Abstract
Calcium balance in chronic kidney disease is poorly understood since its deficiency is a stimulus for secondary hyperparathyroidism and consequent bone loss while calcium excess promotes extra-osseous calcifications. To help resolve this, we evaluated calcium balance in normal individuals and in patients with chronic kidney disease (CKD) on daily diets containing 800 and 2000 mg elemental calcium. Both normal individuals and patients with late stage 3 and stage 4 CKD were in slightly negative to neutral calcium balance on the 800 mg calcium diet. Normal individuals were in modest positive calcium balance on the 2000 mg diet while patients with CKD on the same diet were in marked positive calcium balance at least over the 9 days of study; and significantly greater than the normal individuals. Increased calcium intake significantly decreased 1,25 dihydroxy-vitamin D and intact parathyroid hormone levels but did not alter the serum calcium concentration. Thus, our finding have important implications for both preventing calcium deficiency and loading in individuals with late stage 3 and stage 4 CKD.
Introduction
Serum calcium concentrations are maintained in the normal range until very late in chronic kidney disease (CKD) when it decreases slightly (1,2). However, calcium balance in CKD is poorly understood. Gastrointestinal (GI) calcium absorption has been shown to be lower in CKD subjects on normal calcium intake than in controls yet remains dependent on calcium intake (3). It is generally believed that decreased levels of circulating 1,25 dihydroxy-vitamin D (1,25-D), the principal hormone regulating transcellular calcium absorption in the gastrointestinal tract (GI)(4), results in decreased calcium absorption and net negative calcium balance, providing a stimulus for secondary hyperparathyroidism early in CKD. Late in CKD serum calcium concentrations decrease, as circulating (1,25-D) continues to decrease, lending support to this hypothesis. The decreasing 1,25-D concentrations coupled with a decrease in serum calcium concentrations late in CKD suggest that CKD patients may be in net negative calcium balance and that calcium supplementation may be needed to correct the calcium absorption defect.
To date, there are no adequate calcium balance studies in CKD. In normal adults, calcium balance is relatively neutral when calcium intake ranges between 750 mg to 1740 mg per day, although significant individual variability exists (5). Twenty-four hour urinary calcium excretion generally ranges between 100 to 300 mg per day largely reflecting dietary intake with a fractional GI absorption between 10-60% depending on 1,25-D concentrations and calcium intake (6,7). However, studies that have measured 24 hour urinary calcium excretion in patients with advanced CKD consistently show low 24 hour excretion, usually less than 80 mg/day (8,9). Even CKD patients on escalating doses of calcium based phosphate binders consistently had low urinary calcium excretion often in the range of 20-60 mg/day (10).
Understanding calcium balance is particularly important in CKD as it is characterized by a high prevalence of vascular calcification, which is associated with cardiovascular and all-cause mortality (11). While vascular calcification is thought to result from a complex disruption of normal vascular biology (12), there is ongoing controversy whether supplemental calcium accelerates this process both in CKD and in dialysis patients (13). Furthermore, the increasing concern over phosphorus balance early in CKD and the observational finding that CKD non-dialysis patients treated with phosphate binder have improved survival (14) may increase the use of calcium containing phosphate binders in CKD despite the lack of long term safety data.
Therefore, the purpose of this study was to formally evaluate calcium balance in late stage 3 and stage 4 CKD compared to control subjects. We hypothesized that subjects with advanced CKD are in positive calcium balance on high dietary calcium intake and that this positive balance is not reflected in the serum calcium concentration which is tightly regulated. If true this hypothesis would suggest that exposure to high calcium intake results in positive calcium balance in CKD leading to calcium loading which may precipitate or worsen vascular and soft tissue calcification.
Results
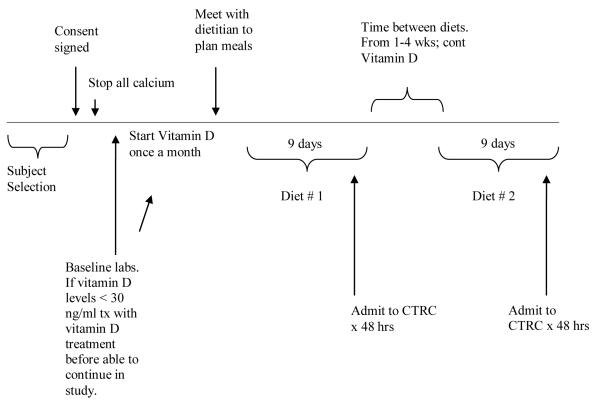
The study timeline is shown in Figure 1. Six subjects with advanced stage 3 or stage 4 CKD and 6 control subjects completed both diet arms. One control subject was dropped from the study due to inability to eat the prescribed diet during the first diet period and was not included in the data analysis. Two other control subjects were consented but failed vitamin D screening or withdrew consent and never started the active diet phase. One CKD subject had an inter-diet collection period that exceeded the study protocol by several months due to an inter-current illness.
Figure 1.
Timeline of study interventions
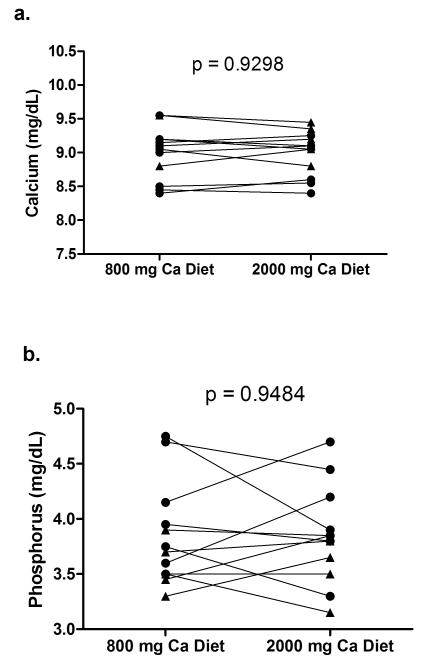
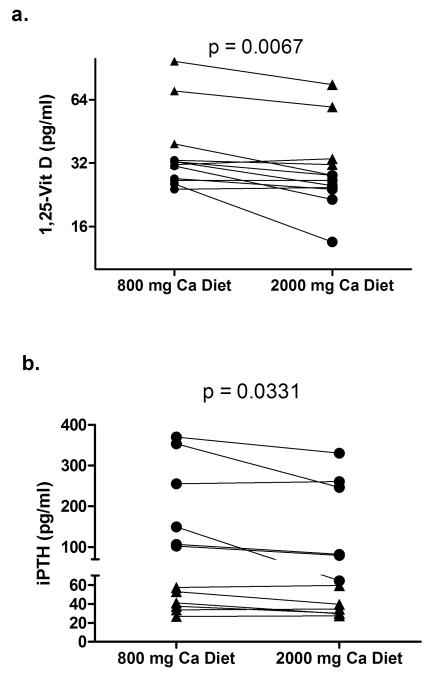
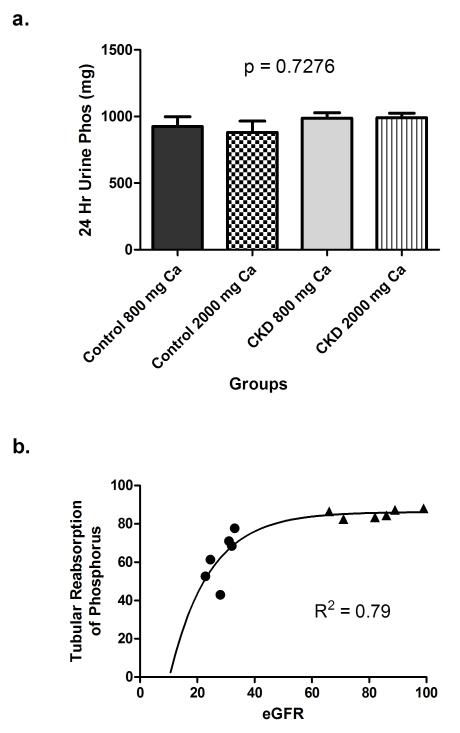
All subjects did a good job with the diets eating only, and most of the food prepared. The average calcium and phosphorus intake for subjects during their 48-hour in-patient diet periods were as follows: CKD- low calcium diet: Ca 812±4, phos 1575±59; high calcium diet: Ca 2009±9, phos 1672±62; Control - low calcium diet: Ca 787±42, phos 1518±136; high calcium diet: Ca 1903±160, phos 1545±125 mg/day. Serum calcium concentrations were not different between CKD and control subjects on either diet and there was no intra-patient difference between the 800 and 2000 mg calcium diets (Figure 2a). Serum phosphorus was significantly higher in CKD subjects on the 800 mg (4.15±0.20 vs. 3.56±0.09 mg/dL, p=0.02) and trended higher on the 2000 mg calcium diet (4.06±0.20 vs. 3.63±0.11 mg/dL, p=0.10), but again, did not show an intra-patient difference between the low and high calcium diets (Figure 2b). However, in response to the increase calcium in the diet, both the serum 1,25-D and the iPTH decreased significantly when subjects were on the 2000 mg calcium diet compared with the 800 mg calcium diet (Table 1, Figure 3). 25-D levels were lower in CKD subjects than controls (33.4±1.9 vs. 43.2±1.8 ng/ml; p=0.001) but did not change between diets in either group. Total urinary phosphorus excretion was not different between CKD and controls and did not vary by calcium diet (Table 2, Figure 4a). However, the tubular reabsorption of phosphorus was significantly lower in CKD than controls (62.4±12.8 vs. 85.4±2.2; p=0.002) and fit closely with the logarithmic exponent as previously reported (15)(Figure 4b).
Figure 2.
Serum calcium (a) and phosphorus (b) on 800 and 2000 mg calcium diets. filled circles = CKD subjects; filled triangles = controls
Table 1.
Baseline information and calcium and phosphorous intake
| 800 mg calcium diet | 2000 mg calcium diet | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (yrs) |
Gender (mg/dL) |
Creatinine ml/min/1.73m2 |
eGFR (MDRD-4) (pg/ml) |
1,25-D (pg/ml) |
iPTH (pg/ml) |
1,25-D (pg/ml) |
iPTH (pg/ml) |
|
| CKD-1 | 51 | M | 4.1 | 20 | 26.5 | 369.5 | 26.5 | 330.0 |
| CKD-2 | 56 | F | 1.6 | 33 | 24.0 | 149.5 | 24.5 | 64.5 |
| CKD-3 | 64 | M | 2.5 | 23 | 32.5 | 102.5 | 25.0 | 79.5 |
| CKD-4 | 62 | M | 2.5 | 25 | 27.0 | 255.0 | 24.0 | 260.5 |
| CKD-5 | 73 | M | 1.8 | 31 | 25.5 | 106.5 | 13.5 | 82.0 |
| CKD-6 | 70 | M | 2.1 | 32 | 31.0 | 353.0 | 21.5 | 246.5 |
| Cont-1 | 47 | F | 0.8 | 82 | 32.5 | 53.1 | 28.0 | 39.7 |
| Cont-2 | 40 | F | 0.7 | 99 | 70.5 | 37.6 | 59.0 | 30.3 |
| Cont-3 | 36 | F | 0.8 | 86 | 97.5 | 33.8 | 75.5 | 34.7 |
| Cont-4 | 67 | F | 0.7 | 89 | 39.5 | 57.5 | 28.0 | 59.5 |
| Cont-5 | 45 | M | 1.3 | 66 | 31.5 | 41.3 | 33.5 | 29.8 |
| Cont-6 | 52 | M | 1.2 | 71 | 33.0 | 26.9 | 31.5 | 27.4 |
Figure 3.
Serum 1,25 dihydroxy-vitamin D (a) and iPTH (b) on 800 and 2000 mg calcium diets. filled circles = CKD subjects; filled triangles = controls
Table 2.
Urine and stool calcium and phosphorus and net balance
| Diets | 800 mg calcium | 2000 mg calcium | 800 mg calcium |
2000 mg calcium |
Estimated Ca Balance |
|||
|---|---|---|---|---|---|---|---|---|
| Urine Ca | Urine P | Urine Ca | Urine P | Stool Ca/P Ratio |
Stool Ca/P Ratio |
800 mg Ca | 2000 mg Ca | |
| CKD-1 | 165 | 1117 | 244 | 937 | 1.02 | 1.16 | 153 | 975 |
| CKD-2 | 49 | 919 | 52 | 1032 | 1.43 | 1.89 | −208 | 908 |
| CKD-3 | 78 | 1097 | 78 | 1064 | 1.39 | 1.55 | −27 | 959 |
| CKD-4 | 112 | 871 | 73 | 851 | 1.12 | 1.60 | −130 | 542 |
| CKD-5 | 55 | 917 | 63 | 1025 | 1.63 | 1.79 | −143 | 812 |
| CKD-6 | 40 | 1001 | 85 | 1036 | 1.19 | 1.54 | 80 | 821 |
| Mean±SD | 83±48 | 987±102 | 99±72 | 991±81 | 1.30±0.23 | 1.59±0.25 | −91± 113 | 759±120 |
| Cont-1 | 245 | 925 | 190 | 1121 | 1.43 | 1.98 | −432 | 761 |
| Cont-2 | 411 | 710 | 349 | 723 | 0.98 | 1.59 | −239 | 184 |
| Cont-3 | 227 | 955 | 591 | 1015 | 1.13 | 1.96 | −117 | 307 |
| Cont-4 | 231 | 890 | 304 | 869 | 1.01 | 1.69 | −74 | 501 |
| Cont-5 | 137 | 820 | 134 | 675 | 0.97 | 1.41 | −79 | 565 |
| Cont-6 | 380 | 1246 | 435 | 1504+ | 1.13 | 1.90 | 79 | 1342+ |
| Mean±SD | 272±104 | 925±180 | 334±166 | 881±189 | 1.11±0.17 | 1.76±0.23 | −144 ±174 | 464±225 |
Indicates data not used in statistical analysis as this urinary phosphorus would require 94% of the dietary phosphorus to have been absorbed. All other urine and stool parameters are within expected ranges.
Figure 4.
24-hour urinary phosphorus excretion (a) and tubular reabsorption of phosphorus (b) filled circles = CKD subjects; filled triangles = controls.
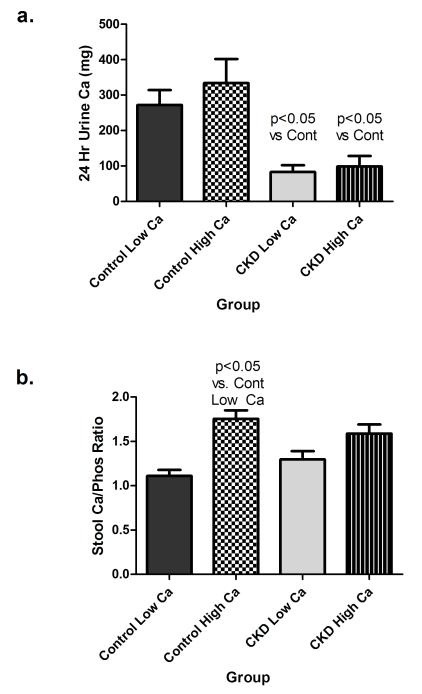
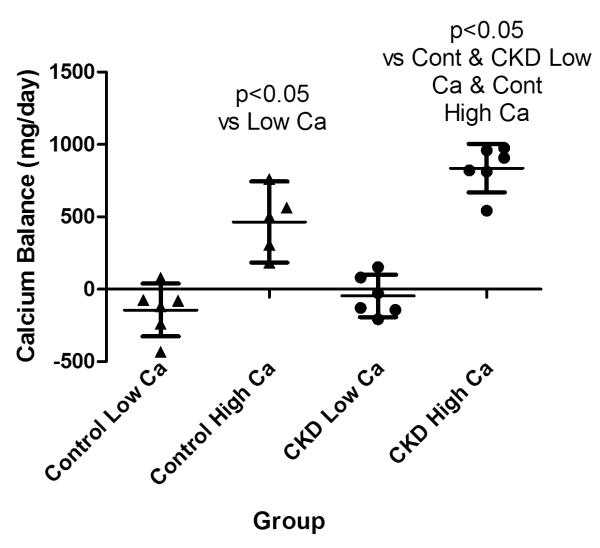
While some patients demonstrated an increase in urinary calcium on the higher calcium intake (Table 2), the intra-patient urinary calcium excretion was not different for CKD or control groups. However, the urinary calcium excretion was significantly lower in CKD subjects compared to controls on both the 800 (83.0±47.6 vs. 272.1±103.6; p<0.05) and 2000 mg (99.0±71.7 vs. 333.9±166.3; p<0.05) calcium diets (Table 2, Figure 5a). The stool calcium/phosphorus ratio was slightly, although not significantly lower in controls vs. CKD subjects on the 800 mg calcium diet possibly suggesting greater calcium absorption in controls consistent with the higher 1,25-D concentrations (Figure 5b). On the 2000 mg calcium diet both control and CKD subjects demonstrated an increase in stool calcium/phosphorus compared with the 800 mg calcium diet as expected, although this was slightly greater and only statistically significant in the control group. There was no difference in the stool calcium phosphorus ratio between groups on either diet (Figure 5b). The fact that CKD subjects did not increase their stool calcium/phosphorus ratio more than controls, strongly suggests that marked down regulation or impairment of GI calcium absorption is not occurring in CKD and cannot account for the low urinary calcium excretion in this group. The estimated percent of ingested dietary calcium absorbed increased from 5±21% to 47±10% in CKD subjects and from 16±26% to 41±11% in controls on the 800 and 2000 mg calcium diets respectively. Estimated calcium balance was slightly negative in CKD subjects on the 800 mg calcium intake (although the 95% CI crossed the neutral line), but markedly positive on the 2000 mg calcium intake (Table 2, Figure 6). Control subjects were also in slightly negative calcium balance on the 800 mg calcium diet, but again this was not statistically significant. Control subjects were also in positive calcium balance on the 2000 mg calcium diets, although significantly less positive than CKD subjects. (Table 2, Figure 6).
Figure 5.
24-hour urinary calcium excretion (a) and stool calcium/phosphorus ratio (b).
Figure 6.
Estimated calcium balance in controls and CKD subjects. filled circles = CKD subjects; filled triangles = controls
Discussion
This is the first study to evaluate calcium balance in advanced CKD. We found that subjects with late stage 3 and stage 4 CKD were in slightly negative to neutral calcium balance on a daily intake of 800 mg, but were in marked positive balance on 2000 mg per day. This finding is in agreement with modeled balance data in dialysis patients predicting negative balance when intake is less than 800 and positive balance when calcium intake exceeds 1500 mg/day (16). Our finding of low urinary calcium excretion in CKD is in agreement with previously reported 24-hour urine calcium excretion in subjects with CKD (8,9). The finding that urinary calcium did not increase in CKD subjects on the high calcium diet while the stool calcium to phosphorus ratio did not differ from controls supports our finding of marked positive calcium balance in the CKD subjects on the high calcium diet and suggests that significant down regulation or impairment of GI calcium absorption is not occurring in CKD. Together, these findings demonstrate that CKD patients receiving 2000 mg of elemental calcium have a substantial increase in calcium absorption resulting in positive balance without escapeover the time period studied. The finding that 1,25-D and iPTH concentrations decreased on the high calcium diet supports the finding that increasing calcium absorption is down regulating these hormones. However despite the decrease in 1,25-D, net calcium absorption increased on the high calcium diet possibly related to absorption via the less regulated, non-saturable paracellular pathway (4).The finding that the dietary calcium absorption was slightly lower in CKD subjects on the low calcium diet than controls is consistent with the lower 1,25-D levels, the principal hormone regulating transcellular calcium absorption in the GI tract when dietary calcium is low. On the high calcium intake, GI calcium absorption increased significantly in both groups as previously reported (17). The percent calcium absorption in this study was quite variable between patients but consistent with early studies reporting absorption between 26-63% on 800 mg/day and between 18-30% on 2000 mg/day (17). Control subjects were in slightly negative to neutral calcium balance on the low calcium diet and in positive balance on the 2000 mg per day calcium diet, although not as positive as CKD subjects. The observation that the serum calcium concentrations did not change in either group is consistent with the knowledge that the serum calcium concentration is tightly regulated (17) and suggests that calcium loading occurs when CKD and possibly control subjects are exposed to high calcium intake. This is consistent with studies in pediatric patients with CKD showing vascular calcium loading in children with advanced CKD (18).
There are a number of limitations in this study. The sample size was small. However, the study was tightly controlled and the findings are consistent across subjects. Due to inaccuracies in the stool dye technique, we were unable to measure a true 24-hour stool sample. While a stable calcium isotope could be used to measure GI calcium absorption the method is expensive. We therefore chose to estimate the 24 hour stool production by assuming that patients were in neutral phosphorus balance, and normalizing the stool calcium to 24-hours based on these calculations. While this was an assumption we could not test, previous research suggests it is correct (15, 19). Furthermore, if incorrect and CKD subjects were in positive rather than neutral phosphorus balance this would only serve to increase their net calcium balance. If CKD subjects were in negative phosphorus balance (due to continued urinary losses in the face of decreased absorption from calcium binding) then our results would overestimate net calcium balance. However, even a 20 or 40% overestimation would still place the CKD subjects in net positive calcium balance. It is possible that both control and CKD subjects may have been accruing bone mass when on the high calcium diet. It is also possibly that the 7 days of diet equilibration was not long enough for subjects to return to a steady state, especially on the high calcium diet, and given more time control subjects may have been able to increase their urinary calcium excretion to achieve balance. There is currently very little literature to define a time course for return to steady state following changes in dietary calcium. However, it has been shown that following a decrease in dietary calcium intake from 2000 mg to 300 mg/day, urinary calcium nadirs by week one and the counter-regulatory hormones, PTH and 1,25-D reach a maximum at 1 week (20). In general, renal conservation of a filtered substance takes longer than decreased reabsorption suggesting that 7 days may be adequate, although we cannot be certain that our control or CKD subjects were in a true steady-state. It is also possible that CKD subjects may require a longer time period than controls to reestablish balance.
However, a long term study (6 weeks) following urinary calcium excretion in CKD subjects treated with increasing dosing of calcium based binders failed to show a significant increase in urinary calcium excretion, suggesting that even after 6 weeks, CKD subjects fail to increase their urinary calcium excretion in response to increased calcium intake (10). Finally, although this study strongly suggests that CKD subjects on a high calcium diet are in positive calcium balance we cannot be certain that this calcium is being deposited in soft tissue. It is possible that bone buffering could be occurring over the time period studied. However, it is unlikely that the bones could continually accumulate calcium in CKD subjects if positive calcium balance were maintained over weeks to months as might occur with long term high calcium intake.
In summary, this study found that subjects with advance stage 3 or stage 4 CKD are in slightly negative to neutral calcium balance on 800 mg calcium intake but in marked positive balance when ingesting 2000 mg of elemental calcium. The increased calcium load when moving from an 800 to a 2000 mg calcium intake resulted in lower 1,25-vitamin D and iPTH but did not result in overall net decreased calcium absorption or an increase in the serum calcium concentration suggesting that the calcium is being deposited in tissue. This finding is consistent with what is known regarding sodium and water balance in CKD where flexibility in urinary excretion is lost and dietary intake needs to be adjusted for patients to remain in balance. If confirmed in larger balance studies these findings suggest that elemental calcium intake should be limited to 800-1200 mg in individuals with late stage 3 or stage 4 CKD not on active vitamin D analogues and possibly to 800 to 1000 mg in subjects receiving active vitamin D (although this was not studied in this trial). Finally, this study underscores the fact that the serum calcium cannot be used as a guide to evaluate calcium balance and normal serum calcium concentrations do not preclude calcium loading. This study suggests that knowledge of dietary calcium intake and the use of active vitamin D need to be considered when calcium is prescribed to patients with CKD. Furthermore in adults with CKD, total elemental calcium intake should be within 800-1200 mg/day to prevent calcium deficiency and calcium loading. These values are close to the current estimated average requirement (800-1000 mg/day) and the recommended dietary allowance 1000-1200 mg/day (RDA – amount needed to meet or exceed the requirement of 97.5% of healthy adults) in the recent Institute of Medicine Report on dietary reference intakes for calcium (21).
Methods
This study used a cross-over designed to evaluate calcium balance on an 800 mg elemental calcium per day diet versus a 2000 mg per day diet in late stage 3 and stage 4 CKD subjects versus controls with normal kidney function. Subjects were required to complete both study periods for inclusion in the data analysis.
Following informed consent a screening 25-D level was measured. If < 30 ng/ml subjects were supplemented with vitamin D (ergocalciferol 50,000 IU weekly) and rescreened at 4 weeks. If the 25-D was still < 30 ng/ml subjects could undergo a second supplementation period followed by rescreening. If the 25-D level was still < 30 ng/ml subjects were excluded. No subjects were receiving any bone modifying medications and all subjects were instructed to discontinue all calcium supplements and any vitamins or antacids containing calcium at least 1 month prior to study entry. Subject who passed the 25-D screening were assigned to the calcium diets in random order. Diet 1: 800 mg elemental calcium and 1600 mg phosphorus; Diet 2: 2000 mg elemental calcium and 1600 mg phosphorus. Each diet phase was a total of 9 days, the first 7 as an out-patient. For each active diet phase, subjects were supplied with all out-patient meals and snacks prepared by the clinical trials research center (CTRC) dietary facilities at the University of Colorado. Subjects were instructed to eat only food and non-water drinks provided but could drink water ad lib. Subjects were instructed to return any uneaten food. Following this outpatient phase, subjects were admitted to the CTRC for 48 hours. The experimental diet was continued during this stay and subjects were given de-ionized water ad lib. During the in-patient stay each subject had two 24-hour urine collections for calcium, phosphorus, and creatinine and fasting morning labs for creatinine, calcium, phosphorus, 25-D, 1,25-D, and iPTH. During this time period all stool was collected and frozen and sent to Covance Laboratory (Madison, WI. USA) for measurement of calcium and phosphorus content for determination of 24-hour stool calcium excretion (see below). Any patient with only 1 stool during the 48 hour stay was asked to collect the next sample at home in an iced container. All but 1 control subject had at least 2 stool samples used in the analysis.
Following the first in-patient stay, subjects resumed their normal diet for a period of at least 1 week but up to 4 weeks before initiating the second diet phase. During the entire study period subjects continued to receive vitamin D supplementation, either their routine home vitamin or ergocalciferol 50,000 IU every month.
Serum and urine calcium, phosphorus, and urine creatinine were measured in the University of Colorado clinical laboratory. Serum creatinine, 25-D, 1,25-D, and iPTH were measured in the Denver Children’s Hospital clinical laboratory. The tubular reabsorption of phosphorus (TRphos) was calculated from the 24-hour urine collections and the fasting phosphorus by the equation: 1-[(Uphos/serum phos) X (serum creat/Ucreat) X 100].
Stool calcium and phosphorus quantification
All stool collected for each subject was combined, weighed, dried, precharred, and ashed overnight in a muffle set to maintain 500°C. The ashed sample was re-ashed with nitric acid, treated with hydrochloric acid, taken to dryness, and put into a solution of 5% hydrochloric acid. The amount of calcium and phosphorus was determined at appropriate wavelengths by comparing the emission of the unknown sample, measured on the inductively coupled plasma spectrometer, with the emission of the standard solutions. The stool calcium/phosphorus ratio was calculated as the total stool calcium divided by the total stool phosphorus. The initial plan for the calculation of the 24 hour stool calcium excretion was to perform a double labeled stool collection by administration of brilliant blue (100 mg) 24 hours prior to CTRC admission and at the midpoint of the hospital stay. Stool collected between the two blue-stained stool samples was to be collected and analyzed and would represent 48 hours of stool. However, due to marked inaccuracy and variation with GI transit time, dye streaking, and sometimes no clear dye free stool this method it was not utilized for the study. Instead 24 hour stool excretion was estimated as follows. It was assumed that both CKD and control subjects were in neutral phosphorus balance. This assumption is in agreement with published findings (15,19) and supported by the finding that all subjects (both CKD and control) excreted equal amounts of phosphorus in the urine during both diet phases. Although dietary calcium and to a lesser extent 1,25-D concentrations are known to exert modulating effects on GI phosphate absorption, based on the fixed and equal phosphorus content of both diets and equal urinary phosphorus excretion we felt the assumption that all patients were in phosphorus balance was reasonable.
Therefore, the phosphorus intake minus the urinary phosphorus excretion allowed for a close estimate of the expected 24-hour stool phosphorus. The total stool phosphorus content was then divided by the expected 24 hour stool phosphorus to determine the time period of the stool analyzed. The total stool calcium was then divided by this time factor to determine the 24-hour stool calcium content for each subject for each diet phase. For example: the expected 24 hour stool phosphorus for a subject with 900 mg of urinary phosphorus would be calculated as intake (1600 mg) minus the 24 hour urine phosphorus (900 mg) = 700 mg. The actual total stool phosphorus content measured (ex: 1100 mg) was divided by 700 mg to determine the number of days the total collected stool represented. In this example; 1100/700 = 1.57 days. The total measured stool calcium was then divided by this number to estimate the 24 hour stool calcium excretion.
Balance calculations
Although calcium is known to be secreted into the GI tract and small amounts are lost in sweat (22) this study used the simplified definition of balance as previous reported (5) as Balance = oral intake-urine output - stool output. As the serum calcium concentrations did not change significantly on either diet it was not felt that the small changes observed needed to be included into the balance equation.
Statistical analysis
Differences within groups between diets were analyzed by the paired t-test. Differences between groups were analyzed by the non-paired t test for two groups or 1-way ANOVA with Tukey post-test for multiple comparisons. Analyses were performed with GraphPad Prism version 5.0 (San Diego, CA. USA). A p value < 0.05 was considered statistically significant.
This study was conducted in accordance with the ethical standards of the responsible committee on human experimentation and was approved by the Institutional Review Boards of the University of Colorado #070959. The trial is registered with ClinicalTrials.gov, Identifier: NCT00974532.
Acknowledgements
The authors would like to that the subjects that participated in this study. Their efforts and dedication to the research process made this study possible. The authors also wish to thank Dr. Rebecca Moore and the staff of the CTRC for their devotion to this trial. We especially thank Archana Mande, MS, RD for her efforts in making this trial a success.
This research was supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Disclosures Dr. Spiegel has received research funding from Amgen, Inc. and Keryx biopharmaceuticals and has served on advisory boards and speakers’ bureau for Amgen, Inc.
References
- 1.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2006;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 2.Craver L, Marco MP, Martinez I, et al. Mineral metabolism parameters throughout chronic kidney disease stages 1–5: Achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–1176. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- 3.Coburn JW, Koppel MH, Brickman AS, et al. Study of intestinal absorption of calcium in patients with renal failure. Kidney Int. 1973;3:264–272. doi: 10.1038/ki.1973.40. [DOI] [PubMed] [Google Scholar]
- 4.Perez AV, Picotto G, Carpentieri AR, et al. Minireview on regulation of intestinal calcium absorption. Digestion. 2008;77:22–34. doi: 10.1159/000116623. [DOI] [PubMed] [Google Scholar]
- 5.Hunt CD, Johnson LK. Calcium requirements: new estimations for men and women by cross-sectional statistical analysis of calcium balance data from metatolic studies. Am J Clin Nutr. 2007;86:1054–1063. doi: 10.1093/ajcn/86.4.1054. [DOI] [PubMed] [Google Scholar]
- 6.Wilz DR, Gray RW, Dominguez JH, et al. Plasma 1,25-(OH)2-vitamin D concentrations and net intestinal calcium, phosphate, and magnesium absorption in humans. Am J Clin Nutr. 1979;32:2052–2060. doi: 10.1093/ajcn/32.10.2052. [DOI] [PubMed] [Google Scholar]
- 7.Smothers RL, Levine BS, Singer FR. Relationship between urinary calcium and calcium intake during calcitriol administration. Kidney Int. 1986;29:578–583. doi: 10.1038/ki.1986.37. eta al. [DOI] [PubMed] [Google Scholar]
- 8.Coburn JW, Maung HM, Elangovan L, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney diseases stages 3 and 4. Am J Kidney Dis. 2004;43:877–890. doi: 10.1053/j.ajkd.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Coyne D, Acharya M, Qiu P, et al. Paricalcitol capsules for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis. 2006;47:263–276. doi: 10.1053/j.ajkd.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Oliverira RB, Cancela ALE, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigrist MK, Taal MW, Bungay P, et al. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 12.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moe SM, Chertow GM. The case against calcium-based phosphate binders. Clin J Am Soc Nephrol. 2006;1:697–703. doi: 10.2215/CJN.00560206. [DOI] [PubMed] [Google Scholar]
- 14.Kovesdy CP, Kuchmak O, Lu JL, et al. Outcomes associated with phosphorus binders in men with non-dialysis-dependent CKD. Am J Kidney Dis. 2010;56:842–851. doi: 10.1053/j.ajkd.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slatopolsky E, Robson AM, Elkan I, et al. Control of phosphate excretion in uremic man. J Clin Invest. 1968;47:1865–1874. doi: 10.1172/JCI105877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bushinsky DA. Contribution of intestine, bone, kidney, and dialysis to extracellular fluid calcium content. Clin J Am Soc Nephrol. 2010;5(Suppl 1):S12–S22. doi: 10.2215/CJN.05970809. [DOI] [PubMed] [Google Scholar]
- 17.Phang JM, Berman M, Finerman GA, et al. Dietary perturbation of calcium metabolism in normal man: compartmental analysis. J Clin Invest. 1969;48:67–77. doi: 10.1172/JCI105975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 19.Coburn JW, Brickman AS, Hartenbower DL, et al. Intestinal phosphate absorption in normal and uremic man: effects of 1,25(OH)2-vitamin D3 and 1alpha(OH)-vitamin D3. Adv Exp Med Biol. 1977;81:549–557. doi: 10.1007/978-1-4613-4217-5_53. [DOI] [PubMed] [Google Scholar]
- 20.Dawson-Hughes B, Stern DT, Shipp CC, et al. Effect of lowering dietary calcium intake on fractional whole body calcium retention. J Clin Endocrinol Metab. 1988;67:62–68. doi: 10.1210/jcem-67-1-62. [DOI] [PubMed] [Google Scholar]
- 21.IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 22.Chu JY, Margen S, Calloway DH, et al. Integumentary loss of calcium. Am J Clin Nutr. 1979;32:1699–1702. doi: 10.1093/ajcn/32.8.1699. [DOI] [PubMed] [Google Scholar]