Abstract
γδ T cells possess innate like properties and are proposed to bridge the gap between innate and adaptive immunity. In this study we explored the role of γδ T cells in cutaneous immunity utilizing a skin transplantation model. Following engraftment of skin expressing cell associated model antigen (ovalbumin) in epithelial keratinocytes, skin resident γδ T cells enhanced graft rejection. While effector function of CD8 T cells was intact in the absence of γδ T cells, cross priming of CD8 T cell to graft derived antigen was impaired in the absence of γδ T cells. The reduced graft rejection and graft priming of γδ T cell deficient mice was evident in both acutely inflamed and well-healed grafting models. Furthermore, expression of the CD40 activation marker on migrating dendritic cells was lower in TCRδ-/- mice compared to wildtype mice, regardless of the presence or absence of inflammation associated with grafting. These results indicate that γδ T cells enhance graft priming and consequently the likelihood of a successful immune outcome in the context of skin graft rejection suggesting that γδ T cells may be an important component of immunity to epithelial cancers or infection.
INTRODUCTION
γδ T cells are a minor subpopulation of T cells in the circulation, however they are highly enriched in epithelial tissues such as skin, gut, lungs and the genitourinary tract (Allison and Havran, 1991). γδ T cell deficient (TCRδ-/-) mice have perturbed epithelial physiology and immune responsiveness upon various biological and non-biological challenges. A skin-resident subset of γδ T cells called dendritic epidermal T cells (DETC) plays a critical role in tumor surveillance following treatment of skin with dimethylbenzanthracine (DMBA) and tetradecanoylphorbol (TPA) (Girardi et al., 2001). The intestinal tract of TCRδ-/- mice has been shown to exhibit increased immunopathology and epithelial damage following infection with Eimeria vermiformis or treatment with dextran sodium sulphate (DSS) in a DSS-induced model of mouse colitis (Chen et al., 2002; Roberts et al., 1996). Ozone treatment and infection with Nocardia asteroides results in increased pulmonary damage and high lethality in γδ T cell deficient mice (King et al., 1999). In the kidneys, the severity of adriamycin-induced nephropathy is dampened by intraepithelial γδ T cells (Wu et al., 2007).
Notwithstanding the fact that the majority of research on intraepithelial γδ T cells has been on the role of γδ cells in cutaneous immunity, our understanding of the involvement of γδ T cells in the immune processes in the skin is incomplete. While the role of skin resident γδ T cells in tumor surveillance, wound healing and homeostasis in the skin has been elaborately described (Girardi et al., 2001; Jameson and Havran, 2007; Jameson et al., 2002; Sharp et al., 2005), the details of how skin resident γδ T cells regulate the function of other skin associated immune cells have not been extensively studied. For example the effects of γδ T cells on cross-presentation of skin derived Ag in generating adaptive immune responses is largely unknown.
Skin transplantation has been used in research as a model to study histocompatibility, cutaneous immunity and the pathopysiology of solid organ transplant rejection (Klein, 1975; Rosenberg and Singer, 1992). The paradigm of skin graft rejection involves the presentation of skin-derived antigens by Ag presenting cells in the lymph node to CD4 and CD8 T cells, which subsequently results in the generation of adaptive immune responses (Lakkis et al., 2000; Rosenberg et al., 1987; Sawada et al., 1997). The role of skin resident γδ T cells in this process has not been described. This study describes the role of γδ T cells in rejection of ovalbumin (OVA) expressing skin grafts. We show that skin resident γδ T cells enhance skin graft rejection. We also show that while cross-priming of CD8 T cells to Ag delivered subcutaneously in conjunction with an adjuvant is intact in TCRδ-/- mice, cross- priming to skin derived Ag is impaired in γδ T cell deficient mice in both acutely inflamed and well-healed skin grafts. Furthermore, we show that while trafficking of skin derived dendritic cells is not impaired in γδ T cell deficient mice, the expression of the CD40 co-stimulatory molecule on migrating DCs is lower in transplanted and naive TCRδ-/- mice compared to wildtype. Therefore we describe a novel role for γδ T cells in cutaneous immunity.
RESULTS
Skin resident γδ T cells enhance rejection of ovalbumin-expressing skin grafts
Immunocompetent mice reject otherwise syngeneic skin expressing OVA in keratinocytes from a keratin 5 promoter (K5mOVA mice) (Azukizawa et al., 2003). To establish the contribution of γδ T cells to skin graft rejection, we generated TCRδ-/-OVA+/- mice by crossing TCRδ-/- (C57BL/6 strain) and K5mOVA (C57BL/6 strain) mice. TCRδ-/- mice were double grafted with skin from TCRδ-/- (control) and TCRδ-/-OVA+/- mice and the rejection rate was compared to that of C57BL/6 (control) and K5mOVA grafts by wildtype C57BL/6 mice. Control grafts in both groups were accepted indefinitely and while 100% of K5mOVA grafts were rejected promptly by C57BL/6 mice as previously described, only 64% of TCRδ-/-OVA+/- grafts were rejected by TCRδ-/- mice (Fig. 1A).
Figure 1. Skin resident γδ T cells enhance rejection of OVA expressing skin grafts.
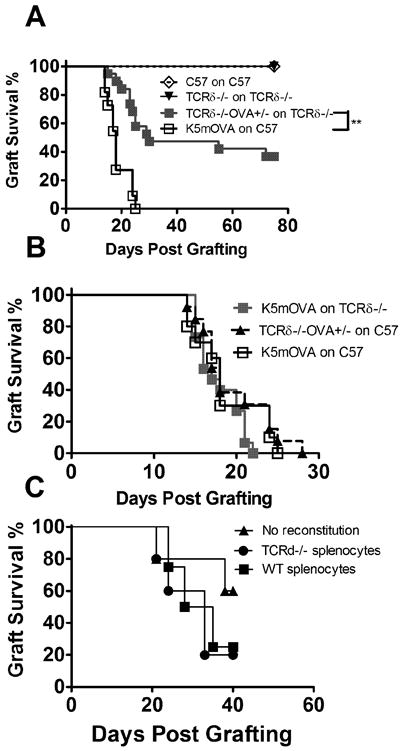
(A) Graft survival for K5mOVA (n=19), C57 (n=10), TCRδ-/-OVA+/- (n=10) and TCRδ-/- (n=19) grafts transplanted onto C57 and TCRδ-/- recipients. (**: P <0.01) (B) Graft survival for K5mOVA grafts transplanted onto C57 (n=10) or TCRδ-/- (n=15) recipients (P =N.S) and TCRδ-/-OVA+/- grafts transplanted onto C57 (n=13) recipients. (C) Graft survival for TCRδ-/-OVA+/- grafts transplanted onto TCRδ-/- animals, recipient of 1×108splenocytes from TCRδ-/- (n=5) or C57 (n=5) mice, or without transferred splenocytes (n=5) (P = N.S.). (Graft survival curves were assessed for significance using Kaplan-Meier survival analysis).
To clarify whether donor or host γδ T cells were contributing to rejection of OVA grafts, K5mOVA skin was transplanted onto TCRδ-/- mice, eliminating host γδ T cells and TCRδ-/-OVA+/- skin (devoid of donor γδ T cells) was grafted onto wildtype mice. In both groups 100% of grafts were rejected robustly by their respective recipients, suggesting the presence of γδ T cells in either the donor or the recipient is sufficient for skin graft rejection and both must be absent for prolonged survival. Robust rejection of K5mOVA grafts by TCRδ-/- mice shows that in this model circulating γδ T cells are redundant and that skin resident γδ T cells enhance rejection of OVA expressing skin grafts (Fig. 1B). To confirm that skin rather than circulating γδ T cells were responsible for enhanced rejection, TCRδ-/- mice were grafted with TCRδ-/-OVA+/- transgenic skin and were administered splenocytes from wildtype or TCRδ-/- animals (one donor spleen per recipient). No difference in the rate of graft rejection was observed (Fig. 1C), further confirming the finding that circulating γδ T cells do not contribute to graft rejection in this model.
Impaired CD8 T cell priming to acutely inflamed graft-derived Ag in TCRδ-/- mice
Skin graft rejection generally requires priming of CD8 T cells to graft-derived Ag and generation of an Ag-specific effector T cell response (Rosenberg et al., 1987; Rosenberg and Singer, 1992; Tak W. Mak, 2006). To determine whether γδ T cells contributed to the priming phase, wildtype and TCRδ-/- mice were transplanted with K5mOVA and TCRδ-/-OVA+/- skin grafts respectively. Twenty days post-grafting, spleens were taken and OVA specific IFNγ secreting CD8 T cell responses were assessed by IFNγ ELISPOT stimulated with the cognate Ag SIINFEKL. Mean ELISPOT number for TCRδ-/- recipients was significantly lower than for C57 mice (Fig. 2A). IFNγ was undetectable from splenic CD8 T cells of TCRδ-/- mice that had not rejected their skin grafts (data not shown), suggesting that acquisition of the capacity to reject a skin graft correlated with acquisition of effector function by Ag-specific CD8 T cells. To confirm this finding in vivo, OVA specific T cell proliferation was assessed by transfer of CFSE labeled Ly5.1+ OT-I cells into wildtype and TCRδ-/- mice, that were thereafter transplanted with TCRδ+/-OVA+/- and TCRδ-/-OVA+/- grafts respectively. Nine days post-grafting, mean proliferation index of adoptively transferred OT-I cells was higher in graft DLNs of wildtype mice indicating faster proliferation in wildtype mice compared to γδ T cell deficient mice (Fig. 2B).
Figure 2. Graft priming, but not systemic priming to OVA is impaired in TCRδ-/- mice.
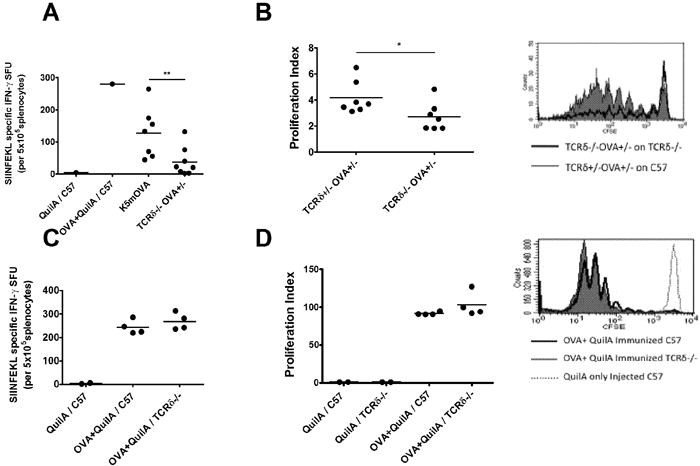
(A) C57 (n=7) and TCRδ-/- (n=8) mice were grafted with K5mOVA and TCRδ-/-OVA+/- skin respectively. Controls mice were immunized with OVA + QuilA or QuilA only. 20 days later SIINFEKL specific IFNγ producing CD8 T cells from the spleen was assessed (**: P <0.01) (B) C57 (n=7) and TCRδ-/- mice (n=7) mice were grafted with TCRδ+/-OVA+/- and TCRδ-/-OVA+/- skin, respectively. All mice received CFSE labelled OT-I cells. 9 days later, OT-I cells proliferation in graft DLNs was analyzed (*: P <0.05). (C) C57 and TCRδ-/- mice (n=4) immunized with OVA + QuilA or QuilA only. SIINFEKL specific IFNγ secreting T cells were assessed in the spleen after 7 days (P = N.S). (D) T cell proliferation in DLN of TCRδ-/- and C57 mice recipient of CSFE labeled OT-I cells and immunized with QuilA or OVA + QuilA, was assessed after 4 days (P = N.S). FACS histograms show representative CFSE dilution. (Un-paired t test was used for statistical analysis).
To determine whether the abrogation in CD8 T cell priming was a systemic defect in TCRδ-/- mice or a phenomenon seen only in priming to skin graft-derived Ag, wildtype and TCRδ-/- mice were immunized SC with OVA and QuilA. IFNγ production by splenic CD8 T cells harvested after 7 days and re-challenged in vitro with SIINFEKL was comparable between wildtype and TCRδ-/- mice (Fig. 2C). Similarly, in vivo proliferation of adoptively transferred CFSE labeled Ly5.1+ OT-I cells in DLNs after OVA and QuilA immunization was comparable between wildtype and TCRδ-/- mice (Fig. 2D). These results indicate that CD8 T cell priming in the absence of γδ T cells is impaired only when Ag expression is restricted to epithelial cells of the skin.
Intact effector CD8 T cell function in TCRδ-/- mice
As γδ T cells were shown to be important in CD8 T cell priming to skin grafts, we examined whether they were also important in the effector phase of the immune response in skin graft rejection. Wildtype and TCRδ-/- mice were pre-immunized with OVA and QuilA and 7 days later each group was transplanted with their relevant OVA expressing grafts (K5mOVA onto C57BL/6 and TCRδ-/-OVA+/- onto TCRδ-/-). All grafts were rejected by day 9 post grafting (Fig. 3A), suggesting that Ag-specific effector functions necessary for skin graft rejection were not significantly impaired in OVA primed mice lacking γδ T cells.
Figure 3. Effector functions of CD8 T cells required for graft rejection are not measurably impaired by the absence of γδ T cells.
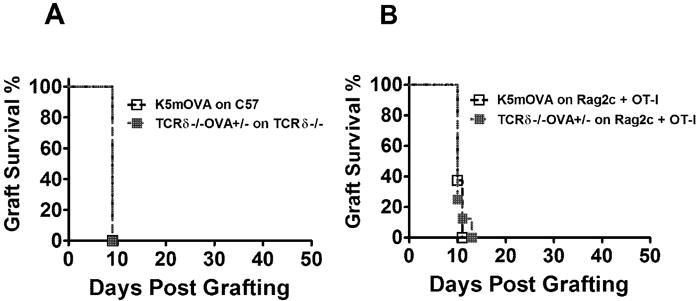
(A) Graft survival for K5mOVA (n=8) and TCRδ-/-OVA+/- (n=8) grafts, transplanted respectively onto C57 and TCRδ-/- mice immunized with OVA + QuilA (P= N.S) (B) Graft survival for K5mOVA or TCRδ-/-OVA+/- (n=8) grafts, transplanted separately onto 2C TCR Tg Rag-/- mice transferred with 1×103 in vitro activated OT-I cells (P=N.S). (Graft survival curves were assessed for significance using Kaplan-Meier survival analysis).
To confirm this finding, RAG/2CTCR Tg mice which lack OVA specific CD8 and γδ T cells (Sha et al., 1988) were transplanted with either K5mOVA or TCRδ-/-OVA+/- skin grafts and simultaneously administered 1×103 in vitro activated OT-I cells which have been previously shown to be necessary and sufficient to enable rejection K5mOVA grafts from a T cell deficient mouse (Broom et al.; Kenna et al., 2008). In both groups recipient mice rejected all skin grafts in a median time of 11 days (Fig. 3B), confirming that effector functions necessary for skin graft rejection are not impaired in the absence of γδ T cells.
Impaired CD8 T cell priming to well-healed graft derived Ag in TCRδ-/- mice
CD8 T cell priming to OVA Ag by freshly placed K5mOVA grafts was impaired in the absence of γδ T cells (Fig. 2A, 2B). To determine whether the local inflammation associated with grafting which contributes to effective priming to skin graft associated Ag (Zhong et al., 2008), is influenced by γδ T cells, we first examined the proliferation of CFSE labeled OT-I cells transferred into TCRδ+/-OVA+/- or TCRδ-/-OVA+/- mice. 42 hours post-transfer, significantly more OT-I cell proliferation was observed in the skin DLNs of γδ T cell replete mice (Fig. 4A).
Figure 4. Impaired OT-I proliferation to skin derived OVA in steady state.
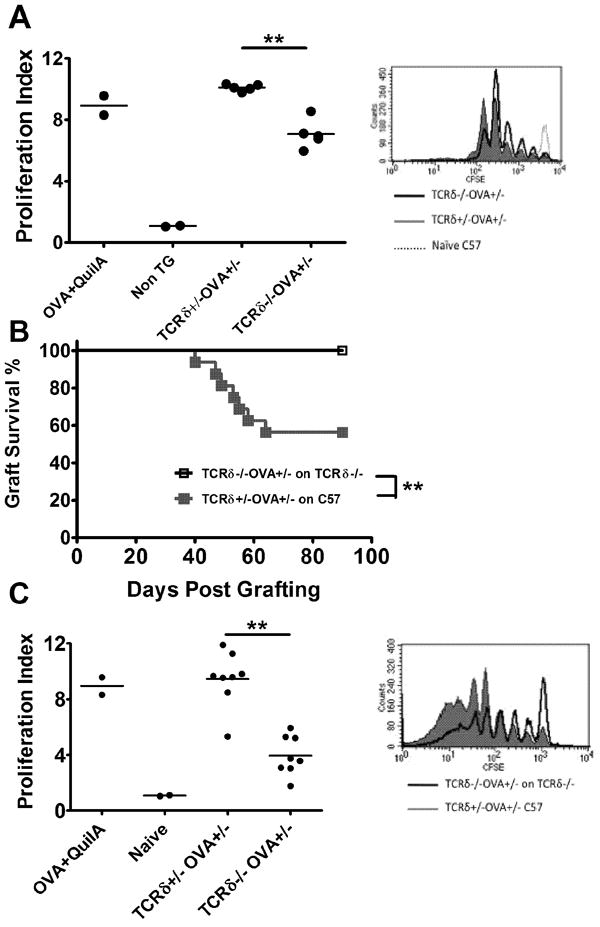
(A) TCRδ+/-OVA+/- (n=5), TCRδ-/-OVA+/- (n=5) and non-Tg mice received CFSE labelled OT-I cells. Control non-Tg mice were immunized with OVA+QuilA. DLN cells were assessed OT-I proliferation in DLNs was assessed 42 hours later (**: P <0.01). (B) Graft survival for TCRδ+/-OVA+/- (n=16) and TCRδ-/-OVA+/- (n=16) grafts transplanted onto CD8 depleted, C57 and TCRδ-/- mice respectively (**: P <0.01). (C) Mice [from Fig 4B] that failed to reject OVA grafts received CFSE labelled OT-I cells. Proliferation in graft DLNs was assessed after 42 hours. (**: P <0.01). FACS histograms show representative CFSE dilution. (Un-paired t test was used for proliferation indices and Kaplan-Meier survival analysis for graft survival curves).
In a second approach, wildtype and TCRδ-/- mice were transplanted with TCRδ+/-OVA+/- and TCRδ-/-OVA+/- skin grafts respectively. All recipient mice were initially treated with a CD8β depleting antibody to deplete CD8 T cells and therefore initially prevent skin graft rejection in the acute inflammatory phase. Graft rejection was monitored until day 90 by which time CD8 T cells had recovered. Whilst nearly 50% of grafts were rejected in the wildtype group, graft rejection was completely absent in the γδ T cell deficient group (Fig. 4B). At day 90, mice that had not rejected their skin grafts were injected with CFSE labeled OT-I cells to determine the proliferation rate of OT-I cells in the presence or absence of γδ T cells. Graft DLNs were taken 42 hours later and proliferation of OT-I cells was analyzed. Proliferation of OT-I cells was slower in the absence of γδ T cells compared to that in their presence (Fig. 4C). These data suggest that inflammation associated with skin grafting enhances T cell priming and graft rejection and in the absence of inflammation, skin graft rejection is further impaired in TCRδ-/- mice. This highlights the potential relevance of γδ T cells in general physiological or chronic inflammatory cutaneous settings in addition to their role in acutely inflamed transplantation model.
Maturation, but not trafficking of migrating DCs is impaired in TCRδ-/- mice
Wound healing and macrophage infiltration into skin is impaired in TCRδ-/- mice (Jameson et al., 2002; Jameson et al., 2005). To assess whether the absence of γδ T cells could affect the migration of skin associated dendritic cells and therefore influence cross-priming of CD8 T cells, we assessed DC migration to lymph nodes in wildtype and TCRδ-/- mice. We observed no differences in the migrating capacity of MHC-IIhi CD11chi DCs to DLNs following FITC painting of syngeneically grafted or non-grafted wildtype and TCRδ-/- skin (Fig. 5A).
Figure 5. CD40 levels on migrating DCs and not DC migration is impaired in TCRδ-/- mice.
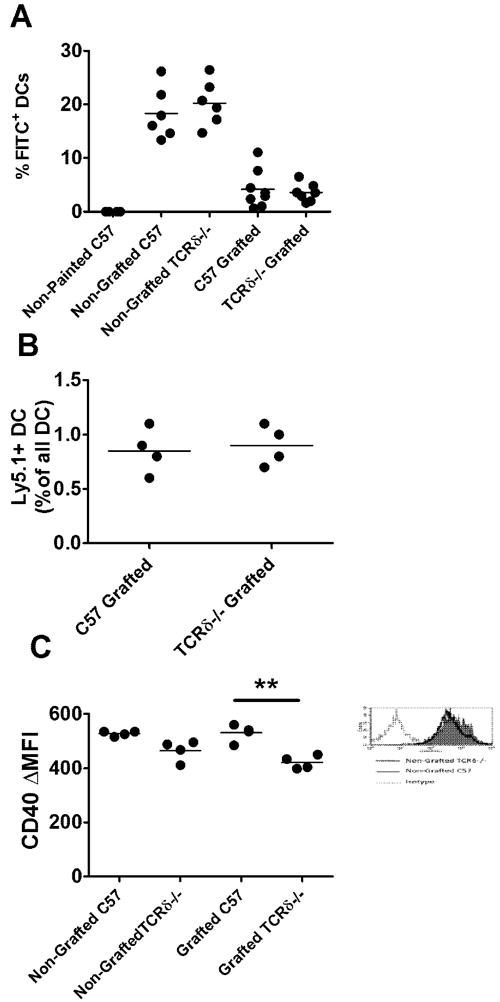
(A) C57 and TCRδ-/- mice, grafted with syngeneic skin, or non-grafted were painted with FITC on graft site or a corresponding site on non-grafted mice. 4 days later, DLNs were analysed for FITC+ migrating DC as a percentage of all DC (P= N.S) (B) Graft DLNs of C57 (n=4) and TCRδ-/- (n=4) mice grafted with congenic Ly5.1+ skin grafts were analysed 4 days later for Ly5.1+ migrating DC as a percentage of all DC (P= N.S) (C) CD40 expression on migrating DC in skin DLNs cells from grafted or non-grafted C57 and TCRδ-/- mice (**: P <0.01; unpaired t test). Histogram shows representative isotype and CD40 staining on migrating DCs.
In a second approach, TCRδ-/- mice were bred with Ly5.1+ Ptprca mice to create congenically marked TCRδ-/- mice. Ly5.2+ C57BL/6 and Ly5.2+ TCRδ-/- mice were transplanted with Ly5.1+ C57BL/6 and Ly5.1+ TCRδ-/- skin. Four days post transplantation, Ly5.1+ migrating skin DC populations were assessed in the DLNs and found to be similar between wildtype and TCRδ-/- mice (Fig. 5B).
Having observed no differences in the migration pattern of skin DCs, the activation state of migrating DCs was subsequently assessed by expression of CD40 co-stimulatory molecule on DCs from non-grafted and syngeneically grafted wildtype and TCRδ-/- mice. Modest but consistently lower levels of CD40 expression were observed in non-grafted and grafted TCRδ-/- mice when compared with non-grafted and grafted wildtype mice, indicating that maturation of DCs may be impaired in the absence of γδ T cells (Fig. 5C).
DISCUSSION
In this study we demonstrate that skin resident γδ T cells enhance rejection of skin grafts expressing cell-associated (OVA). While the replacement αβ T cells present in the epidermis of TCRδ-/- mice cannot compensate for the lack of skin resident γδ T cells, a contribution by recently described dermal γδ T cells (Gray et al., 2011; Sumaria et al., 2011) to graft rejection cannot be ruled out. Furthermore we show that cross-presentation of Ag in the context of skin grafts is impaired in TCRδ-/- mice and this phenomenon is evident in both acute and well-healed grafts. In the same context optimal graft rejection is observed when priming by grafts is bypassed and achieved by systemic immunization.
In contrast, evidence in the literature, suggests a mainly down-regulatory and anti-inflammatory role for γδ T cells in various models (D’Souza et al., 1997; Mombaerts et al., 1993; Mukasa et al., 1995; Shiohara et al., 1996). Based on the studies published by Gorczynski et al on the role of γδ T cells limiting the rejection of small intestinal allografts (Gorczynski et al., 1997; Gorczynski et al., 1996), the authors originally hypothesized that in TCRδ-/- mice there would be an exacerbated immune response to skin grafts and graft rejection would take place more rapidly than in wildtype mice. The different outcome in the current work from that found by others (Gorczynski, 1994) may reflect the micro-anatomical restriction of the antigen to the epidermis in our model. As a result of this restriction, only cross priming by professional APC can lead to priming of the host animal as opposed to a mixture of cross and direct priming in allograft settings. Advantageously, this reductionist system breaks down the process of priming and focuses merely on cross priming by professional APC.
γδ T cells lack TCR diversity and are proposed to recognize evolutionary conserved self molecules that may be up-regulated following stress (Hayday, 2000), therefore any contribution by γδ T cells to skin graft rejection cannot be mediated directly by an Ag-specific response to the expression of OVA in skin grafts. DETCs can exert direct cytotoxic effects on transformed, stressed and cancerous keratinocytes, which express NGK2D ligands or unknown Vγ3 TCR ligands in vivo and in vitro (Havran et al., 1991; Kaminski et al., 1993; Nitahara et al., 2006). The relevance of this is highlighted by the expression of NKG2D ligand on skin grafts acutely after skin transplantation (Kim et al., 2007). However the significance of any potential γδ T cell cytotoxicity in direct effector function against stressed OVA expressing skin grafts is reduced by the observation that γδ T cells are redundant in the effector phase of graft rejection in our model as pre-immunization against OVA protein prior to transplantation abrogates any defect seen in rejection in TCRδ-/- mice.
γδ T cells can contribute to skin graft rejection through the production of cytokines, which in turn may enhance the priming phase of the immune response. In vitro studies have revealed a capacity for DETC to produce a multitude of cytokines such as TNF-α, GM-CSF and IFN-γ (Boismenu et al., 1996; Matsue et al., 1993). However the basal levels or the triggers of heightened cytokine production by DETCs in vivo have not been fully described. Furthermore the necessity, redundancy or usefulness of such cytokines produced by DETC in priming has not been studied in vivo. Induction of Rae-1 -a stress molecule and NKG2D ligand selectively in the epidermal compartment of the skin has been reported to result in activation of DETCs, morphological changes in DETCs and Langerhans cells and a modest up-regulation of CD86 on Langerhans cells (Strid et al., 2008). While the up-regulation of CD86 and the change of cell shape in Langerhans cells is clearly an indirect effect of Rae-1 expression, no functional changes by Langerhans cells in relation to Ag presentation after the induction of Rae-1 expression was reported. Also it is not clear whether these changes were caused by DETC activation or by the infiltration of other NKG2D+ cells to the skin. Based on the expression of NKG2D ligand in skin grafts we found that DETCs at a heightened level of activation five days after grafting evidenced by higher than baseline CD69 expression (data not shown). Inflammation induced Rae-1 expression and subsequent DETC activation may play a role in augmentation of graft rejection. However γδ T cells also enhance rejection of well-healed grafts without evidence of inflammation, thus, there may be multiple mechanisms through which γδ T cells can contribute to enhanced priming to Ag expressed in grafted skin including cytokines produced by non-activated DETC.
One possible mechanism considered for γδ T cells mediated enhancement of rejection was that, in their absence, there was defective migration of DCs from skin to DLNs. This hypothesis was based on the delay that is known to exist in the infiltration of macrophages into wounds and healing of wounds in the skin of γδ deficient mice (Jameson et al., 2002; Jameson et al., 2005). This hypothesis was dismissed using FITC painting and migration of congenically marked (Ly5.1+) DCs in wildtype and TCRδ-/- mice. However reduced proliferation of Ag specific CD8 T cells was observed in TCRδ-/-OVA+/- (OVA Tg and γδ deficient) mice and in mice grafted with well-healed TCRδ-/-OVA+/- skin. This coupled with lower CD40 levels on migrating DCs of TCRδ-/- mice may indicate a tolerogenic form of cross presentation resulting in poor cross-priming, a hypothesis which will be examined in further work. Various studies have reported that cross-presentation of self or cognate Ag by DCs not sufficiently activated by inflammatory stimuli can result in deletional T cell tolerance (Kenna et al., 2008; Waithman et al., 2007). Furthermore due to the lack of growth factors, epidermal cells in TCRδ-/- mice go through three fold higher levels of apoptosis compared to wildtype (Sharp et al., 2005). Considering the tolerogenic effects of apoptosing cells (Green et al., 2009; Griffith et al., 2007) it may be that cross-tolerance outweighs cross-priming to epidermal Ag in TCRδ-/- mice.
The significance of this study is the linking of γδ T cells to an augmented generation of CD8 T cell responses to cutaneous Ag. In line with this finding is a study showing that Vγ1+ cells recruited to the lungs of mice infected intranasally with Bacillus Calmette-Guerin (BCG) promote the development of CD8 cytotoxic T cells (Dieli et al., 2003). Others have shown reduced levels of antibody or antibody producing plasma cells after mucosal OVA sensitization in the absence of γδ T cells (Fujihashi et al., 1996; Svensson et al., 2003). From a more broad perspective our study is in line with recent findings demonstrating a contribution by other innate cells e.g. NK cells, and NKT cells to cutaneous immunity in the context of skin transplantation and regulation of CD8 T cell immunity (Ito et al., 2008; Kroemer et al., 2008; Mattarollo et al., 2010a; Mattarollo et al., 2010b).
Characterization of γδ T cell involvement in the generation of adaptive immune responses in epithelia should facilitate development of immunotherapies utilizing γδ T cells in epithelial diseases including cancers and viral infections.
MATERIALS AND METHODS
Mice
C57BL/6 and congenic (Ly5.1+) Ptprca mice were obtained from the Animal Resources Center (Perth, Australia). K5mOVA Tg mice expressing membrane-bound Ovalbumin driven from the K5 promoter were provided by H. Azukizawa (Osaka, Japan) (Azukizawa et al., 2003). OT-I mice carrying a MHC-I restricted Tg TCR for OVA257-264 (SIINFEKL), originally provided by F. Carbone (Melbourne, Australia) (Hogquist et al., 1994) were crossed with (Ly5.1+) Ptprca mice to generate mice bearing Ly5.1+OT-I cells. RAG/2C mice (a cross between RAG-/- and 2C Tg mice) were provided by B. Fazekas (Sydney, Australia) (Sha et al., 1988). TCRδ-/- mice were obtained from Jackson Laboratory (Bar Harbor, USA). TCRδ-/- were crossed to K5mOVA mice, the F1 generation was backcrossed to TCRδ-/- to generate TCRδ-/-OVA+/- mice. The same breeding strategy was used to generate Ly5.1+ TCRδ-/- congenic mice by crossing Ly5.1+ Ptprca mice with TCRδ-/- mice. All mice were bred under specific pathogen-free conditions at the Princess Alexandra Hospital Biological Research Facility. Age- and sex-matched animals of 6-10 week age were used in experiments. All animal procedures were approved by the University of Queensland Animal Ethics Committee.
Reagents and flow cytometry
OVA257-264 peptide, a H-2Kb restricted CTL epitope with the amino acid sequence SIINFEKL was purchased from Auspep (Melbourne, Australia) with >80% purity, dissolved in 100% DMSO and stored at -20°C.
Anti-mouse monoclonal antibodies (mAb) to CD3 (145-2C11), CD8 (53-6.7), CD45.1 (A20), TCR-Vα2 (B20.1), MHC-II (M5/114.15.2), CD11c (N418) and CD40 (HM40-3) and associated isotype control immunoglobulins were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA). Prior to antibody staining, Fc block (Fcγ III/II receptor; BD Biosciences) was added to the cell suspension for 10 minutes on ice. Stained samples were analysed on FACSCalibur flow cytometer (BD Biosceinces).
Skin transplantation
Skin from donor mice was transplanted onto the flank of recipient mice as previously described (Dunn et al., 1997; Matsumoto et al., 2004). Briefly, donor skin was taken from the dorsal and ventral surfaces of the ear (~1cm2) and placed onto the thoracic flank region of anaesthetized recipients. Grafts were held in place with antibiotic-permeated gauze (Bactigras; Smith and Nephew, London, U.K.) and bandaged with micropore tape and Flex-wrap (Lyppard, Queensland, Australia). Bandages were removed 7 days post-grafting and grafts were monitored daily for loss of distinct border and signs of ulceration or necrosis to >80% of the graft which was used to define graft rejection.
Preparation of single cells suspension from skin for flow cytometry
Naïve or grafted ear skin was digested by floating dermis side down in 1 mg/ml collagenase/dispase solution (Roche, Berlin, Germany) for 1 h at 37°C. At the end of the incubation epidermal sheets were separated from dermis using forceps and transferred into complete RPMI 1640 medium containing 10% FCS and disrupted by vigorous pipetting with a transfer pipette. Cells were then washed through a 70 micron cell strainer (BD Biosciences).
In vivo assays and immunizations
To assess the proliferation of OVA specific CD8+ T cells in vivo, Ly5.1+ OT-I splenocytes were labelled with 2.5 μM CFSE and injected I.V (5×106) into the tail vein of OVA immunized, OVA Tg or OVA grafted wildtype and γδ T cell deficient mice. Respectively, 7 days and 42 hours after transfer into OVA immunized, OVA transgenic or OVA grafted mice, spleens and lymph nodes were harvested and CFSE dilutions in CD45.1+ Vα2+ CD8+ were assessed by FACS. When taking skin DLNs, brachial, axillary and inguinal lymph nodes were harvested based on their proximity to skin grafts and increased size compared to uninvolved, contralateral lymph nodes. Proliferation indices were calculated using ModFit LT software (Verity Software House, Topsham, ME). 1 × 103 in vitro activated OT-I cells, provided by T. Kenna (Kenna et al., 2010) were injected I.V via the tail vein into RAG2/C mice. To evaluate immune responses to soluble Ag, mice were immunized subcutaneously in the tail base with 50 μg OVA (Grade 5; Sigma-Aldrich, St. Louis, MO) and 20 μg QuilA adjuvant (Soperfos Biosector DK-Vedback, Denmark). Negative control mice were immunized with 20 μg QuilA only.
IFN-γ ELISPOT
ELISPOT assays were performed as previously described (Narayan et al., 2007). Briefly, cells from spleens of OVA immunized or OVA grafted mice were cultured overnight in complete RPMI 1640 medium in the presence of 5 ng/ml recombinant mouse IL-2 (BD Biosciences) and 0.1 μM SIINFEKL peptide in ELISPOT assays. IFN-γ spot-forming units were counted using an ELISPOT plate reader and stipulated as SFU per 5×105 splenocytes.
In vivo CD8 T cells depletion
CD8 T cells were depleted by intraperitoneal administration of anti-CD8β depleting antibody (clone 53-5.8) at days -2, 0 and +7 relative to skin transplantation using 100 μg, 100 μg and 150 μg mAb per mouse at each time point respectively. Equal amount of purified rat serum (isotype control) was injected into control mice. Two days after the last injection (Day +9) mice were eye-bled and tested for efficacy of depletion, which was consistently greater than 98%.
FITC painting
Flank skin was shaved with clippers and painted with 50 μl of 5 mg/ml FITC solution in 1:1 in acetone: dibutylphthalate (Sigma-Aldrich, St. Louis, MO). DLNs were harvested 4 days after painting and analyzed by FACS for MHC-IIhi CD11chi FITC+ cells (migrating DCs). In the case of painting grafted mice, bandages were removed from mice 4 days post grafting and mice were painted on the graft at day 5 post grafting with 10 μl of 25 mg/ml FITC solution.
Statistics
Kaplan-Meier plots were used to analyze skin graft survival and a log-rank test was performed to assess the statistical significance of differences between survival curves. For all other data in which statistics were performed, a two-tailed, nonparametric Mann-Whitney test was used for assessment of differences between groups. Differences with a p value of <0.05 were considered to be significant. Prism (Graphpad Software, La Jolla, CA) was used for graphs and statistical analysis.
Acknowledgments
The authors thank Dr. Tony Kenna for providing in vitro activated OT-I cells, Sean Smith for mouse genotyping, Allison Choyce for technical support and staff at the Biological Research Facility at Princess Alexandra Hospital for technical support and animal care.
Financial Support: This work was supported by program grant 080214 from the National Health and Medical Research Council of Australia, NCI grant RFA-CA-08-018, and funding from the Australian Cancer Research Foundation and the Lions Medical Research Foundation. A.R. was recipient of a postgraduate “UQ PhD Confirmation Scholarship” and I.H.F. was recipient of Queensland Government Premiers Fellowship.
Nonstandard abbreviations used
- DETC
Dendritic Epidermal T cells
- γδ T cell
gamma delta T cells
- OVA
Ovalbumin
- mAb
monoclonal antibody
- DLNs
Draining lymph nodes
- IV
Intravenous
- SC
Subcutaneous
- Tg
Transgenic
- Ag
Antigen
- FACS
Flow cytometry
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
References
- Allison JP, Havran WL. The immunobiology of T cells with invariant gamma delta antigen receptors. Annu Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- Azukizawa H, Kosaka H, Sano S, Heath WR, Takahashi I, Gao XH, et al. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol. 2003;33:1879–88. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- Boismenu R, Feng L, Xia YY, Chang JC, Havran WL. Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–92. [PubMed] [Google Scholar]
- Broom JK, Lew AM, Azukizawa H, Kenna TJ, Leggatt GR, Frazer IH. Antigen-Specific CD4 Cells Assist CD8 T-Effector Cells in Eliminating Keratinocytes. J Invest Dermatol. 130:1581–9. doi: 10.1038/jid.2010.17. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–43. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–21. [PubMed] [Google Scholar]
- Dieli F, Ivanyi J, Marsh P, Williams A, Naylor I, Sireci G, et al. Characterization of lung gamma delta T cells following intranasal infection with Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2003;170:463–9. doi: 10.4049/jimmunol.170.1.463. [DOI] [PubMed] [Google Scholar]
- Dunn LA, Evander M, Tindle RW, Bulloch AL, de Kluyver RL, Fernando GJ, et al. Presentation of the HPV16E7 protein by skin grafts is insufficient to allow graft rejection in an E7-primed animal. Virology. 1997;235:94–103. doi: 10.1006/viro.1997.8650. [DOI] [PubMed] [Google Scholar]
- Fujihashi K, McGhee JR, Kweon MN, Cooper MD, Tonegawa S, Takahashi I, et al. gamma/delta T cell-deficient mice have impaired mucosal immunoglobulin A responses. J Exp Med. 1996;183:1929–35. doi: 10.1084/jem.183.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- Gorczynski RM. Adoptive transfer of unresponsiveness to allogeneic skin grafts with hepatic gamma delta + T cells. Immunology. 1994;81:27–35. [PMC free article] [PubMed] [Google Scholar]
- Gorczynski RM, Chen Z, Fu XM, Levy G, Cohen Z. Gamma delta TCR+ cells regulate rejection of small intestinal allografts in rats. Transplant Proc. 1997;29:863–4. doi: 10.1016/s0041-1345(96)00174-1. [DOI] [PubMed] [Google Scholar]
- Gorczynski RM, Cohen Z, Levy G, Fu XM. A role for gamma(delta)TCR+ cells in regulation of rejection of small intestinal allografts in rats. Transplantation. 1996;62:844–51. doi: 10.1097/00007890-199609270-00024. [DOI] [PubMed] [Google Scholar]
- Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. Journal of immunology. 2011;186:6091–5. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TS, Kazama H, VanOosten RL, Earle JK, Jr, Herndon JM, Green DR, et al. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178:2679–87. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science. 1991;252:1430–2. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Ito A, Shimura H, Nitahara A, Tomiyama K, Ito M, Kanekura T, et al. NK cells contribute to the skin graft rejection promoted by CD4+ T cells activated through the indirect allorecognition pathway. International immunology. 2008;20:1343–9. doi: 10.1093/intimm/dxn092. [DOI] [PubMed] [Google Scholar]
- Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–22. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–9. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med. 2005;201:1269–79. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski MJ, Cruz PD, Jr, Bergstresser PR, Takashima A. Killing of skin-derived tumor cells by mouse dendritic epidermal T-cells. Cancer Res. 1993;53:4014–9. [PubMed] [Google Scholar]
- Kenna TJ, Thomas R, Steptoe RJ. Steady-state dendritic cells expressing cognate antigen terminate memory CD8+ T-cell responses. Blood. 2008;111:2091–100. doi: 10.1182/blood-2007-07-103200. [DOI] [PubMed] [Google Scholar]
- Kenna TJ, Waldie T, McNally A, Thomson M, Yagita H, Thomas R, et al. Targeting antigen to diverse APCs inactivates memory CD8+ T cells without eliciting tissue-destructive effector function. J Immunol. 2010;184:598–606. doi: 10.4049/jimmunol.0900032. [DOI] [PubMed] [Google Scholar]
- Kim J, Chang CK, Hayden T, Liu FC, Benjamin J, Hamerman JA, et al. The activating immunoreceptor NKG2D and its ligands are involved in allograft transplant rejection. J Immunol. 2007;179:6416–20. doi: 10.4049/jimmunol.179.10.6416. [DOI] [PubMed] [Google Scholar]
- King DP, Hyde DM, Jackson KA, Novosad DM, Ellis TN, Putney L, et al. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J Immunol. 1999;162:5033–6. [PubMed] [Google Scholar]
- Klein J. Biology of the mouse histocompatibility-2 complex. Principles of immunogenetics applied to a single system. Biology of the mouse histocompatibility-2 complex Principles of immunogenetics applied to a single system. 1975;xii:620. [Google Scholar]
- Kroemer A, Xiao X, Degauque N, Edtinger K, Wei H, Demirci G, et al. The innate NK cells, allograft rejection, and a key role for IL-15. J Immunol. 2008;180:7818–26. doi: 10.4049/jimmunol.180.12.7818. [DOI] [PubMed] [Google Scholar]
- Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686–8. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- Matsue H, Cruz PD, Jr, Bergstresser PR, Takashima A. Profiles of cytokine mRNA expressed by dendritic epidermal T cells in mice. J Invest Dermatol. 1993;101:537–42. doi: 10.1111/1523-1747.ep12365917. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Leggatt GR, Zhong J, Liu X, de Kluyver RL, Peters T, et al. Impaired antigen presentation and effectiveness of combined active/passive immunotherapy for epithelial tumors. J Natl Cancer Inst. 2004;96:1611–9. doi: 10.1093/jnci/djh301. [DOI] [PubMed] [Google Scholar]
- Mattarollo SR, Rahimpour A, Choyce A, Godfrey DI, Leggatt GR, Frazer IH. Invariant NKT cells in hyperplastic skin induce a local immune suppressive environment by IFN-gamma production. J Immunol. 2010a;184:1242–50. doi: 10.4049/jimmunol.0902191. [DOI] [PubMed] [Google Scholar]
- Mattarollo SR, Yong M, Tan L, Frazer IH, Leggatt GR. Secretion of IFN-gamma but not IL-17 by CD1d-restricted NKT cells enhances rejection of skin grafts expressing epithelial cell-derived antigen. J Immunol. 2010b;184:5663–9. doi: 10.4049/jimmunol.0903730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–6. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- Mukasa A, Hiromatsu K, Matsuzaki G, O’Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J Immunol. 1995;155:2047–56. [PubMed] [Google Scholar]
- Narayan S, Choyce A, Fernando GJ, Leggatt GR. Secondary immunisation with high-dose heterologous peptide leads to CD8 T cell populations with reduced functional avidity. Eur J Immunol. 2007;37:406–15. doi: 10.1002/eji.200535688. [DOI] [PubMed] [Google Scholar]
- Nitahara A, Shimura H, Ito A, Tomiyama K, Ito M, Kawai K. NKG2D ligation without T cell receptor engagement triggers both cytotoxicity and cytokine production in dendritic epidermal T cells. J Invest Dermatol. 2006;126:1052–8. doi: 10.1038/sj.jid.5700112. [DOI] [PubMed] [Google Scholar]
- Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, et al. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:11774–9. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AS, Mizuochi T, Sharrow SO, Singer A. Phenotype, specificity, and function of T cell subsets and T cell interactions involved in skin allograft rejection. J Exp Med. 1987;165:1296–315. doi: 10.1084/jem.165.5.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu Rev Immunol. 1992;10:333–58. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- Sawada T, Wu Y, Sachs DH, Iacomini J. CD4+ T cells are able to reject class I disparate allografts. Transplantation. 1997;64:335–40. doi: 10.1097/00007890-199707270-00027. [DOI] [PubMed] [Google Scholar]
- Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988;335:271–4. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–9. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- Shiohara T, Moriya N, Hayakawa J, Itohara S, Ishikawa H. Resistance to cutaneous graft-vs.-host disease is not induced in T cell receptor delta gene-mutant mice. J Exp Med. 1996;183:1483–9. doi: 10.1084/jem.183.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–54. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208:505–18. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L, Lilliehook B, Larsson R, Bucht A. gammadelta T cells contribute to the systemic immunoglobulin E response and local B-cell reactivity in allergic eosinophilic airway inflammation. Immunology. 2003;108:98–108. doi: 10.1046/j.1365-2567.2003.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak Tak W, S ME. The Immune Response Basic and Clinical Principles. Vol. 1194 Elsevier Academic Press; San Diego: 2006. [Google Scholar]
- Waithman J, Allan RS, Kosaka H, Azukizawa H, Shortman K, Lutz MB, et al. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J Immunol. 2007;179:4535–41. doi: 10.4049/jimmunol.179.7.4535. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang YM, Wang Y, Hu M, Zhang GY, Knight JF, et al. Depletion of gammadelta T cells exacerbates murine adriamycin nephropathy. J Am Soc Nephrol. 2007;18:1180–9. doi: 10.1681/ASN.2006060622. [DOI] [PubMed] [Google Scholar]
- Zhong J, Hadis U, De Kluyver R, Leggatt GR, Fernando GJ, Frazer IH. TLR7 stimulation augments T effector-mediated rejection of skin expressing neo-self antigen in keratinocytes. Eur J Immunol. 2008;38:73–81. doi: 10.1002/eji.200737599. [DOI] [PubMed] [Google Scholar]


