Abstract
Malignant glioma is an incurable disease with a relatively short median survival. Several clinical trials have demonstrated that immunotherapy with vaccination is a safe and possibly effective way of prolonging survival. Antibody-based blockade of CTLA-4 ligation on T lymphocytes is associated with enhanced antitumor immunity in animal models of cancer and in patients with advanced melanoma. We hypothesized that sequential therapy with GM-CSF - expressing whole glioma cell vaccination and CTLA-4 blockade is an effective strategy for treating established intracranial gliomas. GL261 glioma cells were injected into the right frontal lobes of syngeneic C57/BL6 mice. At days 3, 6, and 9 after tumor implantation, mice were treated with subcutaneous injection of irradiated GMCSF-expressing GL261 cells. Mice were also treated with intraperitoneal injection of anti-CTLA-4 monoclonal antibodies (mAbs), either at days 3, 6, and 9 or days 12, 15, and 18. Animals were followed for survival. Splenocytes were harvested at day 22 for use in ELISPOT assays. Early treatment of established intracranial gliomas with high-dose CTLA-4 blockade was associated with increased survival in GL261-bearing mice. Later treatment with anti-CTLA-4 mAbs did not significantly improve survival compared to control-treated mice. Early vaccination followed by subsequent CTLA-4 blockade was associated with significantly improved survival versus either treatment alone and intensified tumor-specific immunity as measured by interferon-gamma ELISPOT. Sequential immunotherapy with GM-CSF-expressing irradiated glioma cells and CTLA-4 blockade synergistically prolongs survival in mice bearing established intracranial gliomas.
Keywords: glioma, vaccine, CTLA-4, GVAX, immunotherapy, GM-CSF
Introduction
After repeated efforts, immunotherapy for cancer has recently seen advances in the clinical arena. For instance, phase III trials have shown efficacy and increased survival for active cellular immunotherapy in patients with advanced hormone-refractory prostate cancer (1), and the FDA has granted approval for use in these patients. For more than a decade, blocking ligation of the costimulatory molecule cytotoxic T-lymphocyte Antigen 4 (CTLA-4) has been a promising approach by which to augment and expand the host immunity that exists against some cancers (2). In a multicenter Phase III study published in 2010, CTLA-4 blockade with ipilimumab, a humanized monoclonal antibody against CTLA-4, was associated with significantly improved survival in patients with advanced melanoma. The disease-control rate in the ipilimumab group was 28.5% (3). While cancer immunotherapy approaches are beginning to have a clinical impact, much work needs to be done to refine and better understand both these treatments and interactions between the tumors and the immune system.
Malignant glioma may present a particular challenge. These tumors are very aggressive, and average survival is only 15 months from the time of initial diagnosis (4). Furthermore, they are remarkably immunosuppressive, both systemically (5) and in the tumor microenvironment (6). Despite these obstacles, vaccination strategies (7-9) and CTLA-4 blockade (10) have shown efficacy in preclinical glioma models, and phase II clinical studies of peptide (11) and dendritic cell vaccination (12, 13) have demonstrated clinically correlative biological activity.
Combining cancer vaccination with CTLA-4 blockade has been a preclinical strategy for some time (14), and multiple reports show its promise. In theory, as whole tumor cell vaccination augments the antitumor T-lymphocyte response, subsequently treating with CTLA-4 blockade stands to strengthen the response further. We hypothesize that CTLA-4 blockade after vaccination (“sequential immunotherapy”) is a rational paradigm for potent immunotherapy pote and that this system functions in an intracranial murine glioma model. For vaccination, we employ a platform in which glioma cells are transduced to express high levels of granulocyte-macrophage colony stimulating factor (GMSCF), irradiated, and then injected subcutaneously, known as GVAX (15).
Materials and Methods
Animals
C57BL/6 mice were obtained from Charles River Laboratories. All animal studies were done in accordance with protocols approved by our Institutional Animal Care and Use Committee.
Cell lines
A syngeneic murine tumor cell line, GL261, was obtained from the National Cancer Institute (Frederick, MD). GL261-GMCSF cells, intended for use as vaccine, were created via retroviral transduction and infection as previously described (16, 17). ELISA assay confirmed expression and secretion of murine GM-CSF by GL261-GMCSF cells per manufacturer’s instructions (R&D Systems, Minneapolis, MN). In vitro expression of GM-CSF was measured as 390 (95% confidence interval 287-493) nanograms / 1 × 106 cells / 48 hours. All GL261 cell lines were cultured in RPMI-1640 medium (Mediatech Inc., Manassas, VA) without L-glutamine and supplemented with 10% FCS at 37°C and 5% CO2.
Lentiviral transduction
GL261 cells used for tumor implantation were infected with recombinant lentivirus that was designed to express firefly-luciferase (ffluc) and the fluorescent protein, mCherry, as a tag for expression of both gene products. The ffluc and mCherry-expressing recombinant lentivirus has been described previously (18). Briefly, after exposure to viral supernatant in cell culture medium with polybrene, infected GL261 cells were washed, cultured, and sorted by their expression of mCherry using fluorescence-activated cell sorting on a custom FACSAria (BD Biosciences, San Jose, CA). Expression of firefly luciferasemCherry was confirmed by fluorescence-based excitation on a standard inverted microscope.
Tumor implantation
GL261-ffluc cells were trypsinized, washed with cold RPMI-1640 twice, and counted by trypan blue exclusion. Cells were resuspended in RPMI-1640 at a concentration of 3 × 107 cells/mL. 75,000 GL261-ffluc cells were suspended in 2.5 microliters of RPMI-1640 without supplements and carefully mixed with 2.5 microliters of 3% methylcellulose. The resulting 5 microliter mixture was implanted into the right frontal lobes of C57BL/6 mice aged 6-8 weeks using an automated microsyringe (Hamilton Co., Reno, NV) and a stereotactic mouse frame (Kopf Instruments, Tujunga, CA). All intracranial tumor injections utilized the GL261-ffluc line. Tumors were reliably and statistically significantly detectable by bioluminescence at day 6 after intracranial implantation (data not shown).
Vaccination and anti-CTLA-4 treatment
On days 3, 6, and 9 after tumor implantation, appropriate mice underwent subcutaneous injection of 1 × 106 irradiated (30 Gy) GL261-GMCSF cells in a volume of 100 microliters of phosphate buffered saline (PBS). Control mice were subcutaneously injected with 100 microliters of PBS. On the appropriate days after tumor implantaion, 100 micrograms of anti-CTLA-4-Ig (9H10 clone, BioXCell, West Lebanon, NH) or isotype control (Hamster IgG1) were delivered by intraperitoneal injection in 500 microliters of PBS.
IFN-γ ELISPOT assay
Splenocytes were harvested from mouse spleens 22 days after tumor implantation. 1×106 splenocytes were stimulated for 48 hours in vitro with 1×105 irradiated (35Gy) GL261 cells or in RPMI 1640 medium, supplemented with 10% IFCS, 50 μM 2-ME, 2 mM glutamine, 20 mM HEPES, penicillin-streptomycin in 1-ml tissue culture plates (BD Falcon, San Jose, CA). After stimulation, 1×105 splenocytes from mice in each treatment and control group were loaded in triplicate onto Millipore MultiScreen-HA 96-well filter plates coated with anti-IFN-γ mAb (eBioscience, Inc., San Diego, CA). Plates were incubated at 37°C and 5% CO2 for 24 hours, washed three times with buffer, and incubated with biotinylated anti-IFN-γ monoclonal antibodies for 2 hours at 37°C. Plates were washed 4 times and incubated with Avidin-horseradish peroxidase conjugate for 45 minutes. Plates were washed three times with buffer and, then, twice with PBS before development using BCIP/NBT substrate (Sigma-Aldrich) for 10 minutes. Spots were identified and counted on an AID Version 3.1.1 ELISPOT reader.
Statistical Analysis
Mice were followed daily for survival by a blinded observer, and survival was analyzed with Mantel-Haenszel statistics and Kaplan-Meier curves. For ELISPOT analysis, differences in the numbers of spot-forming splenocytes were examined by the Student’s t-test. All statistical analyses were performed using GraphPad software (GraphPad, La Jolla, CA).
Results
Early CTLA-4 blockade prolongs survival in syngeneic mice bearing intracranial GL261 tumors
Fecci, et al. have previously demonstrated that antibody-based blockade of CTLA-4 binding eradicates SMA-560 glioma tumors implanted in the brains of Vm/DK mice (10). 100 micrograms of antibody were delivered systemically on days 3,6, and 9 after tumor implantation. While the GL261 model that we employed for this study is associated with similar systemic immune effects as are both SMA-560 cells and human glioblastoma (19), we wanted to examine the impact of CTLA-4 blockade in this system. 75,000 viable GL261- ffluc cells were injected into the right frontal lobes of C57/BL6 mice on day 0 and, on days 3,6, and 9, we delivered 100 micrograms anti-CTLA-4 mAb via intraperitoneal injection. At this schedule and dose, most mice survived long-term, whereas all control mice succumbed by day 50, with median survival of 26 days (Figure 1a). However, when syngeneic mice are treated with 100 micrograms of anti-CTLA4 mAb on days 12, 15, and 18 after tumor implantation, survival is equivalent to that of mice treated with control antibody (Figure 1b). High-dose antibody-based CTLA-4 blockade prolongs survival in mice bearing GL261 tumor when tumors are initially taking and are small, but is ineffective against larger, more established tumors.
Figure 1.
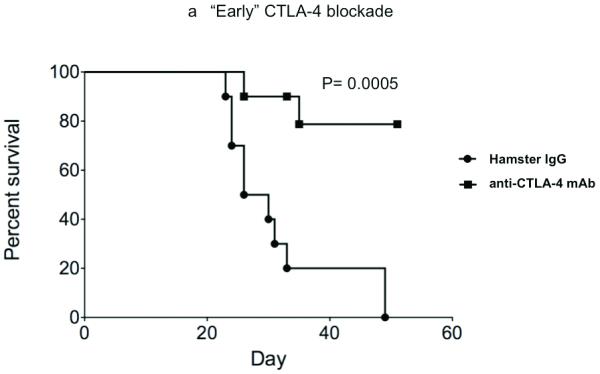
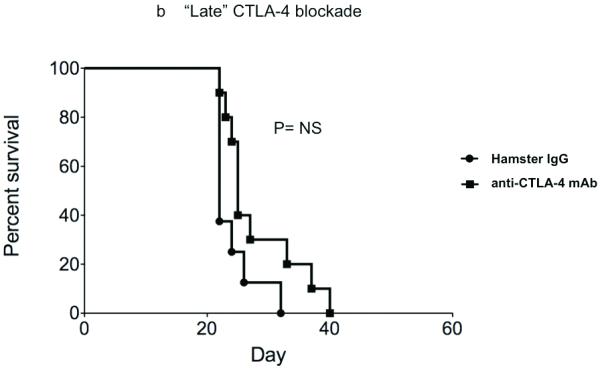
CTLA-4 blockade effectively increases survival in mice bearing recently established intracranial GL261 gliomas, but is less effective when delivered at later timepoints and at lower doses. (A) Intraperitoneal injection of 100 micrograms of anti-CTLA-4 mAb days 3, 6, and 9 after tumor implantation was associated with prolonged survival in C57/BL6 mice bearing intracranial gliomas. (B) CTLA-4 blockade did not significantly improve survival in mice with malignant glioma when antibody was administered on days 12, 15, and 18 after intracranial tumor implantation. (Each experiment documented in figure was performed at least twice).
Following whole tumor cell vaccination with CTLA-4 blockade enhances antitumor immunity in mice bearing established intracranial GL261 tumors
Subcutaneous and intradermal injection of irradiated whole tumor cells that are engineered to express GM-CSF is an established technique of activating effective antitumor immunity, including against intracranial tumors (20). We hypothesized that T-lymphocytes stimulated by vaccination would be further and more durably activated by subsequent systemic CTLA-4 blockade. Therefore, after vaccinating mice with irradiated GL261-GMCSF cells on days 3, 6, and 9 after intracranial tumor implantation, we systemically delivered 100 micrograms of anti-CTLA-4 mAb on days 12,15, and 18 (Figure 2a). By ELISPOT analysis, a significantly higher number of splenocytes harvested from combination-treated tumor-bearing mice secreted IFN-γ than those taken from mice treated with either vaccination or CTLA-4 blockade as monotherapies (Figure 2b). Interestingly, at this late timepoint (day 22), splenocytes from animals treated with CTLA-4 blockade alone were no more reactive than mice from vehicle-treated controls.
Figure 2.
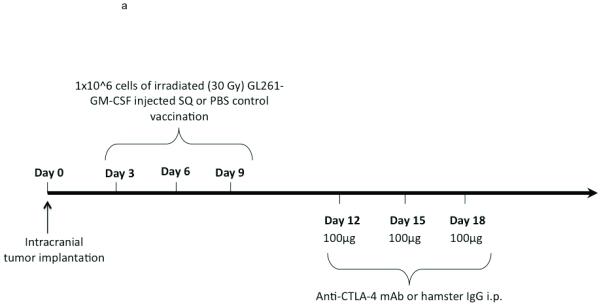
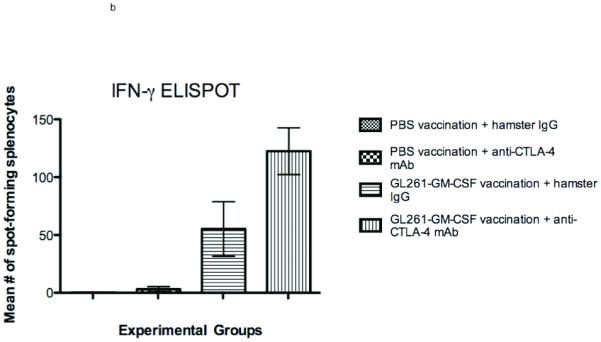
Interferon-gamma ELISPOT. (A) Treatment schedule for sequential combined immunotherapy with GM-CSF-expressing GL261 cells and anti-CTLA-4 mAb. Intracranial implantation of 75,000 GL261-ffluc cells into the right frontal lobe occurred on day 0. (B) Splenocytes harvested from mice treated with sequential vaccination and CTLA-4 blockade were significantly more likely to express interferon-gamma in response to stimulation by irradiated GL261 cells than were mice treated with either vaccination (p=0.03) or CTLA-4 blockade alone (p=0.002)
Sequential treatment with whole-cell vaccination and systemic CTLA-4 blockade enhances survival in glioma-bearing mice
Earlier work has demonstrated the potency of combining GM-CSF-expressing whole tumor cell vaccination with CTLA-4 blockade in subcutaneous tumor models. In each of these models, vaccination and anti-CTLA-4-mAb were given concurrently. It is clear that, in murine glioma models characterized by implantation of a low number of tumor cells and relatively short lifespans, early treatment with CTLA-4 blockade can be highly effective on its own, but that there is little impact when therapy is initiated at later timepoints. We hypothesized that delayed CTLA-4 blockade in the setting of established antitumor immunity would prolong survival, in correlation with the strong interferon-gamma Elispot response that we observed. Having demonstrated no significant survival difference vs. control when GL261-bearing mice were treated with 100 micrograms of 9H10 antibody on days 12, 15, and 18 after tumor implantation, we chose this dose for evaluation in our sequential immunotherapy model. When mice were treated sequentially, following early GL261-GMCSF vaccination with CTLA-4 blockade on days 12,15, and 18 was associated with significant improvement in survival (Figure 3, Table 1).
Figure 3.
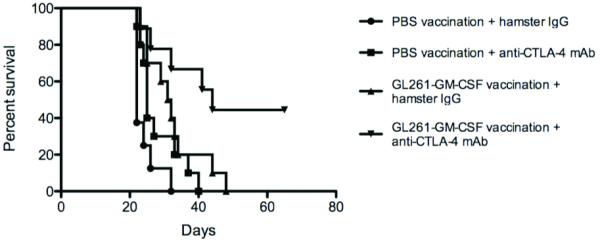
C57/BL6 mice bearing established intracranial GL261 tumors had better long-term survival after treatment with sequential vaccination and CTLA-4 blockade (9H10 antibody given on days 12, 15, and 18) than did mice treated with either as monotherapy (figure is representative of 3 experiments, treating 8-10 mice per group).
Table 1.
Survival analyses after sequential immunotherapy. Mantel-Haenszel analysis demonstrates significant survival differences between groups. CTLA-4 blockade at days 12,15, and 18 alone did not significantly improve survival over control, though there was a trend towards longer survival. Following vaccination with CTLA-4 blockade was significantly better than either modality alone.
| Comparison Group A |
Comparison Group B |
Mantel- Haenszel Survival |
Significance |
|---|---|---|---|
| PBS Vaccination + hamster IgG |
PBS Vaccination + anti-CTLA mAb |
p=.053 | Trend |
| PBS Vaccination + hamster IgG |
GL261-GMCSF vaccination + hamster IgG |
p=.005 | Yes |
| PBS Vaccination + hamster IgG |
GL261-GMCSF vaccination + anti- CTLA-4 mAb |
p=.0008 | Yes |
| PBS Vaccination + anti-CTLA-4 mAb |
GL261-GMCSF vaccination + hamster IgG |
p=.218 | No |
| PBS Vaccination + anti-CTLA-4 mAb |
GL261-GM-CSF vaccination + anti- CTLA-4 mAb |
p=.004 | Yes |
| GL261-GM-CSF vaccination + hamster IgG |
GL261-GM-CSF vaccination + anti- CTLA-4 mAb |
p=.032 | Yes |
Discussion
In the phase III clinical trial establishing the efficacy of CTLA-4 blockade in patients with advanced melanoma, combining ipilimumab with peptide vaccination did not positively impact disease progression or survival when compared to treatment with drug alone (3). Ipilimumab, alone, did not alter progression- free survival in these patients, but afforded improved overall survival. CTLA-4 blockade appears to have activity in the brain, both in case reports of patients with intracranial metastases (21) and in preclinical models of glioma (10), which we have confirmed here. However, blockade of CTLA-4 ligation shortly after a tumor is implanted may not accurately model advanced or even clinically evident cancer. For instance, when we treated mice with “advanced” brain tumors 2 to 3 weeks after implantation, the impact on survival was modest, and there were no cured mice. Disinhibition of tumor-specific lymphocytes by CTLA-4 blockade at this late time point is insufficient to slow the growth of intracranial gliomas.
While early vaccination (days 3, 6, and 9) significantly improved survival in C57/BL6 mice, there are rare cures in this model of treating established intracranial tumors. However, when GMCSF-expressing whole tumor cell vaccination was followed by subsequent CTLA-4 blockade, the effect was clearly synergistic, resulting in almost 50% long-term survival and cure. This series of results is consistent with our hypothesis that whole-cell vaccination causes an expansion in the number of circulating activated tumor-specific immune effectors and in the number of tumor-associated epitopes that are identified. Once these tumor-specific lymphocytes are activated, treatment with systemic anti-CTLA-4 mAbs allows them to be effective for a longer period of time. It is mechanistically telling that tumor-specific response, as measured by splenocyte release of interferon-γ after stimulation by glioma cells, was not appreciably affected by treatment with CTLA-4 blockade alone, but was markedly amplified after sequential vaccination and anti-CTLA-4 mAb.
To the best of our knowledge, this is the first report of combined vaccination and CTLA-4 blockade as treatment for intracranial gliomas. This concept was first reported in subcutaneous tumors by van Elsas, et al. (14) who demonstrated that combining anti-CTLA-4 mAbs with irradiated GM-CSF-secreting B16 melanoma cells effectively treated subcutaneous and metastatic B16 melanoma tumors in syngeneic mice.
Furthermore, the same group has examined, in detail, the mechanisms by which combining GVAX and anti-CTLA mAbs affect antitumor immunity, demonstrating increased tumor infiltration by lymphocytes and inversion of the intratumoral Teffector/Tregulatory ratio (22). Blockade of CTLA-4 on both effector and regulatory lymphocyte subsets contributes to the impact of the combination therapy (23).
As mentioned above, a single previous report documents the efficacy of CTLA-4 blockade as treatment against murine intracranial glioma (10). This strategy has yet to be examined in patients with malignant glioma, though a series of patients with melanoma metastastes to brain have been treated (24).
We also believe that this is the first preclinical demonstration of the synergistic efficacy of delivering whole tumor cell vaccines prior to CTLA-4 blockade, rather than treating essentially at the same time. It is our proposal that CTLA-4 blockade will be most useful in the context of an immune system that is primed against the tumor. In patients, how long this priming takes is unclear, and it may require several booster vaccinations. In mice, however, maximum antitumor immunity is thought to develop 14-21 days after vaccination. Vaccination can play the role of “primer” in patients with malignant glioma, and a number of clinical trials have demonstrated consistent development of host antitumor immunity after treatment with immunotherapy (11, 12).
Although this sequential treatment schedule has not been reported in the preclinical setting, there are suggestions of powerful impact in selected patients with melanoma and ovarian cancer (25). In a clinical study, the majority of patients with advanced melanoma that were treated with CTLA-4 blockade after GVAX generated “clinically meaningful” antitumor immunity, and histopathologic tumor necrosis in patients with ovarian carcinoma was tightly correlated with intratumoral CD8+/FoxP3+ lymphocyte ratios.
The current study demonstrates that following whole tumor cell vaccination with CTLA-4 blockade can successfully treat murine intracranial gliomas. We believe that sequential vaccination and CTLA-4 blockade is a rational immunotherapeutic strategy that merits rigorous clinical investigation, including in patients with malignant glioma.
Acknowledgments
William Curry Financial Support: Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation; Physician Scientist Development Award, Executive Committee on Research, Massachusetts General Hospital
Glenn Dranoff Financial Support: NIH CA143083
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Agarwala SS, Ribas A. Current experience with CTLA4-blocking monoclonal antibodies for the treatment of solid tumors. J Immunother. 33:557–69. doi: 10.1097/CJI.0b013e3181dcd260. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Waziri A. Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin N Am. 21:31–42. doi: 10.1016/j.nec.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Albesiano E, Han JE, Lim M. Mechanisms of local immunoresistance in glioma. Neurosurg Clin N Am. 21:17–29. doi: 10.1016/j.nec.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Mitra S, Wong AJ. The epidermal growth factor variant III peptide vaccine for treatment of malignant gliomas. Neurosurg Clin N Am. 21:87–93. doi: 10.1016/j.nec.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Kim W, Liau LM. Dendritic cell vaccines for brain tumors. Neurosurg Clin N Am. 21:139–57. doi: 10.1016/j.nec.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwalla PK, Barnard ZR, Curry WT., Jr. Virally mediated immunotherapy for brain tumors. Neurosurg Clin N Am. 21:167–79. doi: 10.1016/j.nec.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13:2158–67. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 11.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 28:4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955–64. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 13.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–25. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 14.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinushi M, Hodi FS, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev. 2008;222:287–98. doi: 10.1111/j.1600-065X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 16.Herrlinger U, Kramm CM, Johnston KM, et al. Vaccination for experimental gliomas using GM-CSF-transduced glioma cells. Cancer Gene Ther. 1997;4:345–52. [PubMed] [Google Scholar]
- 17.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maguire CA, Meijer DH, LeRoy SG, et al. Preventing growth of brain tumors by creating a zone of resistance. Mol Ther. 2008;16:1695–702. doi: 10.1038/mt.2008.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maes W, Van Gool SW. Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother. 60:153–60. doi: 10.1007/s00262-010-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampson JH, Archer GE, Ashley DM, et al. Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the “immunologically privileged” central nervous system. Proc Natl Acad Sci U S A. 1996;93:10399–404. doi: 10.1073/pnas.93.19.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodi FS, Oble DA, Drappatz J, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol. 2008;5:557–61. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 22.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margolin KA, Di Giacomo AM, Maio M. Brain metastasis in melanoma: clinical activity of CTLA-4 antibody therapy. Semin Oncol. 37:468–72. doi: 10.1053/j.seminoncol.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]


