Abstract
Background
A key finding from recent studies of epigenetic mechanisms of memory is that increasing histone acetylation after a learning experience enhances memory consolidation. This has been demonstrated in several preparations, but little is known about whether excitatory and inhibitory memories are equally sensitive to drugs that promote histone acetylation and how transcriptional changes in the hippocampal-medial prefrontal cortex (mPFC) network contribute to these drug effects.
Methods
We compare the long-term behavioral consequences of systemic, intra-hippocampal and intra-medial-prefrontal cortex (mPFC) administration of the histone deacetylase (HDAC) inhibitor sodium butyrate (NaB) after contextual fear conditioning and extinction 1 and/or 14 d later in male c57BL/6J mice (n=302). Levels of histone acetylation and expression of the immediate-early gene c-Fos were assessed by immunohistochemistry following infusion of NaB into the hippocampus (n=26).
Results
Across a variety of conditions, the effects of NaB on extinction were larger and more persistent compared to the effects on initial memory formation. NaB administered following weak extinction induced behavioral extinction and infralimbic histone acetylation and c-Fos expression consistent with strong extinction. No similar effect was seen in the prelimbic cortex. The involvement of the infralimbic cortex was confirmed as infusions of NaB into the infralimbic, but not prelimbic cortex, induced extinction enhancements.
Conclusions
These studies show that the memory modulating ability of drugs which enhance acetylation is sensitive to a variety of behavioral and molecular conditions. We further identify transcriptional changes in the hippocampal-infralimbic circuit associated with extinction enhancements induced by the HDAC inhibitor NaB.
Keywords: memory, extinction, reconsolidation, fear conditioning, epigenetics, chromatin, histone acetylation, exposure therapy, prefrontal cortex
Current behavioral therapies for many anxiety disorders, including post-traumatic stress disorder (PTSD), attempt to dampen the powerful and often debilitating affective responses to trauma related cues [1]. This is often achieved through behavioral extinction, in which repeated clinical re-exposure to the anxiety-inducing cues suppresses the original fearful memory. However, extinction is often incomplete and the cue-induced affective response spontaneously recovers over time [2,3]. Thus, a major goal of extinction research is to determine combinations of pharmacotherapy and behavioral interventions that enhance extinction memory formation creating a more robust and persistent decrease in cue-induced affective responses [4]. One complication with this combined approach is that in addition to enhancing extinction, many pharmacological treatments may also enhance the formation of new aversive memories [e.g., 5].
Recent research indicates that histone deacetylase (HDAC) inhibitors enhance memory at a molecular, cellular, and behavioral levels including conditioning, extinction, and recently retrieved conditioned fear memories [e.g., 6,7–9]. These studies indicate a role for histone acetylation in memory enhancements, but several issues remain unresolved. First, little is known about the persistence of the memory enhancing effects. Most studies of HDAC inhibitors and memory have examined performance soon after extinction [but see 10]. Little is known about these enhancements past 7 days [6] and specifically how these enhancements are affected by repeated testing which may weaken spontaneous recovery effects [e.g. 11,12,13]. Second, few studies have compared the effects of HDAC inhibition on initial memory formation to extinction memory formation. A better understanding of HDAC inhibitor induced enhancements of fear memory and fear extinction is critical in evaluating whether HDAC inhibition will preferentially decrease affective responses to environmental stimuli. Studies closely matching multiple factors (including previous learning experiences, internal state, and environmental conditions) are essential in evaluating whether a given treatment will preferentially decrease affective responses to environmental stimuli [13–16].
A remaining challenge for the field is to understand the molecular processes that mediate enhanced extinction effects induced by HDAC inhibition [17]. There is increasing evidence that transcriptional changes in the hippocampus and medial prefrontal cortex (mPFC) as well as signaling from the hippocampus to the mPFC are critical for extinction memory formation and modulation [e.g., 18,19,20]. However, it is unknown whether manipulating chromatin modifications such as histone acetylation in the hippocampus during extinction modulates transcription in specific subregions of the mPFC.
In the following experiments, we investigate the ability of the HDAC inhibitor sodium butyrate (NaB) to produce lasting enhancements in memory following initial learning or extinction under different conditioning (strong or weak), extinction (strong or weak), and administration protocols (pre-session systemic and post-session systemic and intra-hippocampal/mPFC). Because of the critical importance of matching learning experiences when comparing drug effects on fear conditioning and extinction [14,16], different groups received equal total exposure to the context and shocks surrounding NaB administration. We then investigated the effects of intra-hippocampal NaB after extinction on histone acetylation and c-Fos expression in the mPFC to understand how modulating the hippocampus affects transcriptional events in brain regions important for extinction consolidation. Finally, we infused NaB into the mPFC to examine the specificity of these effects.
Methods
Subjects
A total of 328 male C57BL/6 mice (Jackson Laboratory; Bar Harbor, ME (eight-twelve weeks) were housed and cared for under protocols approved by the OHSU IACUC and in accordance with NIH “Principles of Laboratory Animal Care” (detailed in S1).
Cannulations
The bilateral hippocampal cannulation technique followed 13 (see S1). Angled cannulations directed at the mPFC were used to avoid damage to mPFC dorsal structures (detailed in S1).
Injections
Systemic
Sodium butyrate (Millipore, Billerica, MA) was delivered at 1.2 g/kg in1X phosphate buffered saline as vehicle.
Intracranial
Mice received either bilateral intrahippocampal injections or unilateral mPFC (0.25 μL per side) of either NaB (55 mM) or vehicle (sterile saline) over 1 min at a rate of 0.25 μL per min. Injectors were left in place for 30s to ensure diffusion away from the cannula.
Procedure
Fear Conditioning
Mice received 0.35 mA footshocks in a chamber equipped with behavioral monitoring equipment (Context, CTX; described in 13, S1).
Habituation
Mice were habituated to handling and injection procedures as in 13 (detailed in S1).
Matching Approach
To compare NaB effects on conditioning and extinction, groups were matched for total exposure to the context and shocks surrounding NaB administration (Experiments 1, 2, and 3A; detailed in 13). On Day 1, the Extinction group received exposure to the CTX paired with one or two shocks (CTX +) while the Conditioning group was exposed to the CTX in the absence of the shock (CTX−). On Day 2 (reversal) the conditions were reversed such that the Conditioning group received a CTX+ experience while the Extinction group received a CTX− (no shock) experience. With the exception of Experiment 3C (see below), the CTX exposures on Days 1 and 2 were 3 min to equate total CTX exposure in the Conditioning and Extinction experimental groups. Mice received NaB or vehicle treatment either prior to (Experiment 1) or immediately after the reversal session (Experiments 2, 3, and 4). Testing occurred 1 and/or 14 days following the reversal day to examine the initial expression and persistence of the CTX-shock memory. During the test sessions, mice were placed in the CTX for 12 min in the absence of shock (CTX−).
Experiment 1: Pre-session systemic injections with a strong conditioning protocol
The habituation, apparatus, drug injection and general methods used in this experiment are described above. Fifteen min prior to the Day 2 reversal session, mice were injected with either 1.2 g/kg NaB or vehicle to maximally increase acetylation during the critical memory formation time period [15 min to 1 hour post-learning, 21,22]. Mice were assigned to groups that matched levels of Day 1 freezing. Mice were tested 1D and re-tested 14 D following Day 2. A separate group of mice was tested 14 D after Day 2 in the absence of the 1 D test (14 D Initial test).
Experiment 2: Post-session systemic injections
A) Strong conditioning
Methods were identical to Experiment 1, except injections occurred immediately after the Day 2 reversal session to avoid effects of NaB on freezing during that session while isolating effects of NaB on memory consolidation.
B) Weak conditioning
Methods were identical to those used in Experiment 2A except a single 0.35 mA shock was used during conditioning to evaluate whether NaB would enhance consolidation of a weaker contextual fear memory.
Experiment 3. Post-session intrahippocampal injections
A) Strong Conditioning
Conditioning and extinction treatments were identical to those used in Experiment 2. NaB and vehicle infusions were made directly into the hippocampus to evaluate the involvement of the hippocampus in driving NaB mediated memory enhancements. Testing was conducted as above except only the Extinction group was run in the 14 Day Initial test group as the only persistent effect was seen in the Extinction Group in all prior experiments.
B) Weak conditioning
Methods were identical to those used in Experiment 4A except a single .35 mA shock was used during conditioning to evaluate whether NaB would enhance consolidation of a weaker contextual fear memory.
C) Strong extinction
To determine whether NaB could enhance a strong extinction memory, NaB or vehicle was administered after a long 24-min extinction session. Methods were identical to those used in Experiment 4A except a 24-min extinction session was used.
D) Delayed microinfusions
To ensure that the behavioral effects of NaB on extinction were due to its effects on extinction memory consolidation and not a non-specific effect, intrahippocampal injections were administered 4 hours following a 3 min retrieval session [7].
Experiment 4. Histone Acetylation and c-Fos Immunohistochemistry
Immunohistochemistry (IHC) for histone acetylation as well as the product of the immediate early gene c-Fos was performed in select brain regions to determine how NaB infusion into the hippocampus affected the molecular signature of an extinction memory. Briefly, mice were sacrificed 30 min following weak (3 min) or strong (24 min) extinction paired with intrahippocampal NaB or vehicle injection (behavioral methods identical to Experiments 3A and C). Brains were subsequently fixed in formaldehyde and cryoprotected in sucrose. After sectioning the brain into 20 um slices on a cryostat, routine IHC was performed on slices standardized to the same bregma range within the CA1 region of the hippocampus and mPFC [described in 23]. Histone acetylation and c-Fos expression were analyzed using antibodies to acetylated Lys14 on histone H3 (1:1000 dilution; Millipore) or c-Fos (1: 2,000 dilution; Santa Cruz Biotechnology). The Vectastain ABC kit (Vector Laboratory, Burlingame, CA) and metal enhanced DAB kit (Pierce, Rockford, IL) were used for immunoreaction detection. Three slices per brain region were analyzed in all experiments with data (either density or cell counts; see below) averaged per animal across slices.
Experiment 5. mPFC Infusions
Procedures were identical to those used in Exp. 3A except that injections were directed at the mPFC.
Data Analysis
Fear was evaluated by measuring freezing behavior (absence of movement ≥3s) using the infrared activity monitors. Freezing was analyzed in three-minute blocks in all sessions. Due to rapid within-session extinction during test sessions, data during the first three minutes (the duration of the context exposures during Days 1 and 2) are presented [7,24]. Quantification of c-Fos was performed by counting c-Fos positive nuclei in each brain region. Histone acetylation was quantified by density of staining (pixel density) due to high level of constitutive acetylation. Images were corrected for variability in staining by calibrating quantification based on highest and lowest (background) density of staining between experimental groups. Group differences were analyzed with a 2X2 analysis of a variance (ANOVA) with Drug Treatment and Conditioning Order (Experiments 1,2, 3A) or Drug Treatment and Extinction Duration (Experiment 4) as between subjects factors. Simple planned comparisons were tested using a student’s t-test. For all statistical tests the α was set ≤ 0.05.
Results
Experiment 1: Pre-session systemic injections with a strong conditioning protocol
In this and all subsequent experiments, there was very little freezing (<5%) during Day 1, before shocks were delivered (data not shown). During Reversal, the Extinction groups showed high levels of freezing independent of drug treatment, whereas in the Conditioning groups, NaB-treated mice froze more than vehicle-treated mice (Figure 1). This was confirmed by a significant main effect of Drug [F(1,38)=6.39, p=0.016] driven by the higher freezing in the NaB Conditioning group [t(19)=2.95, p=0.008] and lack of significant difference between the Extinction groups [ p>0.3]. This was not due to NaB having non-specific effects on locomotion, response to the shock (Figure S1 in the Supplement) or anxiogenic effects of NaB [19,25]. This suggests that the increased freezing may be a non-specific action of the drug during conditioning or a pre-existing difference in baseline levels of freezing between NaB and Veh treated mice.
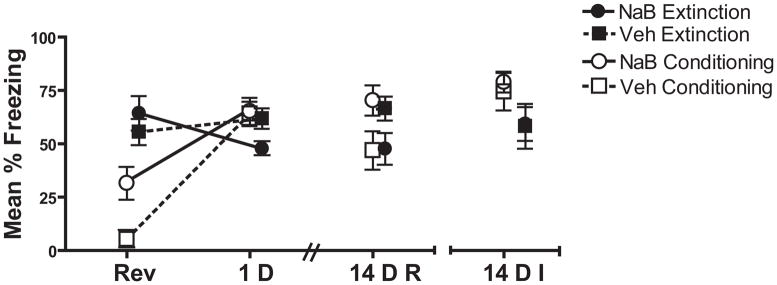
Figure 1. Pre-Extinction NaB injections induced persistent extinction enhancements to 14 D in the presence of repeated testing.
Mice in the Conditioning group who received NaB (n=11) injections prior to the 2 context-shock pairings froze significantly more than Veh (n=11) on Reversal. When injections preceded memory retrieval, Veh (n=11) and NaB (n=10) treated mice (Extinction Group) did not differ in performance on Reversal. When tested 1D later, NaB treated mice in the Extinction group showed a significant decrement in freezing relative to vehicle treated controls indicative of enhanced extinction. This effect persisted when mice were re-tested 14D later (14DR) but not when the 14 D test was the initial test (14 DI). No reliable difference between NaB and Veh treated mice was seen on any test in the Conditioning Group.
NaB delivered prior to extinction decreased freezing during the 1D and 14D re-test and, when delivered prior to the conditioning session, increased freezing during the 14D re-test. During Test 1, NaB treated mice in the Extinction group froze significantly less than vehicle-treated mice [t(19)=2.35, p=0.03], but there was no drug effect in the Conditioning group. The lack of difference in the Conditioning NaB and Veh treated mice is not likely due to latent inhibition induced by pre-exposure as mice pre-exposed to the context prior to conditioning showed no difference from those that were not pre-exposed (Figure S2 in the Supplement).
During the 14D re-test, mice in the conditioning group that received NaB displayed greater freezing. NaB generated a persistent decrease in freezing within the Extinction groups (Figure 1, 14DR). A significant Drug Treatment × Conditioning Order interaction confirmed this effect [F(1,39)=6.78, p=0.013]. This persistent extinction enhancement was not observed in mice that received the 14D test as their first test after NaB treatment [Figure 1, 14DI; ps >0.1]. Thus, long- term enhancements were revealed by repeated testing, but were not present when the 14D test was not preceded by a 1D test.
Experiment 2: Post-session systemic injections
A) Strong conditioning protocol
During the first test, the NaB treated mice showed a significant extinction enhancement (Figure 2A). There was no interaction or main effect of Conditioning Order or Drug Treatment [ps >0.1], or significant difference between drug and vehicle treated Extinction mice. However, a difference score between Reversal and the 1D test revealed that NaB treated extinction mice showed a significantly greater decrease in freezing from Reversal to Test than did the vehicle treated mice [t(22)=2.53 p=0.019]. No difference between groups was observed on the 14 D test when this test was either a retest or initial test [all ps >0.1].
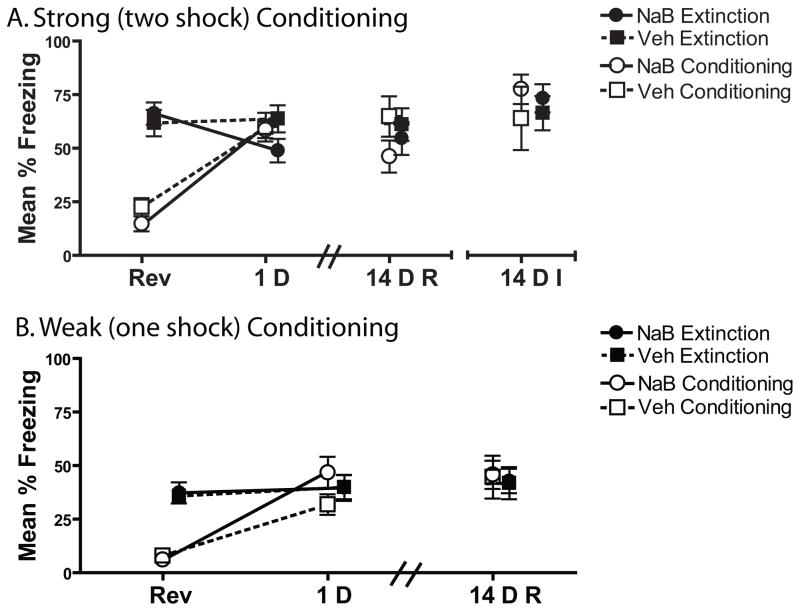
Figure 2. Post-Extinction Systemic NaB injections cause an initial extinction enhancement.
A) Mice received two shocks on CTX+ days. During Reversal, NaB and Veh treated mice did not differ within Conditioning (NaB n = 12, Veh n=12) or Retrieval groups (NaB n=12, Veh n=13). Mice injected with NaB immediately after retrieval showed an extinction enhancement relative to vehicles when tested 1D later (1D). This effect was not persistent to 14D when the mice were re-tested (14DR) or when the 14 D test was the initial test (14DI). B) To examine whether NaB would enhance a weak CTX-shock memory all mice received only one shock on CTX+ days. Freezing levels were identical within Conditioning and Extinction Drug groups. Mice injected with NaB (n=13) immediately following weak conditioning (1 CTX-shock pairing) did not differ from Veh (n=13) treated mice when tested 1D later. No difference between Drug groups was observed when mice were tested again 14D (14DR) later or when the 14 d test was the first test (14DI). There was no difference between NaB (n=14) and Veh (n=14) treated mice on any test in the Extinction group.
B) Weak conditioning protocol
During Test 1, only the NaB treated Conditioning group showed an increase in freezing from Reversal day (Figure 2B), but this was not reliably different from vehicle treated mice [p=0.09]. Examination of the first minute of the 1D test showed that the NaB Conditioning mice [M=60.1, SE = 7.8] froze significantly more than the Veh treated Conditioning mice [M=38.8, SE=5.9] suggesting that NaB caused a modest conditioning memory enhancement under very sensitive temporal parameters [t(24)=2.2, p=0.038]. No effect of NaB was observed in the Extinction group [ps>.1].
During the first 3 min of either the 14 D Re-Test or 14 D Initial Test, no differences were observed between any groups [all ps>0.1]. Although the weaker conditioning protocol produced lower levels of freezing compared to the stronger, 2-shock protocol, NaB still had no significant effect on a newly formed fear memory suggesting that these null effects were not due to a behavioral ceiling.
Experiment 3. Post-session intrahippocampal infusions
A) Strong conditioning protocol
Intrahippocampal injection of NaB induced a persistent extinction enhancement (Figure 3A). A Conditioning Order × Drug interaction [F(1,34)=4.75, p=0.04] during the 1 D test confirmed the initial extinction enhancement as the NaB treated Extinction mice froze significantly less than vehicle-treated mice [t(15) = 4.1, p=0.001] while there was no difference between Drug groups in the Conditioning Group [p>0.05].
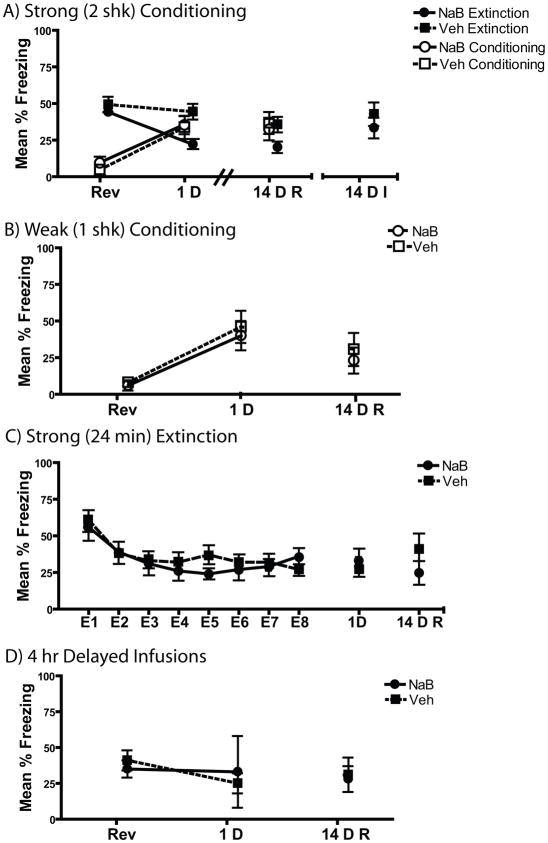
Figure 3. Intra-hippocampal NaB injections selectively cause persistent extinction enhancements only in the presence of weak extinction and repeated testing.
A) During Reversal, mice receiving either NaB or Veh following conditioning (n=9 NaB and Veh groups) or retrieval (NaB n=9; Veh n=8) did not differ. Mice receiving post-extinction NaB injections showed a significant extinction enhancement relative to controls when test 1D later. This extinction enhancement persisted to 14 D only when mice were retested (14DR). No conditioning enhancement was seen on any test. B) NaB (n=9) infused into the hippocampus immediately after weak (1 shk) conditioning did not result in a significant difference in freezing from Veh (n=9) when test 1 and 14 D later. C) Strong (24 min) extinction led to persistent decreases in freezing 1 and 14D later. Post-extinction NaB (n=10) hippocampal infusions was not able to induce any change in freezing relative to Veh (n = 12). D) Infusion of NaB (n=5) into the hippocampus 4 hr following 3 min extinction had no significant effect on freezing relative to Veh (n=8) when tested 1D later.
When re-tested 14 days later, this effect persisted with NaB treated mice in the Extinction group freezing less than vehicle treated controls. While there was no interaction or main effect of Conditioning Order and Drug Group [all p>0.1] there was significantly less freezing in the NaB treated Extinction mice [t(15)=2.65, p=0.018]. In contrast, when the 14 D test was the initial test, this effect was not present [p=0.69]. No differences were observed in the Conditioning group on either of the 14D retention tests [p>0.05]. Effects of hippocampal NaB are consistent with the results of Experiment 1 which showed a persistent extinction enhancement only when mice were repeatedly tested.
B) Weak conditioning protocol
Post-session intra-hippocampal NaB did not enhance a newly formed weak contextual fear memory when tested either 1D or 14D following acquisition [Figure 3B; ps >0.6].
C) Strong extinction protocol
NaB infused directly into the hippocampus following strong extinction did not enhance fear extinction when tested 1D or re-tested 14D following extinction (Figure 3C). The long (24 min) extinction produced robust extinction and no difference between Drug groups during the extinction session (p=0.2). The groups did not differ during either the 1D or 14D test (ps>0. 25).
D) Delayed intrahippocampal injections
When injections were administered 4 h after extinction, there was no difference between NaB and vehicle treated animals [Figure 3D, t(10)=.37 p=0.72] indicating that these effects were due to NaB’s effects on extinction and not some nonspecific drug effect.
Hippocampal Acetylation and c-Fos
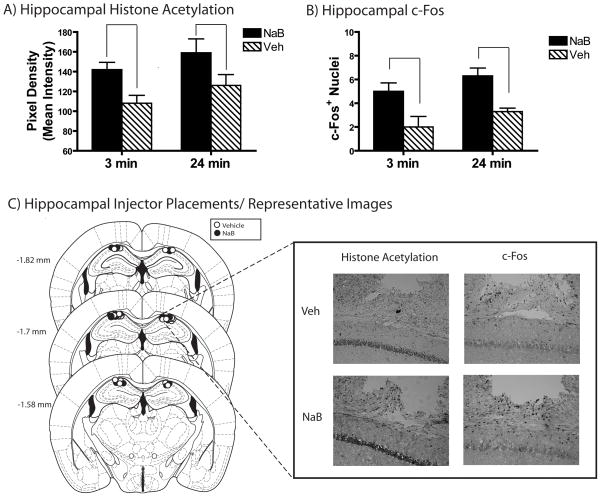
NaB targeted dorsal hippocampal CA1 [26] enhanced acetylation and c-Fos in CA1 (right panel Figure 4A; left panel shows injector placements). A significant main effect of Drug Treatment confirmed greater acetylation stain density [F(1,23)=10, p=0.005] and c-Fos+ nuclei [F(1,15)=17.02, p=0.002] in NaB treated mice across extinction durations. There was no interaction between Drug Treatment and Extinction Duration or main effect of Extinction Duration on acetylation of c-Fos [all ps >0.1; Figures 4B & C].
Figure 4. Injecting NaB Into the Dorsal Hippocampus Increases CA1 Histone Acetylation.
A) NaB (n = 6,7) injected into the dorsal hippocampus enhanced acetylation (A) and c-Fos (B) in the dorsal hippocampus relative to vehicle (n=6,7) regardless of whether extinction was strong (3 min) or weak (24 min). C) Individual injector placements are shown in the left panel. Representative immunohistochemistry demonstrating that dorsal hippocampal infusions of NaB increases H3 Lys14 acetylation and c-Fos in the dorsal hippocampus are shown in the inset right panel. As no difference was observed between Extinction Duration or interaction between Extinction Duration and Drug Treatment, only representative images from NaB and Veh injections are shown. Stereotaxic image reproduced with permission from [26].
mPFC Histone Acetylation and c-Fos Expression
Infralimbic Cortex
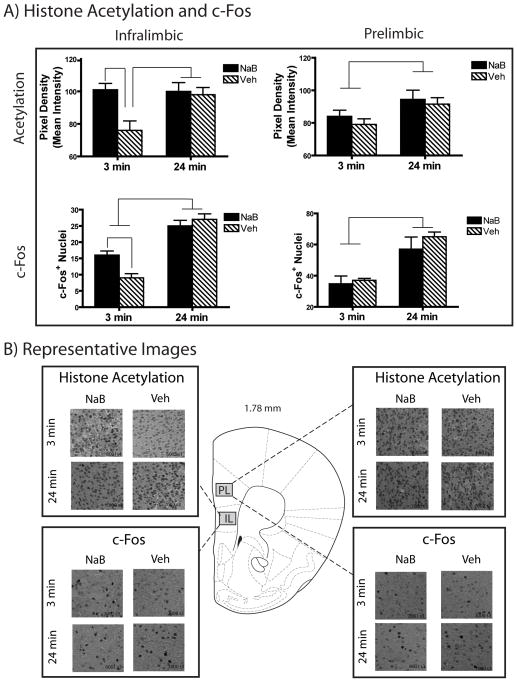
Strong extinction (24 min) resulted in more histone acetylation as well as c-Fos+ nuclei in the infralimbic cortex [26] than did weak extinction (3 min). Furthermore, intra-hippocampal NaB increased acetylation and c-Fos following weak extinction but not following strong extinction (Figure 5A left panels).
Figure 5. Intra-hippocampampal NaB Enhanced Histone Acetylation and c-Fos Expression Following Weak Extinction in the Infralimbic Cortex but not Prelimbic Cortex.
A) Mice receiving strong extinction (24 min) showed greater H3 Lys14 acetylation in both the infralimbic cortex and prelimbic cortex compared to weak extinction (3 min; top panels). Intra-hippocampal NaB enhanced histone acetylation in the infralimbic cortex following weak extinction (n = 7) above Veh levels (n=6) bringing them to levels commensurate with strong extinction (NaB group n=7, Veh group n=6; top left panel). In contrast to the infralimbic effects, intrahippocampal NaB infusion had no effect on prelimbic acetylation following either weak or strong extinction relative to vehicle (top right panel).
Mice receiving strong extinction (24 min) showed greater c-Fos expression in both the infralimbic and prelimbic cortices than did weak extinction (3 min; top panels). Similar to the acetylation findings, intra-hippocampal NaB enhanced c-Fos following weak extinction (n = 5) above Veh levels (n = 4) with no effect following strong extinction (NaB n = 4, Veh n = 3; bottom left panel). No effect of hippocampal NaB on c-Fos in the prelimbic cortex was seen (bottom right panel).
C) Representative histone acetylation and c-fos immunohistochemistry images from of the infralimbic and prelimbic cortices. A unilateral sample is presented here for illustration, however the IHC was quantified in the entire (bilateral) infralimbic and prelimbic cortices. Stereotaxic image reproduced with permission from [26].
A significant Extinction Duration X Drug interaction [F(1,25) = 5.88, p =0.024] combined with a main effect of Extinction Duration (F(1,25)=4.94, p=0.037) and Drug [F(1,25)=7.81, p=0.037] confirmed the differences in histone acetylation intensity. Simple main effects revealed that indeed the Veh treated 3-min extinction group had significantly lower levels of infralimbic acetylation than NaB or Veh treated 24 min groups or the 3-min NaB treated group [all ps ≤ 0.01].
The c-Fos results were confirmed with an Extinction Duration X Drug interaction [F(1,15) = 6.906, p=0.022] and main effect of Extinction Duration [F(1,15)=66.8, p <−.001] with no main effect of Drug [p=0.141]. Simple main effects revealed that both NaB and Veh treated 24 min extinction groups showed significantly more c-Fos positive neurons than both the NaB and Veh treated 3-min extinction groups [all ps<0.01]. The 3-min NaB group showed a greater number of infralimbic c-Fos positive neurons than the 3-min vehicle treated mice [p=0.01].
Prelimbic Cortex
In contrast to the infralimbic IHC, no effect of intra-hippocampal NaB was seen in the prelimbic cortex. Only elevated acetylation and c-Fos was found following 24-min extinction vs. 3-min extinction (Figure 5A right panels). A main effect of Extinction Duration on both acetylation [F(1,15)= 25.6, p<0.001] and c-Fos [F(1,25) =6.5 p=0.018] confirmed this with no interaction or effect of drug in any group [all ps>0.3].
Representative images of infralimbic and prelimbic IHC are shown in Figure 5B, respectively. Together, these results suggest that transcriptional modulations in the hippocampus drive infralimbic transcription supporting extinction.
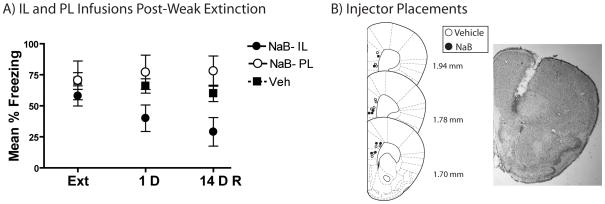
mPFC Infusion
Infusion of NaB into the infralimbic but not prelimbic cortex immediately following extinction induced a persistent extinction enhancement (Figure 6A). A significant of effect Drug Infusion Placement indicated a difference at both 1 and 14 D tests [F(2,19)=3.63, p=0.049 and F(2,19)= 5.14, p=0.019]. Further analysis indicated that at both 1D and 14 D tests mice receiving infralimbic NaB froze significantly less than vehicle [t(15)=2.29, p=0.037 and t(15)=2.47, p=0.027]. No effect of prelimbic infusion was seen on any test day [all ps>0.2].
Figure 6. Infralimbic but not Prelimbic NaB Infusions Caused Persistent Extinction Enhancements.
A) During retrieval mice receiving NaB into the infralimbic (n=6) or prelimbic cortex (n=3) following extinction did not differ from vehicle (n=9). Only the mice injected with NaB following extinction froze significantly less than vehicle on the 1 and 14D tests. B) Cannula placements with a representative angled placement in the infralimbic cortex (inset). Stereotaxic image reproduced with permission from [26].
Mice were identified as receiving infralimbic or prelimbic NaB infusions depending on injector tip placement [Figure 6B; 26]. Mice receiving vehicle infusions into prelimbic and infralimbic did not differ on any day and were thus combined into a single vehicle group.
Summary of Behavioral Findings
Table 1 shows the p-values for NaB induced enhancement in expression of the conditioning or extinction memory relative to Veh. NaB was able to induce persistent extinction enhancements under a range of conditions (pre-session systemic injections, post-session intracranial infusions) while enhancements of the acquisition memory were more restricted across procedures.
Table 1. Summary of effects of NaB during conditioning and extinction.
P-values for differences between NaB and vehicle during the 1 and 14D tests. NaB induced persistent extinction enhancements under a range of conditioning (pre-session systemic injections, post-session intracranial infusions), whereas enhancements in the conditioning memory were more restricted across preparations.
| Initial Test (1 D) | Persistence (14 D†) | |||
|---|---|---|---|---|
| Conditioning | Extinction | Conditioning | Extinction | |
| Pre-session systemic (Exp 1) | p>.05 | p=.03 | .013* | .013 |
| Post-session systemic (Exp 2) | ||||
| Strong Conditioning | p>.05 | p=.019 | p>.05 | p>.05 |
| Weak Conditioning | p>.05 | p>.05 | p>.05 | p>.05 |
| Post-Session Intrahippocampal (Exp 3) | ||||
| Strong Conditioning | p>.05 | p=.001 | p>.05 | p=.018 |
| Weak Conditioning | p>.05 | NT | p>.05 | NT |
| Strong Extinction | NT | p>.05 | NT | p>.05 |
| Post-Session mPFC (Exp 5) | ||||
| Infralimbic | NT | p=.037 | NT | p=.027 |
| Prelimbic | NT | p>.05 | NT | p>.05 |
All persistent extinction enhancements were only found if the mice were repeatedly tested (14D Re-Test) and not if the 14 D test was the initial test (14 D Initial Test).
The NaB induced freezing enhancement at 14 D is confounded by the pre-conditioning NaB injections which resulted in freezing greater than Veh at baseline. NT signifies “not tested” as certain tests were not required in all conditions.
Discussion
The key finding from these experiments was that the HDAC inhibitor sodium butyrate promoted long-term extinction, as revealed through behavioral and molecular measures. When a brief extinction session, that on its own had little impact on behavior, was followed by intra-hippocampal NaB administration, the behavioral and molecular consequences of that session were similar to those induced by a long extinction session. NaB infusion into the hippocampus drove increases in histone acetylation and c-Fos expression consistent with strong extinction in the infralimbic, but not prelimbic cortex. The involvement of the infralimbic cortex was confirmed as infusions of NaB into the infralimbic, but not prelimbic cortex, induced persistent extinction enhancements.
The other important finding was that HDAC inhibitor-induced extinction enhancements occurred under a wider range of conditions (pre- or post-session systemic injections, post-session intra-hippocampal injections) compared to the initial conditioning effects. Our findings suggest that NaB can enhance memories that form during initial learning and extinction, but the long-term effects of this drug are sensitive to several behavioral parameters, including conditioning/extinction strength and testing conditions. These findings add to other recent demonstrations of the limitations of HDAC inhibitor-induced memory enhancements [27–29].
We showed that modulating acetylation and c-Fos expression in the hippocampus is sufficient to drive transcriptional changes in the infralimbic cortex and that these changes are associated with strong extinction. A remaining question is whether these hippocampal driven changes in the mPFC are necessary to promote strong extinction. Our basic finding is consistent with studies showing the hippocampus and infralimbic cortex interact to promote fear extinction [19,30]. Within this network, we observed changes in acetylation at L14 of H3 as well as c-Fos expression, which are generally associated with permissive, transcriptionally active chromatin states. These chromatin states are associated with downstream increases in the expression of genes critical for excitatory and inhibitory memory formation [e.g., BDNF, Nr4a1; 31,32–34]. Interestingly, a recent study indicates that inhibiting enzymes that remove acetyl groups (e.g., p300) in the mPFC enhances extinction memory [20] demonstrating the need for future studies characterizing the global chromatin state required for extinction memory formation.
The specificity of this effect to the infralimbic but not prelimbic cortex is consistent with growing evidence that enhanced extinction is driven by transcriptional events in the infralimbic but not prelimbic cortex [20,35] as well as the involvement of the hippocampus in mediating such changes specifically in the infralimbic cortex [19]. Furthermore, anatomical studies in rats show the dorsal hippocampus (CA1) has more projections to the infralimbic than the prelimbic cortex, which may explain why the molecular effects of CA1 NaB infusion were present in the infralimbic and not the prelimbic cortex [30].
An interesting caveat to the persistent extinction enhancements was that enhancements only persisted if the 14D retention test was the second test. This suggests that the first test functions as a second extinction session that, combined with the previous pairing of NaB with the first extinction session, weakens spontaneous recovery of fear behavior on the 14 D test. This difference in recovery is typical when there are differences in total extinction prior to testing [36] and is consistent with many other studies that have demonstrated more persistent effects following repeated testing [11–13,37]. Because attenuated spontaneous recovery often corresponds to other measures of recovery such as renewal and reinstatement, future studies using discrete fear conditioning will be useful in examining the conditions under which extinction enhancements are vulnerable to recovery [38].
From a theoretical perspective it is possible that the learning that occurs during extinction is simply more vulnerable to pharmacological manipulations compared to initial conditioning. Some studies have demonstrated that the rate of extinction may be slower compared to the rate of initial acquisition [16]. A slower rate of learning during extinction would theoretically leave more room for enhancements than would the relatively fast rate of learning associated with initial acquisition. In turn, this would translate into smaller drug-induced enhancements in initial consolidation.
Indeed, recent studies indicate that the memory enhancing effects of NaB are critically dependent on the strength of learning and the subsequent memory. For example, NaB transforms a weak or impaired memory into a robust long-lasting memory [39,40]. Sensitivity of memories to the enhancing effects of NaB has also been shown at the molecular level—NaB transforms relatively low levels of histone acetylation following weak training into robust levels of acetylation commensurate with strong training and memory expression [41]. These studies are also consistent with our finding that the ability of NaB to enhance an extinction memory at both behavioral and molecular levels depends on the strength of the extinction memory; if the learning during extinction is strong, increases in histone acetylation in the hippocampus may not have further downstream effects on changes in the infra-limbic cortex. In light of our current results this suggests that the strength of the memory may be a critical determinant in the ability of HDAC inhibitors to enhance memory.
From a preclinical perspective, our findings suggests that HDAC inhibitors like NaB may be more likely to enhance fear memory extinction than exacerbate future fear expression when paired with exposure-based therapies. From a basic science perspective, we found that extinction enhancements are linked to powerful changes in the molecular expression of the memory in key brain regions involved in extinction. Together, our findings demonstrate promise for the future clinical application of HDAC inhibitors, like NaB to exposure-based therapies.
Supplementary Material
Acknowledgments
This work was support by National Institutes of Health grants R01MH077111 and R01DA025922 to K.M.L as well as AG023477, Vertex Pharmaceutical Scholarship and F31MH087031 to J.M.S. Further support came from AA010760 and AA016647 to A.E. R. J.D.R. was supported by NIDA T32DA007262.
Footnotes
Disclosure/Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clin Psychol Rev. 2009;29:715–26. doi: 10.1016/j.cpr.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q J Exp Psychol B. 2004;57:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- 3.Leung HT, Westbrook RF. Spontaneous recovery of extinguished fear responses deepens their extinction: a role for error-correction mechanisms. J Exp Psychol Anim Behav Process. 2008;34:461–74. doi: 10.1037/0097-7403.34.4.461. [DOI] [PubMed] [Google Scholar]
- 4.Davis M, Barad M, Otto M, Southwick S. Combining pharmacotherapy with cognitive behavioral therapy: traditional and new approaches. J Trauma Stress. 2006;19:571–81. doi: 10.1002/jts.20149. [DOI] [PubMed] [Google Scholar]
- 5.Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–6. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–76. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–31. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–59. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 9.Barrett RM, Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem. 2008;15:460–7. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35:870–80. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estes WK. Processes of memory loss, recovery, and distortion. Psychol Rev. 1997;104:148–69. doi: 10.1037/0033-295x.104.1.148. [DOI] [PubMed] [Google Scholar]
- 12.Parsons RG, Davis M. Temporary disruption of fear-potentiated startle following PKMzeta inhibition in the amygdala. Nat Neurosci. 2011;14:295–6. doi: 10.1038/nn.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stafford JM, Lattal KM. Direct comparisons of the size and persistence of anisomycin-induced consolidation and reconsolidation deficits. Learn Mem. 2009;16:494–503. doi: 10.1101/lm.1452209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lattal KM, Stafford JM. What does it take to demonstrate memory erasure? Theoretical comment on Norrholm et al. (2008) Behav Neurosci. 2008;122:1186–90. doi: 10.1037/a0012993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–6. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- 16.Rescorla RA. Comparison of the rates of associative change during acquisition and extinction. J Exp Psychol Anim Behav Process. 2002;28:406–15. [PubMed] [Google Scholar]
- 17.Stafford JM, Lattal KM. Is an epigenetic switch the key to persistent extinction? Neurobiol Learn Mem. 2011;96:35–40. doi: 10.1016/j.nlm.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2011;328:1288–90. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marek R, Coelho CM, Sullivan RK, Baker-Andresen D, Li X, Ratnu V, et al. Paradoxical enhancement of fear extinction memory and synaptic plasticity by inhibition of the histone acetyltransferase p300. J Neurosci. 2011;31:7486–91. doi: 10.1523/JNEUROSCI.0133-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–74. [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–65. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- 24.Lattal KM, Honarvar S, Abel T. Effects of post-session injections of anisomycin on the extinction of a spatial preference and on the acquisition of a spatial reversal preference. Behav Brain Res. 2004;153:327–39. doi: 10.1016/j.bbr.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–14. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 3. Academic Press; 2007. [Google Scholar]
- 27.Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reolon GK, Maurmann N, Werenicz A, Garcia VA, Schroder N, Wood MA, et al. Posttraining systemic administration of the histone deacetylase inhibitor sodium butyrate ameliorates aging-related memory decline in rats. Behav Brain Res. 2011;221:329–32. doi: 10.1016/j.bbr.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 31.Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, et al. Hippocampal Focal Knockout of CBP Affects Specific Histone Modifications, Long-Term Potentiation, and Long-Term Memory. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5:905–15. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 33.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–86. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–40. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J Neurosci. 2010;30:13586–96. doi: 10.1523/JNEUROSCI.0849-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weidemann G, Kehoe EJ. Savings in classical conditioning in the rabbit as a function of extended extinction. Learn Behav. 2003;31:49–68. doi: 10.3758/bf03195970. [DOI] [PubMed] [Google Scholar]
- 37.Luttges MW, McGaugh JL. Permanence of retrograde amnesia produced by electroconvulsive shock. Science. 1967;156:408–10. doi: 10.1126/science.156.3773.408. [DOI] [PubMed] [Google Scholar]
- 38.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 39.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–82. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 40.Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A. 2009;106:9447–52. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Federman N, Fustinana MS, Romano A. Histone acetylation is recruited in consolidation as a molecular feature of stronger memories. Learn Mem. 2009;16:600–6. doi: 10.1101/lm.1537009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.