline treated controls,
P<0.05 vs “double” TG at baseline
Table 2.
Albuminuria (micrograms albumin per 24 hours)
| V14Rho studies |
N19Rho studies |
|||
|---|---|---|---|---|
| Controls | “Double” TG | Controls | “Double TG | |
| Baseline | 48 ± 6.6 | 52 ± 7.3 | 31 ± 6.2 | 58 ± 9.1 |
| 2 wks of doxycycline | 40 ± 5.2 | 112 ± 26*† | 27 ± 10 | 196 ± 61* |
| 2 wks after doxycycline | 28 ± 19 | 24 ± 8.6‡ | 27 ± 10 | 49 ± 18 |
| 4 wks after doxycycline | 35 ± 19 | 31 ± 17‡ | 32 ± 19 | 11 ± 7.5‡ |
P<0.001 vs doxycycline treated controls,
P<0.01 vs baseline V14 “double” TG mice ,
P<0.05 vs “double” TG at after 2 weeks of doxycycline treatment,
We next determined the effects of more prolonged transgene induction on albuminuria. For these studies, mice were treated for 6 weeks with doxycycline and albuminuria was examined at baseline and 2, 4 and 6 weeks after doxycycline treatment. As shown in Figure 2C, induction of V14Rho caused a progressive increase in albuminuria over the 6 week treatment period compared to control animals. By the 6-week time point, albuminuria was significantly increased compared to the 2-week time point in V14Rho “double” TG mice. Induction of the N19Rho transgene also caused an increase albuminuria compared to control animals (Figure 2D). The increase in albuminuria, however, was not significantly different during doxycycline treatment in N19Rho “double” TG mice at the 3 time points examined.
Glomerular histomorphology and glomerular ultrastructure
Light microscopic examination of kidney sections revealed minimal abnormalities (Figure 3A, 3B and 3C). Transmission electron microscopy (TEM) revealed a few areas of foot process (FP) flattening in the control mice (Figure 3D and 3G). In contrast, large areas of FP effacement involving the majority of glomeruli available for examination were detected in both V14Rho (Figure 3E and 3H) and N19Rho (Figure 3F and 3I) “double” TG mice. To quantitate the severity of FP effacement, we assessed the number of patent slit diaphragms per µm of glomerular basement membrane (GBM). There was a significant decrease in the number of patent slit diaphragms per µm GBM in both V14Rho “double” TG mice (2.25 ± 0.21 [controls] vs 1.24 ± 0.23 [V14Rho “double” TG]; P<0.01) and N19Rho “double” TG mice (2.19 ± 0.20 [controls] vs 1.22 ± 0.25 [N19Rho “double” TG]; P<0.01). The light microscopic and ultrastructural findings were similar in “double” TG mice and controls after either 2 or 6 weeks of doxycycline treatment.
Figure 3.
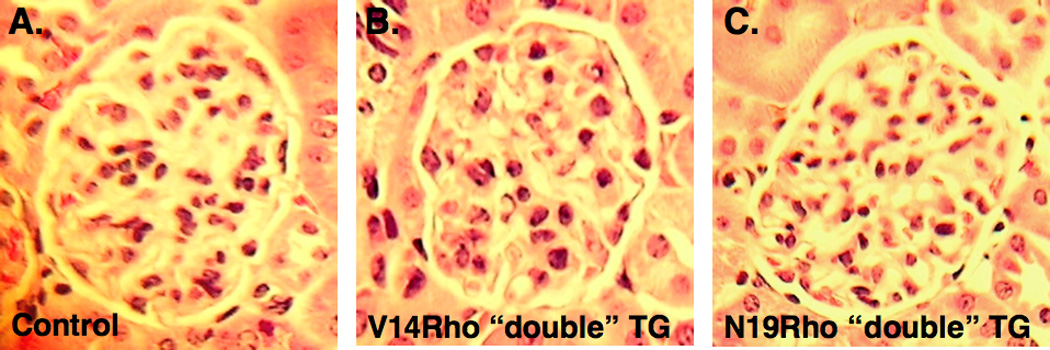
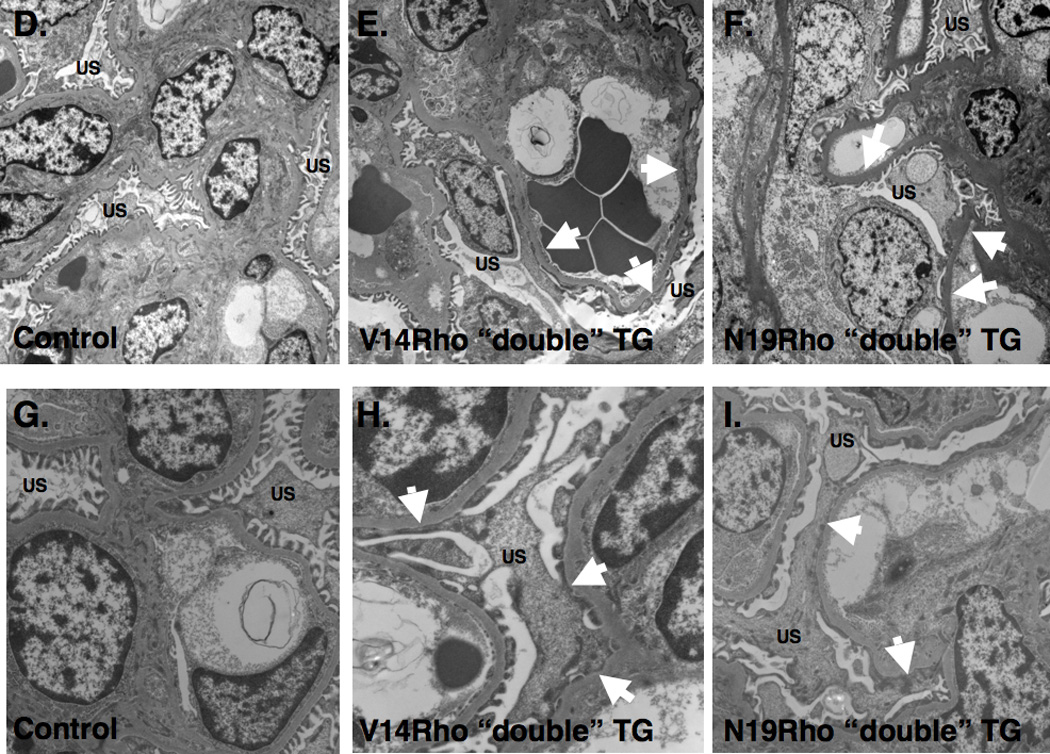
Renal histology. Panels A, B and C show glomerular histology in controls, V14Rho “double” TG mice and N19Rho “double” TG mice, respectively, 2 weeks after treatment with doxycycline. Glomerular histology was similar in all groups at the light microscopic level. Panels D–I shows glomerular ultrastructure in controls, V14Rho “double” TG mice and N19Rho “double” TG mice 2 weeks after treatment with doxycycline. Low power views are shown in panels D–F and higher magnification images are shown in panels G–I. In both N19Rho and V14Rho “double” TG mice large areas of FP effacement (arrows) were detected involving approximately 40% of the GBM length. In contrast, foot processes were well preserved in control mice treated with doxycycline although a few areas of foot process flattening were observed. US is urinary space.
Effect of the transgenes on actin polymerization
Rho GTPases are important regulators of actin polymerization (7), and maintenance of the actin cytoskeleton plays a critical role in sustaining glomerular filtration barrier function (4–6). To investigate the effect of Rho A on actin polymerization, we introduced V14Rho or N19Rho into cultured podocytes using protein transduction (23) by tagging the proteins with the TAT human immunodeficiency virus (HIV) protein sequence [V14Rho(+) or N19Rho(+)]. Cell impermeable proteins lacking the TAT sequence were used as controls [V14Rho(−) or N19Rho(−)]. For the experiments, cultured podocytes were treated with V14Rho or N19Rho TAT proteins and then actin polymerization was monitored by phalloidin staining (rhodamine). As shown in Figure 4A, a few stress fibers were seen in podocytes treated with a control Rho A protein lacking the TAT sequence. In podocytes treated with V14Rho(+), the number of stress fibers was increased (Figure 4B); whereas, in cells treated with N19Rho(+) the number of stress fibers were reduced (Figure 4C). Quantitation of actin polymerization (F actin) is shown in Figure 4D. V14Rho(+) induced a significant increase in actin polymerization compared to control podocytes. In contrast, actin polymerization was significantly decreased by treatment with N19Rho(+) compared to either control podocytes or podocytes treated with V14Rho(+). Podocyte morphology was similar in untreated podocytes and podocytes treated with control TAT proteins (not shown).
Figure 4.
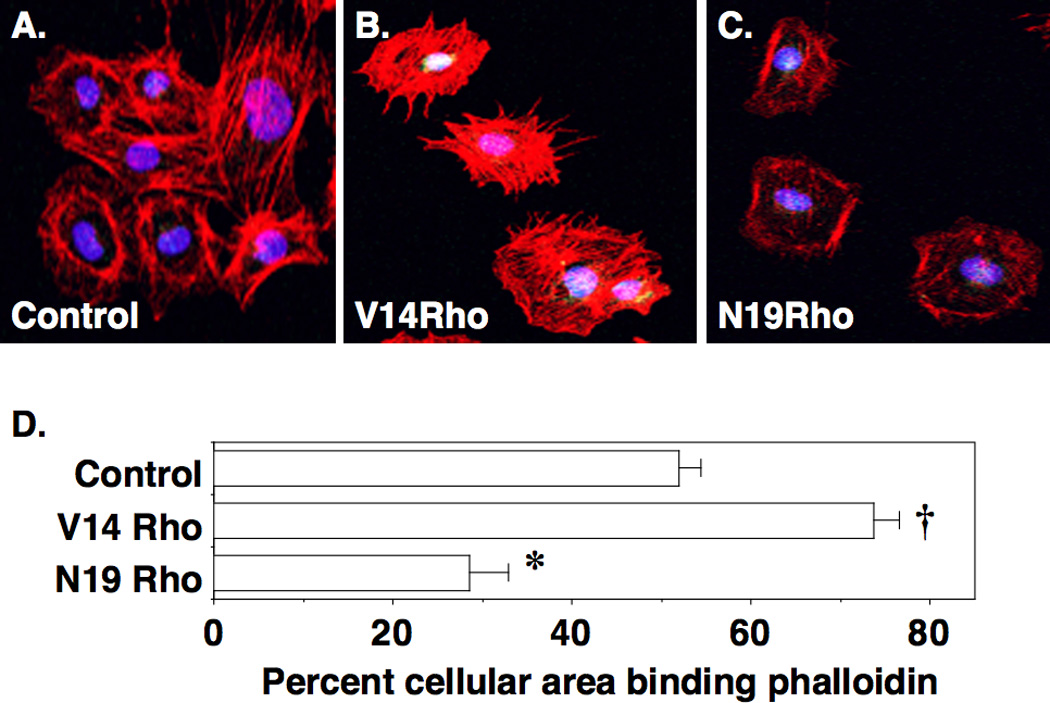
Effect of V14Rho and N19Rho on actin polymerization in podocytes. A few stress fibers are seen in podocytes treated with control proteins. In contrast, V14Rho(+) treatment enhanced the number of stress fibers; whereas, treatment with N19Rho(+) caused a decrease in stress fiber formation. Quantitation of the percent cellular area binding phalloidin is shown in panel D. There was a significant increase in the percent cellular area binding phalloidin in V14Rho(+) treated podocytes compared to cells treated with either control proteins or N19Rho(+). Treatment with N19Rho(+) significantly decreased phalloidin binding compared to cells treated with either control proteins or V14Rho(+). *P< 0.01 vs cultured podocytes treated with either control TAT proteins or N19Rho(+), †P<0.01 vs cultured podocytes treated with either control TAT proteins or V14Rho(+)
Effect of the transgenes on synaptopodin expression
Synaptopodin is an actin-associated protein that is expressed exclusively by podocytes in the kidney (24). To determine if the effects of the transgenes on actin polymerization (Figure 4) affected synaptopodin expression in vivo, we first measured glomerular synaptopodin levels by immunoblotting in mice treated with doxycycline for 6 weeks. As shown in Figure 5A, synaptopodin levels were decreased by induction of either V14Rho or N19Rho compared to controls. The podocyte protein podocalyxin was not affected by induction of either transgene. Quantitation of synaptopodin protein and mRNA levels is shown in Figure 5B and 5C, respectively. Synaptopodin protein levels were decreased at both “double” TG lines compared to controls. A similar pattern was seen for synaptopodin mRNA levels at the 6-week time point. Despite the marked reduction in synaptopodin protein levels by immunoblotting, synapotopodin was detectable by IHC in both control mice as well as “double” TG mice (Figure 5D), although the intensity of the immunofluorescence tended to by decreased in “double” TG animals. In V14Rho “double” TG mice, synaptopodin staining also tended to be more granular (Figure 5D, bottom panel) compared to the linear staining observed in N19Rho “double” TG mice and controls (Figure 5D top and middle panels). We also investigated synaptopodin expression at the 2-week time point. As shown in Figure S1 in the supplementary section, synaptopodin mRNA and protein levels were both decreased in N19Rho “double TG mice after 2 weeks of doxycycline treatment. In contrast, synaptopodin mRNA and protein levels were better preserved at this time point in V14Rho “double” TG mice suggesting that the tempo of synaptopodin loss was delayed in these animals.
Figure 5.
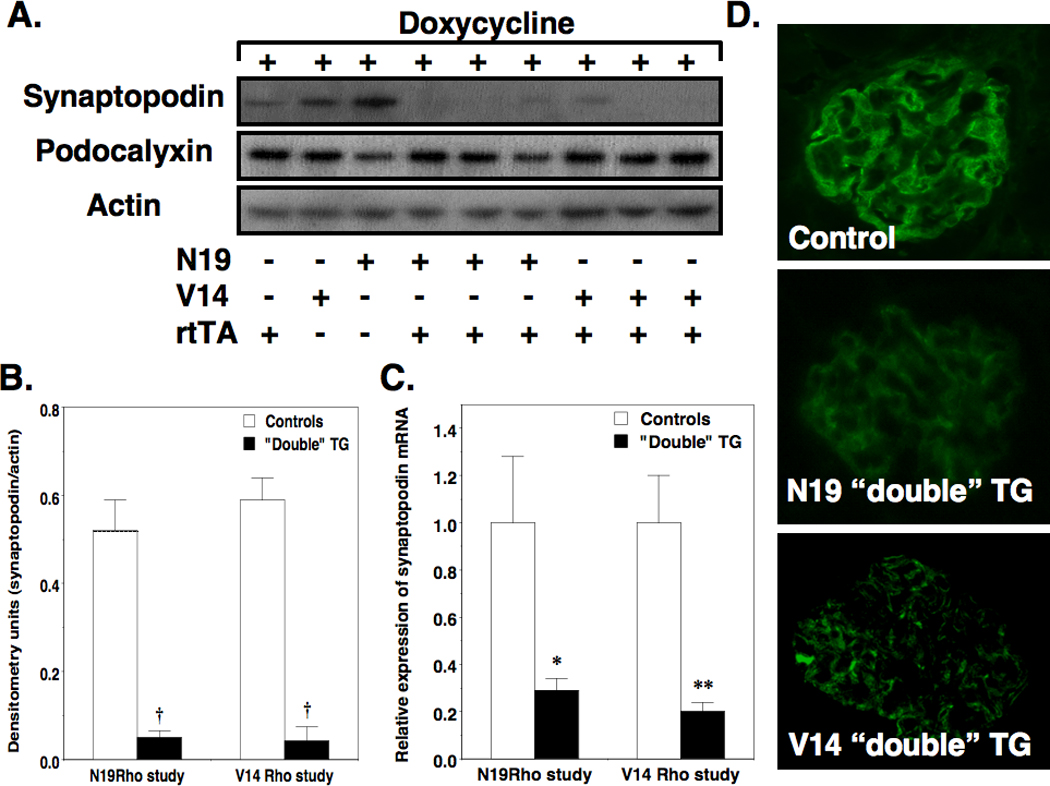
Effect of the Rho transgenes on synaptopodin and podocalyxin expression. Panel A shows representative immunoblots of synaptopodin and podocalyxin protein levels in enriched glomerular preparations after 6 weeks of doxycycline treatment. Synaptopodin levels were difficult to detect in both N19Rho and V14 Rho “double” TG mice compared to controls. In contrast, podocalyxin levels were not changed in any of the groups. Quantitation of the immuoblots is shown in panel B. Induction of the Rho A transgenes in “double” TG mice significantly reduced glomerular synaptopodin protein levels compared to controls. As shown in panel C, a similar reduction in synaptopodin mRNA levels was seen in both N19Rho and V14Rho “double” TG mice compared to controls. In panel D, synaptopodin was detected by immunohistochemistry in all groups, although the immunofluorescence tended to be reduced in the “double” TG mice. Moreover, the staining appeared more granular in the V14 “double” TG animals. Four to 6 samples were studied per group. *P<0.05 or **P<0.01 vs controls, †P<0.001 vs controls
Effect of the transgenes on expression of Rho A
The Rho GTPase family includes members belonging to the Rho, Rac and cdc42 GTPases (25). In podocytes, the Rho A protein is stabilized by interacting with the podocyte protein synaptopodin (5). We, therefore, determined if decreased synaptopodin levels in “double” TG mice affected expression of Rho GTPase family members in enriched glomerular preparations after 6 weeks of doxycycline treatment. As shown in Figure 6A and 6B, Rho A levels were unaffected by transgene induction in N19Rho “double” TG animals. In contrast, Rho A levels were significantly decreased in V14 “double” TG mice compared to either controls or N19Rho “double” TG animals. As shown in the inset to Figure 6B, the changes Rho A protein levels represented endogenous Rho A expression because longer exposure of the immunoblots detected expression of the slightly larger Rho A transgenes. Rac1 levels were not changed by transgene induction (Figure 6A) and cdc42 was difficult to detect in the glomerular preparations (not shown).
Figure 6.
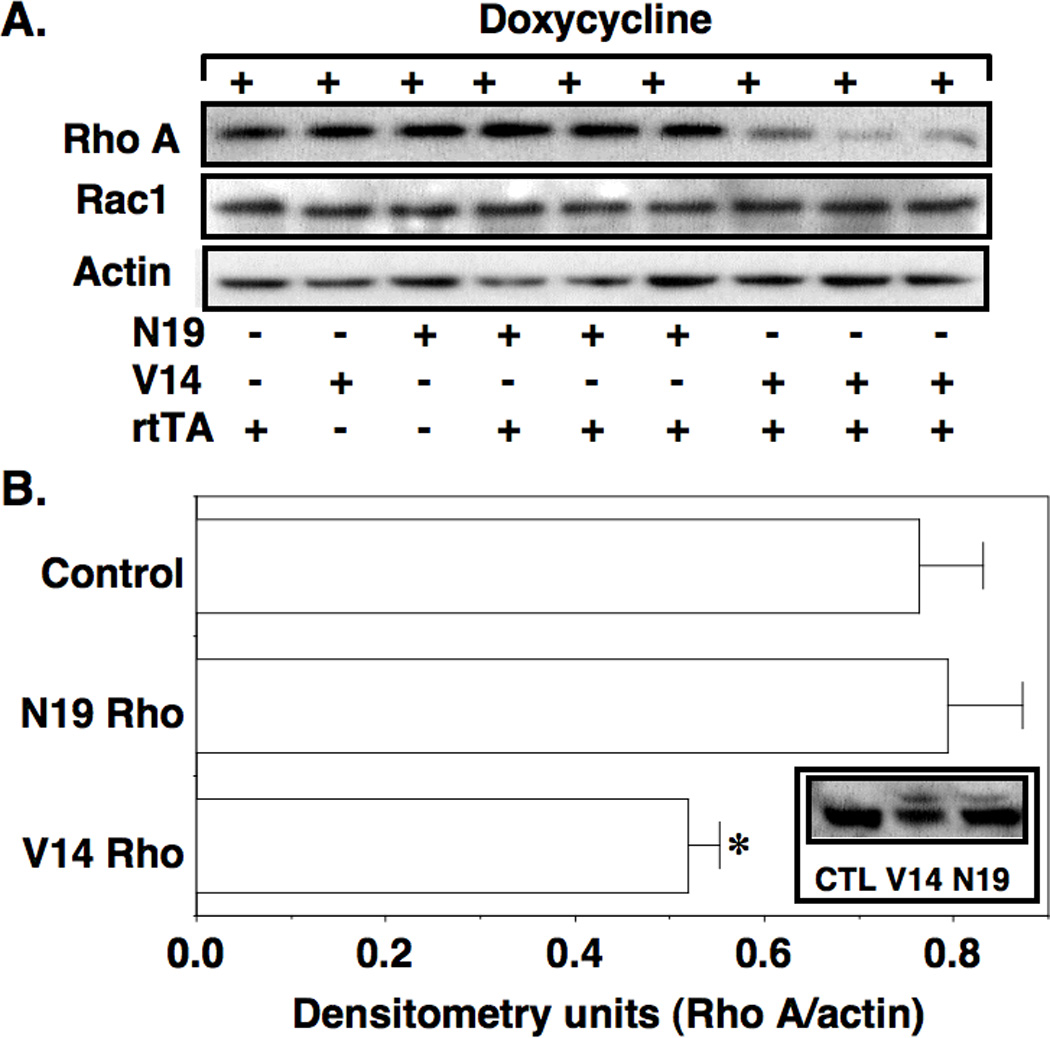
Effect of the Rho transgenes on expression of Rho GTPase family members. In panel A, Rho A levels were decreased in V14 Rho “double” TG mice compared to controls. In contrast, Rac1 expression was similar in all groups examined. Quantitation of the Rho A immuoblots is shown in panel B. Induction of the V14Rho transgene in “double” TG mice significantly reduced glomerular Rho A protein levels compared to controls as well as N19Rho “double” TG mice. The changes Rho A protein levels represented endogenous Rho A expression because longer exposure of the immunoblots detected expression of the slightly larger Rho A transgenes (inset). Nine samples were studied per group, *P<0.05 vs V14Rho controls or N19Rho “double” TG mice
Effect of Rho A on podocyte apoptosis
Rho A plays an important role in regulating cell death, in part, by inhibiting the prosurvival and anti-apoptotic phophatidylinositol-3-kinase (PI3K)/Akt pathway (25–27). This negative regulatory effect is mediated by the downstream Rho A effectors Rho kinases or ROKs (25), which phosphorylate and activate PTEN (Phosphatase and tensin homolog deleted on chromosome ten) (28). PTEN, in turn, dephosphorylates and inactivates Akt (25). To investigate the effect of Rho A on podocyte apoptosis in vitro, we treated cultured podocytes with V14Rho or N19Rho TAT proteins and then measured apoptosis as described in the Methods Section. As shown in Figure 7A, V14Rho(+) enhanced podocyte apoptosis compared to V14Rho(−) and this apoptotic effect was blocked by the Y27632. In contrast, N19Rho(+) had no significant effect on apoptosis of cultured podocytes compared to cells treated with N19Rho(−) (7.4±3.2 [N19Rho(+)] vs 7.2±1.7 [N19Rho(−)] % apoptosis above baseline; P=NS). To assess PTEN phosphorylation levels, we immunoprecipitated PTEN and then immunoblotted for both total and phospho-threonine in cultured podocytes (Figure 7B). Densitometric quantitation of the immunoblots is shown in Figure 7C. Phospho-PTEN levels were significantly increased in cultured podocytes 10 and 30 minutes after treatment with V14Rho(+) compared to cells treated with V14Rho(−).
Figure 7.
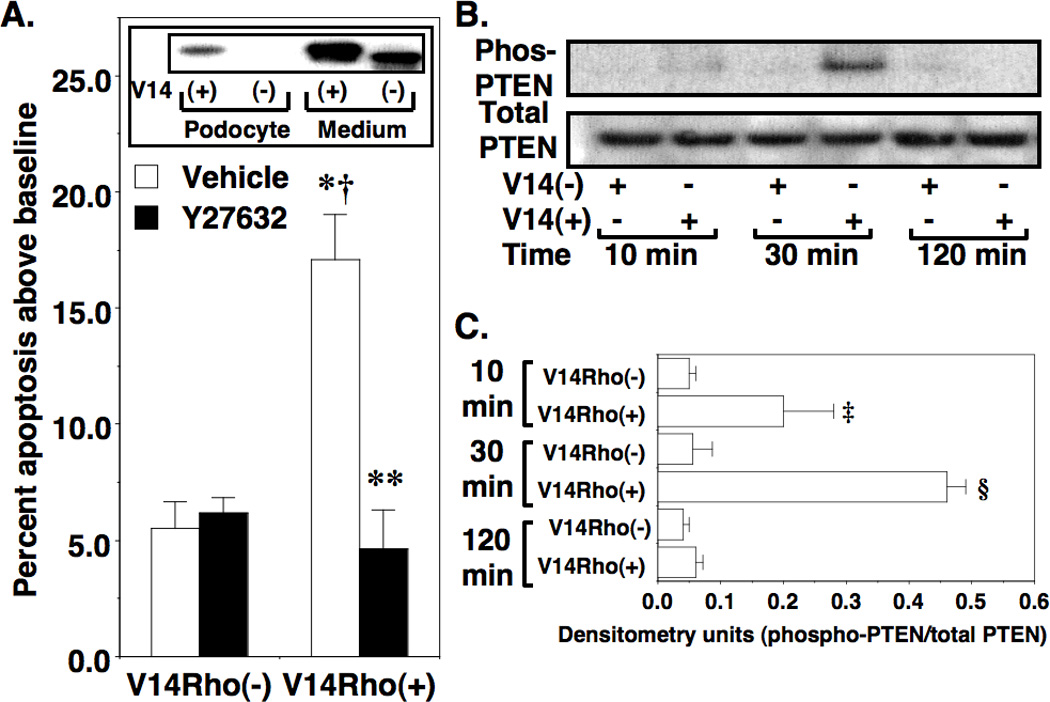
Effect of V14Rho(+) on podocyte apoptosis. In panel A, treatment with V14Rho(+) significantly increased podocyte apoptosis compared to cells treated with V14Rho(−), and Y27632 significantly reduced podocyte apoptosis induced by V14Rho(+). Immunoblotting of the HA-tagged V14Rho proteins in either culture medium or podocyte lysates is shown in the inset. The HA-tagged V14Rho(+) was effectively transduced into cultured podocytes. There is a slight difference in molecular size of V14Rho(+) and V14Rho(−) due to the presence or absence of the TAT sequence. In panel B, total PTEN and phospho-PTEN levels were assessed in immortalized podocytes after treatment with V14Rho(+) or V14Rho(−). Densitometric quantitation of the immunoblots is shown in Figure C. Phospho-PTEN levels were significantly increased in cells treated with V14Rho(+) at the 10-minute and 30-minute time points. Four to 7 samples were studied per group for the apoptosis experiments. Four to 5 samples were studied per group for the immunoblotting studies. *P<0.001 vs cells treated with V14Rho(−) and vehicle, †P<0.01 vs cells treated with V14Rho(−) and Y27632, **P<0.001 vs cells treated with V14Rho(+) and vehicle ‡P<0.05 or §P<0.01 vs vs cells treated with V14Rho(−)
To determine if Rho A activation caused podocyte apoptosis in vivo, we assessed both podocyte number and podocyte apoptosis after 6 weeks of doxycycline treatment. We were, however, unable to detect podocyte apoptosis and the number of podocytes per glomerular profile was similar in controls and either N19Rho” double” TG mice (8.1 ± 0.4 [controls] vs 8.5 ± 0.5 [N119Rho; P = NS) or V14Rho” double” TG mice (8.4 ± 0.2 [controls] vs 8.1 ± 0.2 [N119Rho; P = NS).
Effect of transgene induction on glomerular nephrin expression
The podocyte protein nephrin plays a critical role in maintaining the integrity of the glomerular filtration barrier (29–31), and its expression is decreased in some (32–40), although not all (32), proteinuric glomerular diseases. In addition to its structural role, nephrin is also a signaling molecule (27, 41, 42). In this regard, nephrin may inhibit podocyte apoptosis by activating the prosurvival PI3K/Akt pathway (25, 41). In this scenario, a decrease in glomerular nephrin expression might promote apoptosis of glomerular podocytes. We, therefore, measured nephrin mRNA levels using quantitative RT-PCR and glomerular mRNA prepared from mice treated for 6 weeks with doxycycline. As shown in Figure 8A, induction of the V14Rho transgene caused a reduction in nephrin mRNA levels. In contrast, nephrin mRNA levels were similar in N19 “double” TG mice compared to controls. Panels B and C of Figure 8 show representative immunoblots of nephrin protein levels in glomerular preparations from N19Rho and V14Rho “double” TG mice and controls (“single” TG and non-TG mice) treated for 6 weeks with doxycycline. Quantitation of the immunoblots is shown in Figure 8D. There was a significant decrease in nephrin protein levels in V14Rho “double” TG compared to controls. In contrast, nephrin protein levels were similar in N19Rho “double” TG compared to control animals. Similar results were obtained at the 2-week time point (Supplementary Figure 2). With longer exposure nephrin protein appears as a doublet.
Figure 8.
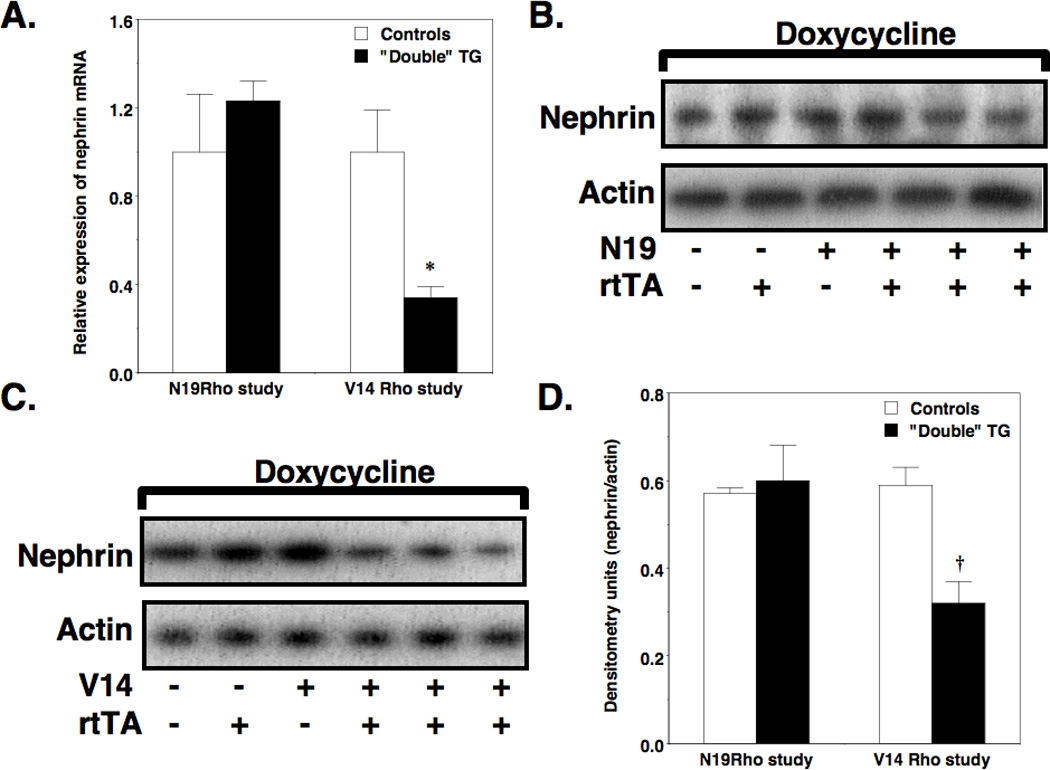
Effect of the V14Rho and N19Rho transgene glomerular nephrin expression. In panel A, induction of the V14Rho transgene in “double” TG mice significantly reduced glomerular nephrin mRNA levels compared to controls. Panels B and C show representative immunoblots of nephrin protein levels in enriched glomerular preparations from N19Rho and V14Rho “double” TG mice and controls. Quantitation of the immunoblots by densitometry is shown in panel D. Induction of the V14Rho transgene in “double” TG mice significantly reduced glomerular nephrin protein levels compared to controls. Four to 6 samples were studied per group. *P<0.01 vs V14Rho controls, †P<0.05 vs V14Rho controls
Discussion
In the present studies, we found that both Rho A activation as well as Rho A inhibition specifically in glomerular podocytes caused albuminuria and FP effacement. These data, taken together with recently published studies (20), suggest that Rho activity in podocytes must be tightly regulated to maintain podocyte function. Given that excessive Rho GTPAse activity likely contributes to glomerular damage in disease states (11–19), the observation that Rho A inhibition also promotes podocyte injury has implications for treatment strategies in glomerular disease processes. In this regard, chemically distinct ROK inhibitors attenuate renal damage in a variety of experimental models (11–19). Moreover, ROK inhibition reduces glomerular injury in animal models without adversely affecting glomerular filtration barrier function (11–15, 18, 19). In contrast, Rho A inhibition caused albuminuria and FP effacement in the present studies. While we cannot exclude “off-target” effects of transgene over-expression, these data are consistent with the notion that treatment strategies that inhibit Rho A may be difficult to employ clinically because of the potential for damaging the integrity of the glomerular filtration barrier; whereas, strategies that target downstream effectors of Rho A such as ROKs may attenuate the detrimental effects of excessive Rho A activity in glomerular diseases without adversely affecting glomerular filtration barrier function.
While both Rho A activation and Rho A inhibition promoted albuminuria and FP effacement, the mechanisms appeared to be different. For example, V14Rho reduced glomerular nephrin expression, increased the number of stress fibers and promoted podocyte apoptosis. In contrast, N19Rho had little effect on glomerular nephrin levels or podocyte apoptosis but reduced stress fiber formation. These findings suggest that the mechanisms of proteinuria induced by Rho A activation are different from the mechanisms that promote proteinuria during Rho A inhibition. In this regard, a major function of glomerular podocytes is to provide structural support to the glomerular tuft (43). By modulating actin polymerization, Rho GTPases likely play a pivotal role in regulating podocyte morphology, which a growing literature suggests, is important for maintaining the integrity of the glomerular filtration barrier (4–6). In proteinuric renal diseases, a reduction in the activity of Rho GTPases may lead to a phenotypic change in the glomerular podocyte characterized by a loss of stress fibers and enhanced formation of lamellipodia and filopodia as a result of activation of the Rho GTPase family members Rac1 and cdc42 (4–6). Indeed, in humans with minimal change disease (MCD), there a few light microscopic abnormalities but the podocyte undergoes a phenotypic switch characterized by FP effacement and, in some patients, microvillus protrusions on the apical surface reminiscent of filopodia (32). Despite this apparent change in podocyte morphology, expression of podocyte proteins such as nephrin (32) are not changed in MCD with the exception of the actin-associated protein synaptopodin which may be decreased (44). While we did not detect microvillus protrusions in the present studies, the N19Rho”double” TG mice did exhibit: 1. Normal light microscopic findings, 2. FP effacement, and 3. Preservation of nephrin expression despite a significant decrease in glomerular synaptopodin levels. Thus, activation of the N19Rho transgene resulted in many of the features of patients with MCD.
In contrast to Rho A inhibition, enhanced Rho A activity stimulated actin polymerization as well as reduced glomerular nephrin expression and caused apoptosis of cultured podocytes. Although we were unable to demonstrate that induction of V14Rho in podocytes caused either apoptosis or a decrease in podocyte number in vivo, in glomerular diseases, podocyte apoptosis is an important mechanism leading to a decrease in podocyte number (32, 43, 45–47). While the mechanisms that promote podocyte apoptosis are likely complex, the prosurvival PI3K/Akt pathway protects podocytes from apoptosis (25–27); and PI3K activity is negatively regulated by Rho A through its downstream effector ROK by phosphorylating and activating the Akt inhibitor PTEN (25). Moreover, the decrease in glomerular nephrin expression following induction of the V14Rho transgene may also promote apoptosis of glomerular podocytes because nephrin directly stimulates the prosurvival PI3K/Akt pathway (25, 41). Given that cellular outcome is likely dependent on differential activation of signaling pathways that promote either cell survival or apoptosis, a decrease in PI3K/Akt signaling might alter this balance and promote podocyte apoptosis which, some investigators contend, may cause instability of the glomerular tuft and glomerulosclerosis (32). Indeed, recent studies suggest that high levels of Rho activation in podocytes promote focal glomerulosclerosis (20); whereas, lower levels of Rho activation cause FP effacement without light microscopic abnormalities (20). While we did not observe glomerulosclerosis in our study, it is possible that the level of Rho activation in our experiments may have been insufficient to promote sclerosis. Alternately, differences in experimental design including genetic background of the mice may have contributed to the lack of glomerulosclerosis observed in the present experiments compared to published studies (20).
Lastly, both Rho A activation and Rho A inhibition caused a reduction in glomerular synaptopodin expression in “double” TG mice after 6 weeks of treatment with doxycycline. Synaptopodin stabilizes Rho A protein levels by a protein interaction between Rho A and synaptopodin which, in turn, prevents Rho A from interacting with the ubiquitin ligase Smurf1 and proteosomal degradation (5). We, therefore, investigated the effect of decreased synaptopodin expression on the level of Rho GTPase proteins. We found that endogenous Rho A protein levels were decreased in V14Rho “double” TG mice, consistent with the notion that synaptopodin stabilizes the Rho A protein. In contrast, Rho A protein levels were not affected by expression of the N19Rho transgene despite a decrease in synaptopodin levels in N19Rho “double” TG mice. While we can only speculate on mechanism, the protein-protein interaction between Rho A and Smurf1 preferentially favors an interaction between inactive Rho A proteins (GDP bound Rho A) such as the dominant-negative N19Rho construct (48). Indeed, V14Rho binds Smurf1 poorly or not at all (48). Based on these observations, we speculate that induction of N19Rho competes with endogenous inactive Rho A proteins (GDP bound Rho A) and, in turn, competitively antagonizes degradation of endogenous Rho A in N19Rho “double” TG mice. This competitive antagonism does not occur in the V14Rho “double” TG mice and, as a result, a decrease in synaptopodin levels in these animals enhances degradation of endogenous Rho A.
In summary, we found that either Rho A activation or Rho A inhibition had similar adverse effects on glomerular filtration barrier function and reduced podocyte synaptopodin expression but the mechanisms of these detrimental effects appeared to be different. Enhanced Rho A activity increased actin polymerization as well as caused a reduction in glomerular nephrin expression and promoted podocyte apoptosis. In contrast, inhibition of Rho A caused a loss of podocyte stress fibers but did not alter glomerular nephrin expression and did not cause podocyte apoptosis. These data suggest that some basal level of Rho A activity has beneficial effects in podocytes, perhaps by stabilizing the glomerular architecture. The level of Rho A activity, however, must be tightly regulated because enhancing Rho A activity above basal levels also has adverse effects on glomerular filtration barrier function. Taken together with published studies (11–20), these findings suggest that Rho A plays an important role in modulating the integrity of the glomerular filtration barrier both under basal conditions and during disease states.
Methods
Materials
Alexa Fluor 568 phalloidin was obtained Molecular Probes (Eugene, OR), the urine albumin and creatinine kits were obtained from AssayPro (St. Charles, MO) and Exocell (Philadelphia, PA), respectively, the ROCK inhibitor Y27632 (38) was obtained from Calbiochem (La Jolla, CA), and the TAT protein DNA constructs (49) were obtained from Becker-Hapak, Washington University (23).
Creation of an inducible V14Rho and N19Rho transgenes
The V14Rho and N19Rho transgenes were created by subcloning a fragment of the HA tagged TAT constructs into a previously described construct (50).
PCR
Screening for TG mice and expression of transgene mRNA were performed using PCR or RT-PCR, respectively, and the primer pairs AAGGACCAGTTCCCAGAGGT and GAAATTGGACAGCAAGAAAG.
Experimental procedures
The following experimental procedures were performed as previously described (37, 38, 50–53): 1. Culture of mouse podocytes and treatment with TAT proteins, 2. Creation of FVB/NJ TG mice and induction of the transgenes, 3. Light, electron microscopy and quantitation of slit diaphragm patency, 4. Isolation of glomerular preparations, 5. Immunoblotting and immunohistochemistry, 6 Detection of apoptosis (in vivo and in vitro), 7. Quantitation of glomerular podocytes, 8. Immunoprecipitation of PTEN 9. Real-time quantitative RT-PCR and 10. Quantitation of polymerized actin in cultured podocytes. All animal procedures were approved by the Animal Care and Use Committee of Duke University Medical Center. Additional details are provided in the Supplementary Section.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). For comparison between two groups, statistical significance was assessed by a t-test using the InStat computer program (GraphPad Sofware, Inc.). For comparisons between more than two groups, statistical analysis was performed using a one way analysis of variance (ANOVA) followed by a Bonferonni multiple comparisons post test (54) using the InStat program.
Supplementary Material
Acknowledgements
These studies were supported by a Merit Review from Veterans Health Administration, Office of Research and Development (BX000791-01). Dr. Spurney also received salary support through a grant from the National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases (RO1-DK075688 and RO1-DK087707).
Footnotes
Disclosures
The authors have no financial interests to disclose.
References
- 1.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 2.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavenstadt H. Roles of the podocyte in glomerular function. Am J Physiol Renal Physiol. 2000;278:F173–F179. doi: 10.1152/ajprenal.2000.278.2.F173. [DOI] [PubMed] [Google Scholar]
- 4.Yanagida-Asanuma E, Asanuma K, Kim K, et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171:415–427. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asanuma K, Yanagida-Asanuma E, Faul C, et al. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 6.Lu TC, He JC, Wang ZH, et al. HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem. 2008;283:8173–8182. doi: 10.1074/jbc.M708920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- 8.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz M. Rho signalling at a glance. J Cell Sci. 2004;117:5457–5458. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- 10.Seasholtz TM, Brown JH. RHO SIGNALING in vascular diseases. Mol Interv. 2004;4:348–357. doi: 10.1124/mi.4.6.8. [DOI] [PubMed] [Google Scholar]
- 11.Kanda T, Wakino S, Hayashi K, et al. Effect of fasudil on Rho-kinase and nephropathy in subtotally nephrectomized spontaneously hypertensive rats. Kidney Int. 2003;64:2009–2019. doi: 10.1046/j.1523-1755.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagatoya K, Moriyama T, Kawada N, et al. Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction. Kidney Int. 2002;61:1684–1695. doi: 10.1046/j.1523-1755.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- 13.Nishikimi T, Matsuoka H. Molecular mechanisms and therapeutic strategies of chronic renal injury: renoprotective effect of rho-kinase inhibitor in hypertensive glomerulosclerosis. J Pharmacol Sci. 2006;100:22–28. doi: 10.1254/jphs.fmj05003x5. [DOI] [PubMed] [Google Scholar]
- 14.Satoh S, Yamaguchi T, Hitomi A, et al. Fasudil attenuates interstitial fibrosis in rat kidneys with unilateral ureteral obstruction. Eur J Pharmacol. 2002;455:169–174. doi: 10.1016/s0014-2999(02)02619-5. [DOI] [PubMed] [Google Scholar]
- 15.Sun GP, Kohno M, Guo P, et al. Involvements of Rho-kinase and TGF-beta pathways in aldosterone-induced renal injury. J Am Soc Nephrol. 2006;17:2193–2201. doi: 10.1681/ASN.2005121375. [DOI] [PubMed] [Google Scholar]
- 16.Shibata S, Nagase M, Fujita T. Fluvastatin ameliorates podocyte injury in proteinuric rats via modulation of excessive Rho signaling. J Am Soc Nephrol. 2006;17:754–764. doi: 10.1681/ASN.2005050571. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai N, Kuroiwa T, Ikeuchi H, et al. Fluvastatin prevents podocyte injury in a murine model of HIV-associated nephropathy. Nephrol Dial Transplant. 2009;24:2378–2383. doi: 10.1093/ndt/gfp012. [DOI] [PubMed] [Google Scholar]
- 18.Hidaka T, Suzuki Y, Yamashita M, et al. Amelioration of crescentic glomerulonephritis by RhoA kinase inhibitor, Fasudil, through podocyte protection and prevention of leukocyte migration. Am J Pathol. 2008;172:603–614. doi: 10.2353/ajpath.2008.070196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koshikawa S, Nishikimi T, Inaba C, et al. Fasudil, a Rho-kinase inhibitor, reverses L-NAME exacerbated severe nephrosclerosis in spontaneously hypertensive rats. J Hypertens. 2008;26:1837–1848. doi: 10.1097/HJH.0b013e328305086c. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L, Jiang R, Aoudjit L, et al. Activation of RhoA in Podocytes Induces Focal Segmental Glomerulosclerosis. J Am Soc Nephrol. 22:1621–1630. doi: 10.1681/ASN.2010111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gingrich JR, Roder J. Inducible gene expression in the nervous system of transgenic mice. Annu Rev Neurosci. 1998;21:377–405. doi: 10.1146/annurev.neuro.21.1.377. [DOI] [PubMed] [Google Scholar]
- 22.Shigehara T, Zaragoza C, Kitiyakara C, et al. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14:1998–2003. doi: 10.1681/ASN.V1481998. [DOI] [PubMed] [Google Scholar]
- 23.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24:247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- 24.Mundel P, Heid HW, Mundel TM, et al. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 2007;55:61–75. doi: 10.1007/s00005-007-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Sun N, Aoudjit L, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73:556–566. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 27.Huber TB, Hartleben B, Kim J, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23:4917–4928. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Dong X, Wang Z, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 29.Kestila M, Lenkkeri U, Mannikko M, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 30.Putaala H, Soininen R, Kilpelainen P, et al. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Rantanen M, Palmen T, Patari A, et al. Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol. 2002;13:1586–1594. doi: 10.1097/01.asn.0000016142.29721.22. [DOI] [PubMed] [Google Scholar]
- 32.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 33.Aaltonen P, Luimula P, Astrom E, et al. Changes in the expression of nephrin gene and protein in experimental diabetic nephropathy. Lab Invest. 2001;81:1185–1190. doi: 10.1038/labinvest.3780332. [DOI] [PubMed] [Google Scholar]
- 34.Benigni A, Tomasoni S, Gagliardini E, et al. Blocking angiotensin II synthesis/activity preserves glomerular nephrin in rats with severe nephrosis. J Am Soc Nephrol. 2001;12:941–948. doi: 10.1681/ASN.V125941. [DOI] [PubMed] [Google Scholar]
- 35.Kawachi H, Koike H, Kurihara H, et al. Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int. 2000;57:1949–1961. doi: 10.1046/j.1523-1755.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 36.Luimula P, Ahola H, Wang SX, et al. Nephrin in experimental glomerular disease. Kidney Int. 2000;58:1461–1468. doi: 10.1046/j.1523-1755.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Fields TA, Pazmino K, et al. Activation of Galpha q-coupled signaling pathways in glomerular podocytes promotes renal injury. J Am Soc Nephrol. 2005;16:3611–3622. doi: 10.1681/ASN.2005020167. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Ellis MJ, Fields TA, et al. Beneficial effects of the Rho kinase inhibitor Y27632 in murine puromycin aminonucleoside nephrosis. Kidney Blood Press Res. 2008;31:111–121. doi: 10.1159/000121531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan H, Takeuchi E, Taylor GA, et al. Nephrin dissociates from actin, and its expression is reduced in early experimental membranous nephropathy. J Am Soc Nephrol. 2002;13:946–956. doi: 10.1681/ASN.V134946. [DOI] [PubMed] [Google Scholar]
- 40.Barisoni L, Kriz W, Mundel P, et al. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 41.Chuang PY, He JC. Signaling in regulation of podocyte phenotypes. Nephron Physiol. 2009;111:9–15. doi: 10.1159/000191075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verma R, Kovari I, Soofi A, et al. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116:1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava T, Garola RE, Whiting JM, et al. Synaptopodin expression in idiopathic nephrotic syndrome of childhood. Kidney Int. 2001;59:118–125. doi: 10.1046/j.1523-1755.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- 45.Susztak K, Raff AC, Schiffer M, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 46.Verzola D, Gandolfo MT, Ferrario F, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72:1262–1272. doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- 47.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 48.Wang HR, Zhang Y, Ozdamar B, et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 49.Chellaiah MA, Soga N, Swanson S, et al. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J Biol Chem. 2000;275:11993–12002. doi: 10.1074/jbc.275.16.11993. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Flannery PJ, Rosenberg PB, et al. Gq-dependent signaling upregulates COX2 in glomerular podocytes. J Am Soc Nephrol. 2008;19:2108–2118. doi: 10.1681/ASN.2008010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Chang JH, Paik SY, et al. Calcineurin (CN) activation promotes apoptosis of glomerular podocytes both in vitro and in vivo. Mol Endocrinol. 25:1376–1386. doi: 10.1210/me.2011-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spurney RF. Role of C-terminal serines in desensitization and phosphorylation of the mouse thromboxane receptor. J Biol Chem. 1998;273:28496–28503. doi: 10.1074/jbc.273.43.28496. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Gesty-Palmer D, Fields TA, et al. Inhibition of WNT signaling by G protein-coupled receptor (GPCR) kinase 2 (GRK2) Mol Endocrinol. 2009;23:1455–1465. doi: 10.1210/me.2009-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


