Abstract
Prostaglandin E2 (PGE2) is an arachidonic acid (AA)-derived signaling molecule that can influence host immune responses to infection or vaccination. In this study, we investigated PGE2 production in vitro by cells infected with the poxvirus vaccine strain, modified vaccinia Ankara virus (MVA). Human THP-1 cells, murine bone marrow-derived dendritic cells, and murine C3HA fibroblasts all accumulated PGE2 to high levels in culture supernatants upon infection with MVA. We also demonstrated that MVA induced the release of AA from infected cells, and this was, most unusually, independent of host cytosolic phospholipase A2 activity. The accumulation of AA and PGE2 was dependent on viral gene expression, but independent of canonical NF-κB signaling via p65/RelA. The production of PGE2 required host cyclooxygenase-2 (COX-2) activity, and COX-2 protein accumulated during MVA infection. The results of this study provide insight into a novel aspect of MVA biology that may affect the efficacy of MVA-based vaccines.
Keywords: poxvirus, vaccine, lipid mediator, cyclooxygenase-2, cytosolic phospholipase A2, NF-κB
INTRODUCTION
Prostaglandins (PGs) are a group of fatty acid-derived signaling molecules that regulate a myriad of physiological and pathological processes. Viruses of many types have been shown to induce the production of PG upon infection (Reynolds and Enquist, 2006; Steer and Corbett, 2003). In some cases, PGs may affect the outcome of a virus infection, either by mediating direct, cell-autonomous effects on viral replication, or through modulation of innate and/or adaptive immune responses. Unsurprisingly, examples of viral inhibition of PG production in response to exogenous stimuli have also been described (Culver and Laster, 2007; Savard et al., 2000), which suggests that some viruses have the capacity to manipulate this important host response to their own advantage.
One of the most ubiquitous and best-characterized PG subtypes is prostaglandin E2 (PGE2). The biological responses associated with PGE2 signaling have long been known to include key roles in the mediation of inflammation (Dubois et al., 1998; Ivanov and Romanovsky, 2004; Portanova et al., 1996). More recently, PGs have also been found to have important roles in shaping both cell-mediated and humoral immune responses. In most cases, PGE2 has been shown to polarize the immune system towards generation of a T helper type 2 (Th2)-biased response. Examples of PGE2-mediated effects include the reduction of Th1 cytokine production by T cells, macrophages, and NK cells, and the promotion of IgG1 and IgE class-switching by B cells (reviewed by Harizi and Gualde, 2005; Harris et al., 2002; Phipps et al., 1991). Additionally, both human and murine dendritic cells (DCs) have been shown to produce PGE2, as well as respond to PGE2 signaling (Fogel-Petrovic et al., 2004; Harizi et al., 2001, 2002). PGE2 promotes migration and survival of DCs, and suppresses their ability to produce IL-12p70 (reviewed by Harizi and Gualde, 2005; Harris et al., 2002). Thus, PGE2 can play a critical role during innate and adaptive immune responses, and has functions complementary to those of more widely studied, protein-based signaling molecules.
Modified vaccinia Ankara virus (MVA), a promising live-virus vaccine platform, is a highly attenuated form of vaccinia virus (VAC) strain Ankara that is avirulent in humans (Moss et al., 1996; Stickl et al., 1974), yet remains effective as a vaccine against virulent poxviruses (Earl et al., 2004; Mayr et al., 1978; Wyatt et al., 2004). Due to its safety and immunogenicity, MVA is also a candidate vaccine vector for cancer immunotherapy, and for prophylaxis against various infectious diseases (reviewed by Gomez et al., 2008; Rimmelzwaan and Sutter, 2009; Sutter and Staib, 2003). During its attenuation by repeated passage in cell culture, MVA lost many accessory genes found in related poxviruses, and therefore it often differs greatly from them in its capacity to disrupt host responses (Antoine et al., 1998; Blanchard et al., 1998). Because of the potential for widespread use of MVA in human populations as a vaccine, or vaccine vector, there is a need to increase our understanding of the characteristics of MVA during its host–virus interactions.
Although substantial research has been conducted on the diverse immune modulation strategies of poxviruses, the PG response following poxvirus infection has received comparatively little attention. Recent in vivo studies with VAC have shown directly that PGE2 is an important determinant of the extent and type of immune responses initiated upon virus infection, or as a consequence of vaccination with live-virus vaccines and vaccine vectors (Bernard et al., 2010; Chang et al., 2009). In vitro, the synthesis of PGs from exogenously added eicosanoid precursors was found to be enhanced during infection of monkey kidney cells with several poxviruses, including MVA (Palumbo et al., 1993, 1994). However, these studies did not address whether poxvirus infection alone was sufficient to induce PG production, nor were the biochemical pathways used for PG biosynthesis investigated in poxvirus-infected cells.
In the absence of exogenously added precursor molecules, de novo synthesis of PGs normally is initiated by the enzymatic release of arachidonic acid (AA) from membrane glycerophospholipids (reviewed by Smith, 1989). Several cellular phospholipases may be involved in this process; however, cytosolic phospholipase A2 (cPLA2) is often regarded as the most important enzyme because cells obtained from cPLA2 knock-out mice are severely deficient in PG production in response to a variety of stimuli (Gijon et al., 2000; Sapirstein and Bonventre, 2000). Phospholipase-released AA is then converted into the intermediate prostaglandin H2 (PGH2) by cellular cyclooxygenase (COX) enzymes. There are two predominant isozymes of COX; COX-1 is most often constitutively expressed, and has roles in tissue homeostasis, while COX-2 typically has low basal expression, but is readily inducible (Smith et al., 1996; Tsatsanis et al., 2006). The final step of PG biosynthesis is the enzymatic conversion of COX-generated PGH2 to PGE2, or other PG subtypes, by specific PG synthases (Park et al., 2006).
In the current study we describe MVA-induced production of PGE2 by human THP-1 cells, as well as by murine bone marrow derived DCs and a murine fibroblast cell line. We found that MVA induced the accumulation of COX-2 protein in infected cells and caused AA to be released from cellular membranes by a mechanism that was independent of host cPLA2 activity. The production of PGE2 by MVA-infected cells was dependent on COX-2 activity but independent of canonical NF-κB signaling via p65/RelA. The production of PGE2 in response to infection with MVA may contribute to the immune response generated by MVA-based vaccines.
RESULTS
MVA infection alone does not lead to the accumulation of PGE2, or arachidonic acid, in culture supernatants of BS-C-1 cells
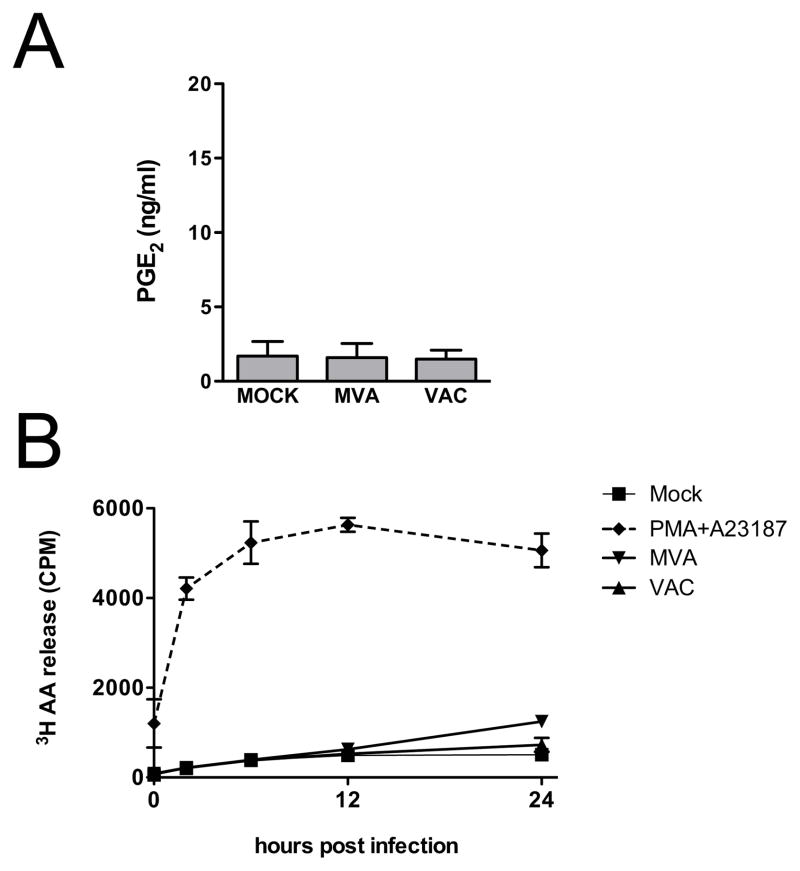
Previous reports have described the accumulation of PGE2 in poxvirus-infected BS-C-1 cell cultures following treatment with the calcium ionophore A23187 and addition of radiolabeled linoleic acid, an eicosanoid precursor (Palumbo et al., 1993, 1994). However, it is not clear from the results of these studies whether poxvirus infection alone was sufficient to induce PG synthesis, nor was it determined whether PGE2 was contained within the cells or released into the culture supernatant where it could effect signaling. Consequently, we evaluated the ability of MVA to induce the accumulation of PGE2 in the culture supernatants of BS-C-1 cells under normal infection conditions in vitro, in the absence of additional exogenous treatments. BS-C-1 cells were infected with MVA at 5 PFU/cell, and the concentration of PGE2 in culture supernatants was measured by ELISA 24 h after infection. As shown in Fig.1A, MVA infection alone was not sufficient to cause the accumulation of PGE2 in culture supernatants. Because MVA is highly divergent from other poxviruses and replication-deficient in most mammalian cells, as a control, we also investigated the ability of replication-competent VAC (strain Western Reserve) to induce PGE2 production by BS-C-1 cells. As seen for MVA, VAC infection did not result in the accumulation of PGE2 in BS-C-1 cell culture supernatants. These data indicate that neither MVA, nor VAC, can induce PGE2 production by BS-C-1 cells under normal infection conditions.
FIG. 1.
MVA infection does not induce PGE2 production or release of AA by BS-C-1 cells. (A) BS-C-1 cells were mock infected, infected with MVA, or infected with VAC, each at 5 PFU/cell. Culture supernatants were collected 24 h after infection and concentrations of PGE2 were measured by ELISA. Data are means and SEM from three independent experiments. (B) BS-C-1 cells were radiolabeled with [3H]AA overnight and then mock infected, treated with PMA and A23187 (10 ng/ml PMA, 10 μM A23187), or infected with MVA or VAC, each at 5 PFU/cell. Culture supernatants were collected 0, 2, 6, 12, and 24 h after infection and 200 μL aliquots were subjected to liquid scintillation counting to determine release of [3H]AA (CPM). Data plotted are means and SEM, from three independent experiments.
In the absence of exogenously added eicosanoid precursor molecules, the biosynthesis of PGE2 is dependent on the enzymatic release of AA from membrane glycerophospholipids. Thus, we reasoned that the failure of BS-C-1 cells to produce PGE2 under our experimental conditions might result from an inability of poxvirus infection to stimulate AA release from cellular membranes. To evaluate this possibility, the liberation of AA from virus-infected cells was measured by liquid scintillation counting of [3H]AA accumulation in the culture supernatant of radiolabeled BS-C-1 cells at various times after infection. The release of AA could be stimulated by treatment of BS-C-1 cells with phorbol 12-myristate 13-acetate (PMA) and A23187 together, as shown in Fig. 1B. In contrast, there was no detectable release of AA above that seen from mock-infected control cells when cells were infected with VAC, and AA release was only weakly stimulated between 12 and 24 h after infection with MVA (Fig. 1B). These results suggest that the inability of BS-C-1 cells to produce measurable concentrations of PGE2 upon infection with MVA or VAC is likely due to the lack of virus-induced AA release.
MVA infection induces PGE2 production by human THP-1 cells, murine DCs and a murine fibroblast cell line
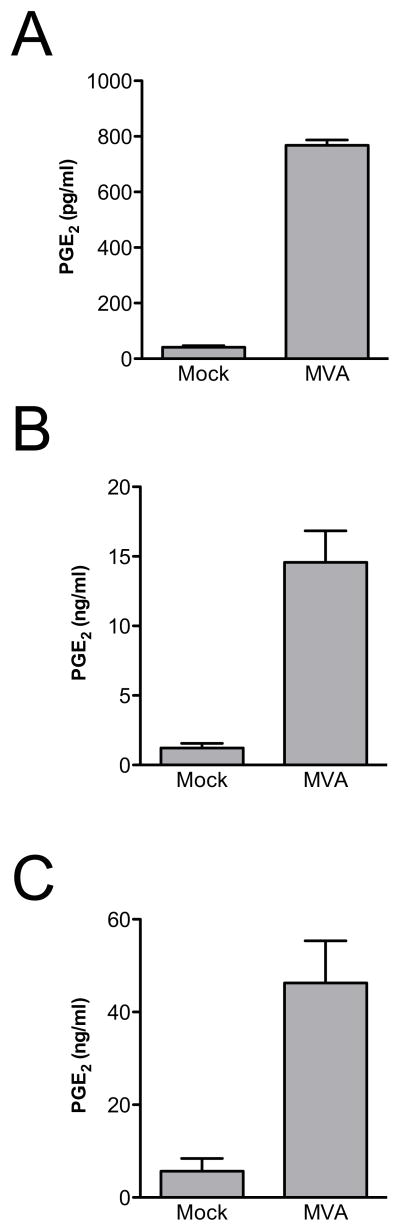
To evaluate whether the inability of MVA to induce PGE2 production in BS-C-1 cells represented a cell type-dependent effect, we investigated additional cell culture systems. Upon differentiation, human THP-1 cells become macrophage-like, and in previous studies they have been shown to produce chemokines, and certain cytokines, in response to infection with MVA (Delaloye et al., 2009, Lehmann et al., 2009). THP-1 cells were differentiated by treatment with PMA, and then either mock-infected, or infected with MVA at 5 PFU/cell. Culture supernatants were collected 24 h after infection, and PGE2 concentrations were determined by ELISA. As shown in Fig. 2A, MVA infection of THP-1 cells caused robust accumulation of PGE2 in culture supernatants.
FIG. 2.
Human and murine cells produce PGE2 in response to MVA infection. Human THP-1 cells that had been differentiated with PMA (A), murine DCs (B), or C3HA murine fibroblasts (C) were mock-infected, or infected with MVA at 5 PFU/cell. Culture supernatants were collected 24 h after infection and PGE2 concentrations were measured by ELISA. Data are means and SEM from three (A,C) or five (B) experiments.
Because MVA is intended to be used as a vaccine, we were also interested in its effect on PG production by cells that are directly involved in the generation of vaccine-induced immune responses. DCs have critical roles in initiating and influencing adaptive immune responses, and it has recently been shown that MVA preferentially targets DCs over other subsets of hematolymphoid cells both in vitro and in vivo (Liu et al., 2008). We therefore investigated whether infection of murine DCs with MVA could induce a PGE2 response. Murine DCs were generated in vitro from bone marrow cells, and were either mock-infected, or infected with MVA at 5 PFU/cell. Consistent with previous reports (Liu et al., 2008), we found that MVA was taken up by murine DCs and expressed viral genes, but the cells were non-permissive for viral replication (data not shown). The PGE2 produced by murine DCs was measured in culture supernatants 24 h after infection. As shown in Fig. 2B, we found abundant accumulation of PGE2 in the culture supernatants of MVA-infected murine DCs.
Further characterization of MVA-induced PGE2 production would be facilitated by the ability to work in an established, adherent cell line that does not require differentiation, so we also evaluated the potential use of C3HA cells as a model system. This mouse fibroblast line was used previously to study the biochemical pathways responsible for PG production during adenovirus infection (Culver and Laster, 2007). In common with many other cell lines (Blanchard et al., 1998; Carroll and Moss, 1997), we found that C3HA cells were not infected productively by MVA, but both early and late viral genes were expressed (data not shown). As for the other cell types investigated, C3HA cells were mock-infected, or infected with MVA at 5 PFU/cell, and the accumulation of PGE2 was measured 24 h after infection. As shown in Fig. 2C, MVA infection induced the accumulation of high levels of PGE2 in C3HA cell culture supernatants.
These results show that MVA can induce the production of PGE2 in vitro by cells of both human and murine origin, which may have important implications for the immune response generated by MVA-based vaccines.
Viral gene expression is required for MVA-induced PGE2 production
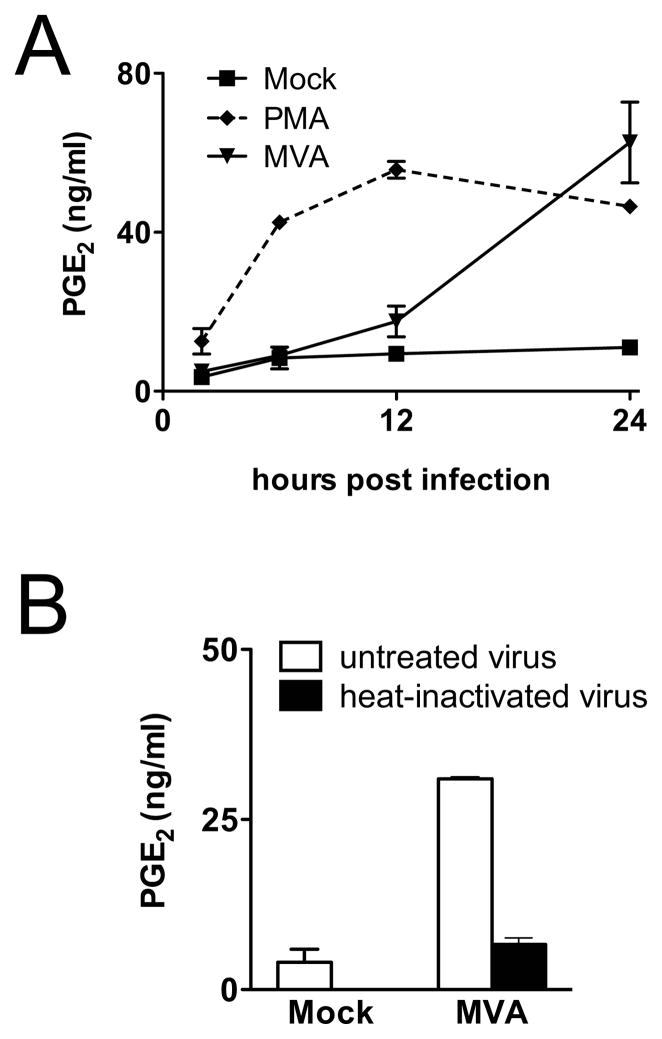
To establish the kinetics of MVA-induced PGE2 production in C3HA cells, the accumulation of PGE2 in culture supernatants was measured by ELISA at various times post infection. As shown in Fig. 3A, PGE2 production was minimal until approximately 12 h post infection. Whereafter, between 12 h and 24 h post infection there was a dramatic increase in PGE2 production. The long delay between MVA infection and the onset of PGE2 accumulation was in marked contrast to the response seen in control cells treated with PGE2-inducing ligands, such as PMA, or lipopolysaccharide (LPS) (Fig. 3A, and data not shown). This delayed response suggested that production of PGE2 in response to poxvirus infection might not be mediated through interaction of the virus particles with a cellular receptor but instead might require entry of the virus into the cell and expression of viral gene products. To address this question, inoculations were performed with inactivated virus preparations. Mild heat treatment (55°C, 1 h) of poxvirus particles renders them unable to initiate viral protein synthesis after entry into cells, but does not disrupt their structure (Harper et al., 1978). Infection of C3HA cells with heat-inactivated MVA resulted in levels of PGE2 accumulation that were not significantly different from those of mock-infected control cells (Fig. 3B), which indicates that viral gene expression is required for the production of PGE2 in response to infection by MVA. In contrast, C3HA cells infected with MVAΔudg mutants that are defective in late gene expression (Garber et al., 2009) produced high levels of PGE2 (data not shown). Taken together, these results suggest that expression of one or more viral early gene products is sufficient to induce PGE2 production in MVA-infected cells.
FIG. 3.
Viral gene expression is required for MVA-induced PGE2 production. (A) C3HA cells were mock-infected, treated with PMA (10 ng/ml), or infected with MVA at 5 PFU/cell. Culture supernatants were collected 2, 6, 12, or 24 h after infection and concentrations of PGE2 were measured by ELISA. Representative data from three independent experiments are shown. (B) Cells were mock-infected, or infected with MVA or heat-inactivated (HI) MVA preparations, each at 5 PFU/cell. Culture supernatants were collected 24 h after infection and concentrations of PGE2 were determined by ELISA. Data are means and SEM from two independent experiments.
AA release from MVA-infected C3HA cells follows kinetics similar to those of PGE2 production and also requires viral gene expression
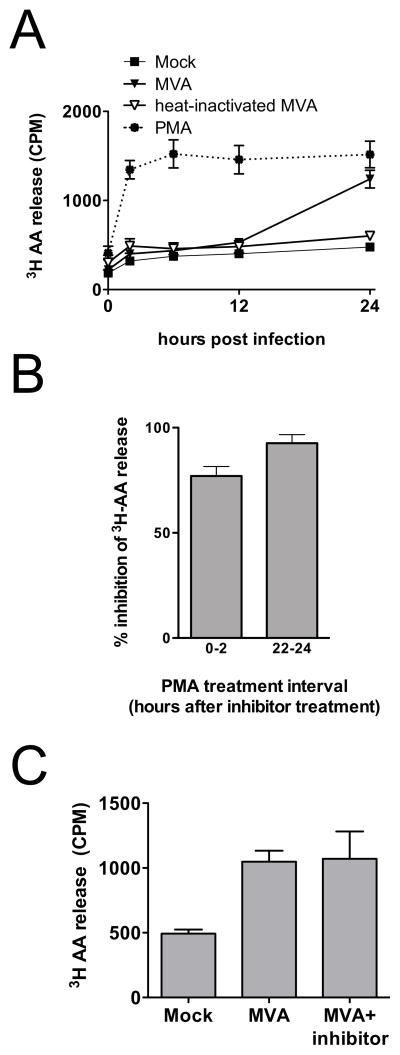
Relatively few studies have explored the liberation of AA in response to virus infection. As shown in Fig. 4A, the release of AA from MVA-infected C3HA cells was markedly delayed relative to the onset of infection, with the preponderance occurring between 12 h and 24 h post infection. The kinetics of AA release in response to MVA infection closely paralleled those of PGE2 accumulation. Experiments conducted with heat-inactivated virus preparations showed that, as for the production of PGE2, the release of AA from MVA-infected cells was almost entirely dependent on viral gene expression (Fig. 4A). The dependence of the initiation of AA release on viral gene expression likely contributes to the relatively slow response to poxvirus infection when compared to the responses induced by treatment of C3HA cells with proinflammatory ligands, such as PMA or LPS (Fig. 4A, and data not shown). These ligands (Barbour et al., 1998; Gijon et al., 2000), as well as the few viruses that have been investigated (Culver and Laster, 2007; Liu et al., 2005), cause AA release via activation of cPLA2. This prompted us to evaluate the role of cPLA2 in MVA-induced AA release.
FIG. 4.
MVA infection stimulates delayed AA release from murine fibroblasts. (A) C3HA cells were radiolabeled with [3H]AA overnight and then mock-infected, treated with PMA (10 ng/ml), or infected with MVA or heat-inactivated (HI) MVA preparations, each at 5 PFU/cell. Culture supernatants were collected 0, 2, 6, 12, and 24 h after infection and 200 μl aliquots were subjected to liquid scintillation counting to determine release of [3H]AA (CPM). Data plotted are means and SEM from six independent experiments for mock-infected and virus-infected cultures, and from two independent experiments for HI virus cultures. (B) C3HA cells were radiolabeled with [3H]AA overnight and treated with cPLA2 inhibitor (2.5 μM), or solvent control (DMSO). Cells were stimulated with PMA (10 ng/ml) either 15 min or 22 h after the addition of cPLA2 inhibitor. Culture supernatants were collected 2 and 24 h after addition of the cPLA2 inhibitor and 200 μl aliquots were subjected to liquid scintillation counting to determine the release of [3H]AA (CPM). Data plotted are expressed as mean percent inhibition by cPLA2 inhibitor treatment compared to solvent control, calculated from the three independent experiments. (C) C3HA cells were radiolabeled with [3H]AA and mock infected, or infected with MVA in the presence of cPLA2 inhibitor (2.5 μM), or solvent control (DMSO). Infected cells were incubated for 24 h in medium supplemented with cPLA2 inhibitor, or DMSO, and 200 μl aliquots were subjected to liquid scintillation counting to determine release of [3H]AA (CPM). Data are means and SEM from two independent experiments.
Because of the relatively long time-course required to study AA release from MVA-infected cells, we first conducted control experiments to determine whether a cell-permeable, cPLA2-specific inhibitor (Seno et al., 2000) would remain effective throughout a 24-h incubation. Identical cultures of C3HA cells were labeled with [3H]-AA and then treated with either the cPLA2 inhibitor (2.5 μM) or the solvent control (DMSO) and incubated at 37°C. The relative effectiveness of the cPLA2 inhibitor between 0–2 h and 22–24 h of incubation was determined by PMA-stimulation of matched inhibitor-treated and control cultures during each of these time windows. As shown in Fig. 4B, the cPLA2 inhibitor was able to block PMA-stimulated AA release throughout the 24 h time-course of the experiment. However, the cPLA2 inhibitor did not reduce the accumulation of AA in culture supernatants of MVA-infected C3HA cells (Fig. 4C). These data suggest that, in contrast to other viruses that have been studied, MVA-induced AA release is primarily dependent on a phospholipase, or phospholipases, other than cPLA2.
MVA-induced PGE2 production requires COX-2 activity and COX-2 accumulates in infected cells
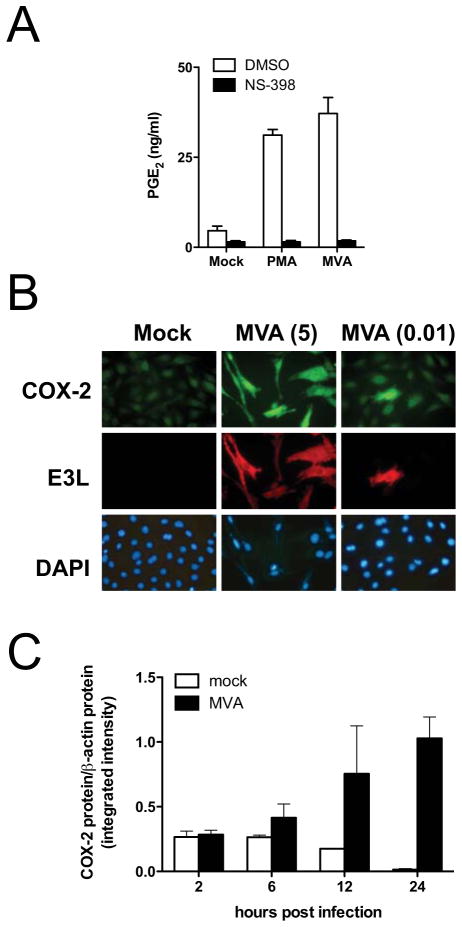
Of the two predominant COX isoforms, COX-2 is most often associated with inducible PGE2 synthesis (Smith et al., 1996). We used a cell-permeable, COX-2-selective inhibitor, NS-398, to evaluate the contribution of COX-2 activity to the production of PGE2 during MVA infection. We found that NS-398 effectively suppressed the accumulation of PGE2 in MVA-infected cell cultures, as well as in PMA-stimulated control cultures (Fig. 5A). These results show that essentially all of the PGE2 production by MVA-infected C3HA cells is dependent on the activity of COX-2.
FIG. 5.
MVA-induced COX-2 is required for PGE2 production by infected murine fibroblasts. (A), C3HA cells were mock-infected, treated with PMA (10 ng/ml), or infected with MVA at 5 PFU/cell in the presence of solvent control (DMSO), or the COX-2-specific inhibitor, NS-398 (1 μM). Culture supernatants were collected 24 h after infection and PGE2 concentrations were measured by ELISA. Data are means and SEM from three independent experiments. (B) C3HA cells were infected with MVA at 5 PFU/cell (5), or 0.01 PFU/cell (0.01), and 24 h after infection, cells were fixed, permeabilized, and immunostained with anti-E3L primary mAb and TRITC-labeled secondary antibody to detect infected cells, and anti-COX-2 primary antibody and FITC-labeled secondary antibody to measure COX-2 accumulation. DAPI staining was used to visualize uninfected as well as infected cells. Images were captured with constant exposure times to show intensity differences, and representative fields are shown. (C) C3HA cells were mock-infected, or infected with MVA at 5 PFU/cell. Cell lysates were prepared 2, 6, 12, or 24 h after infection. Lysates (10 μg) were resolved by SDS-PAGE and immunoblotted to detect COX-2 and β-actin. The near-infrared fluorescence intensity (integrated intensity) of each COX-2-specific band was measured with the Li-Cor Odyssey system, and was normalized to respective β-actin controls. Protein quantification data are means and SEM from three (2 h, 6 h, 12 h), or five (24 h), independent experiments.
Next, we utilized immunofluorescence microscopy to determine if COX-2 protein accumulation was induced specifically in MVA-infected C3HA cells. By 24 h after infection with MVA (5 PFU/cell), C3HA cells had noticeably elevated COX-2 protein accumulation compared to mock-infected controls (Fig. 5B). MVA infection was confirmed by immunofluorescent detection of the viral E3L protein in these cells. To determine whether COX-2 accumulation was induced in a cell-autonomous manner by the virus infection or whether uninfected bystander cells in the culture also accumulated COX-2, cells were infected with MVA at 0.01 PFU/cell. COX-2 accumulated above background levels in virus-infected cells, but not in uninfected cells in the culture (Fig. 5B). This result shows that the accumulation of COX-2 is induced specifically during MVA infection of C3HA cells. Further, in combination with our results showing that COX-2 activity was required for MVA-induced PGE2 synthesis, it suggests that the PGE2 detected in culture supernatants is produced predominantly by MVA-infected cells and not by uninfected bystander cells within the culture system.
To evaluate the time-course of COX-2 accumulation during MVA infection in more detail, equal amounts (10 μg) of mock-infected or MVA-infected C3HA cell lysates, collected at various times post infection, were subjected to quantitative immunoblot analysis for the COX-2 protein (Fig. 5C). This analysis revealed that the level of COX-2 protein present in mock-infected cells declined steadily during the 24 h incubation in medium containing low serum. In contrast, the level of COX-2 in MVA-infected cells began to rise by 6 h post infection, and by 24 h post infection it was elevated ~80-fold relative to the level in mock-infected cells. Thus, these results confirm those obtained by immunofluorescence microscopy and show that COX-2 protein levels begin to rise in MVA-infected cells prior to the release of AA, or the accumulation of PGE2.
MVA-induced COX-2 accumulation and PGE2 biosynthesis are not dependent on canonical NF-κB signaling via p65/RelA
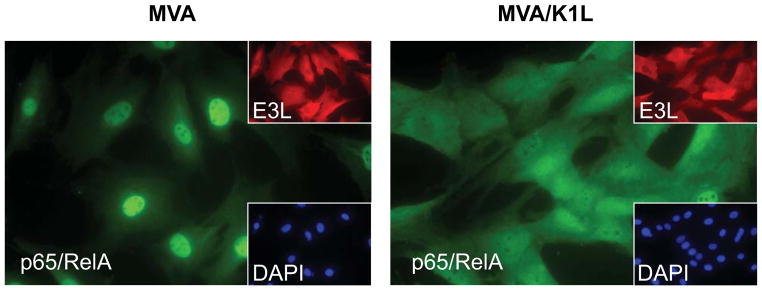
Transcriptional regulation of COX-2 synthesis can involve a multitude of transcription factors, which include NF-κB, AP-1, NFAT, CRE, and others (Iniguez et al., 2000; Kang et al., 2006). The contribution of each of these transcription factors to the regulation of COX-2 gene expression likely varies among particular types of cells and different stimuli. For several viruses, including encephalomyocarditis virus (ECMV), dengue virus, hepatitis C virus, and latent Epstein-Barr virus (EBV), the activation of NF-κB signaling has been implicated either in COX-2 induction, or the accumulation of PGE2, in response to infection (Liou et al., 2008; Murono et al., 2001; Steer et al., 2003; Waris and Siddiqui, 2005). Alternatively, interference with the activation of NF-κB has been implicated in the suppression of PGE2 production that occurs upon EBV lytic infection (Savard et al., 2000). MVA is unique in that it activates canonical NF-κB signaling via p65/RelA, while related orthopoxviruses, including VAC, encode multiple factors that suppress NF-κB activation (Oie and Pickup, 2001). To test directly whether canonical NF-κB signaling via p65/RelA was required for MVA-induced production of PGE2, we utilized a recombinant form of MVA (MVA/K1L) that contains a functional copy of the K1L gene from VAC (strain Western Reserve) in place of the disrupted MVA K1L gene. We and others have demonstrated that expression of the VAC K1L gene is sufficient to suppress MVA-induced IκBα degradation and subsequent activation of NF-κB (Lynch et al., 2009; Shisler and Jin, 2004). As described for other cell types, the infection of C3HA cells with MVA resulted in the activation of NF-κB signaling, which could be demonstrated by translocation of p65/RelA to the nucleus of infected cells (Fig. 6). In marked contrast, p65/RelA remained in the cytoplasm of cells infected with MVA/K1L (Fig. 6), thus confirming that canonical NF-κB signaling is suppressed in C3HA cells by expression of the K1L gene.
FIG. 6.
Expression of the VAC K1L gene in MVA prevents MVA-induced NF-κBp65 nuclear translocation. C3HA cells were mock-infected, or infected with MVA or MVA/K1L each at 5 PFU/cell. At 4 h after infection, cells were fixed, permeabilized, and immunostained with anti-E3L primary mAb and TRITC-labeled secondary antibody to detect infected cells, and anti- p65/RelA primary Ab and FITC-labeled secondary antibody to discern cellular localization of p65/RelA. DAPI staining was used to visualize nuclear regions. Representative fields are shown.
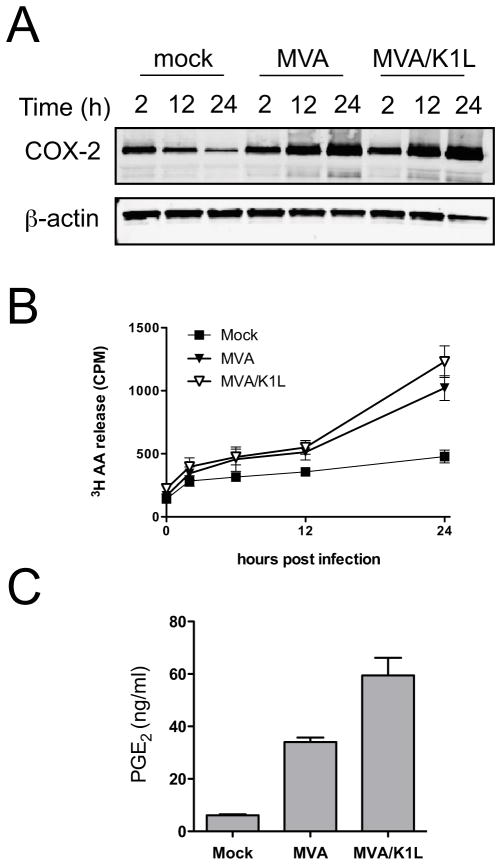
The results described above substantiate the use of direct comparisons between MVA and MVA/K1L to determine the contribution of canonical NF-κB signaling to MVA-induced PGE2 biosynthesis. The analysis of cell lysates by immunoblotting revealed that similar amounts of COX-2 protein were present in C3HA cells infected with MVA or MVA/K1L (Fig. 7A). This indicated that, in contrast to the findings made for some other viruses, the activation of canonical NF-κB signaling via p65/RelA is not required for COX-2 protein accumulation during MVA infection. Additionally, there was no difference in the kinetics of AA liberation from cells infected with MVA or MVA/K1L (Fig. 7B), nor was there any reduction in the accumulation of AA or PGE2 in culture supernatants of MVA/K1L-infected cells (Fig. 7C). Thus, neither COX-2 accumulation nor PGE2 biosynthesis are dependent on the activation of canonical NF-κB signaling in MVA- infected C3HA cells.
FIG. 7.
MVA-induced PGE2 biosynthesis is not dependent on NF-κB activation. (A) C3HA cells were mock-infected, or infected with MVA or MVA/K1L at 5 PFU/cell. Cell lysates were prepared 2, 12, or 24 h after infection. Lysates (10 μg) were resolved by SDS-PAGE and immunoblotted to detect COX-2 and β-actin. Immunolabeled protein bands were visualized using the Li-Cor Odyssey system. Three independent experiments were conducted and a representative gel image is shown. (B) C3HA cells were radiolabeled with [3H]AA overnight and then mock-infected, or infected with MVA or MVA/K1L, each at 5 PFU/cell. Culture supernatants were collected 0, 2, 6, 12, and 24 h after infection and 200 μl aliquots were subjected to liquid scintillation counting to determine release of [3H]AA (CPM). Data plotted are means and SEM, from four independent experiments. (C) C3HA cells were mock infected, or infected with MVA or MVA/K1L, each at 5 PFU/cell. Culture supernatants were collected 24 h after infection and concentrations of PGE2 were determined by ELISA. Data are means and SEM from three independent experiments.
DISCUSSION
This study extends previous reports (Palumbo et al., 1993, 1994) and demonstrates for the first time that MVA infection can result in PGE2 production in the absence of exogenous PG precursors and A23187. Most notably, MVA infection was shown to induce PGE2 production from human macrophage-like cells and from murine DCs. This study is also the first to describe release of the endogenous eicosanoid precursor, AA, from cellular membranes in response to poxvirus infection. We showed that COX-2 accumulation is induced during MVA infection and that COX-2 is required for PGE2 production by MVA-infected cells. Finally, we found that the ability of MVA to activate canonical NF-κB signaling via p65/RelA did not contribute to MVA-induced COX-2 accumulation or PGE2 production.
The ability of poxvirus-infected cells to metabolize AA has been demonstrated in previous studies conducted with BS-C-1 cells (Palumbo et al., 1993, 1994). Our results extend this observation, and demonstrate that MVA infection of human THP-1 cells, murine DCs, or murine C3HA fibroblasts, results in the release of AA from cell membranes, metabolism of AA, and accumulation of PGE2. However, MVA infection alone was unable to stimulate either the release of AA from BS-C-1 cell membranes, or the accumulation of PGE2 in culture supernatants of BS-C-1 cells. We therefore speculate that, in the absence of exogenously added precursor molecules, a lack of available substrate (AA) accounts for the failure of poxvirus-infected BS-C-1 cells to produce PGE2. The dichotomy between MVA-induced PGE2 production in THP-1 cells, murine DCs and C3HA cells, and the lack of PGE2 production in MVA-infected BS-C-1 cells, implicates the contribution of cell-specific factors in influencing the poxvirus-induced PG response. The underlying causes of such cell type specificity are likely to be multifactorial. For example, AA release and induction of COX-2 expression may be activated via distinct signal transduction pathways in response to MVA infection. As a result, a cell type-specific failure to activate either one of these signal transduction pathways could prevent MVA-induced PGE2 production.
The infection of human THP-1 macrophage-like cells or murine DCs with MVA caused high levels of PGE2 to accumulate in culture supernatants compared to mock-infected controls, and these results were recapitulated in the C3HA mouse fibroblast cell line. Accordingly, C3HA cells were used as a model system to gain insight into the molecular factors involved in MVA-induced PGE2 biosynthesis. We found that the kinetics of MVA-stimulated AA release closely paralleled those of PGE2 accumulation, which is consistent with the possibility that the availability of AA, the initial substrate for the pathway, is rate-limiting for PGE2 biosynthesis. Additionally, we found that COX-2 specifically accumulated in MVA-infected cells, and COX-2 enzyme activity was required for MVA-induced PGE2 production. These results are all consistent with established models of inducible PGE2 production (Smith, 1989; Smith et al., 1996).
One of the more striking findings of this study was that in MVA-infected cells, AA release and PGE2 accumulation were delayed by approximately 12 h relative to the onset of infection. And, even more remarkably, AA release was found to be independent of cPLA2 enzyme activity. The delayed release of AA observed upon MVA infection contrasts with the more rapid, cPLA2-dependent, release described following infection of C3HA cells with adenovirus (Culver and Laster, 2007) or by stimulation of these cells with PMA, or LPS. Experiments with heat-inactivated MVA preparations and MVAΔudg mutants demonstrated that the delayed response may, at least in part, be due to the requirement for viral early gene expression for the activation of AA release and subsequent PGE2 synthesis. Conceivably, it might take up to 12 h for one or more viral proteins to accumulate to a critical threshold level that can directly activate the signal transduction leading to AA release and PGE2 production. Equally, though, AA release may be delayed simply because it is an indirect effect of viral infection. A third possibility, which is not mutually exclusive with the others, is that the utilization of non-cPLA2-dependent mechanisms for AA release may contribute to the atypical kinetics that we observed for MVA-infected cells. It remains to be determined whether AA release from MVA-infected cells relies on known cellular or viral phospholipases (Baek et al., 1997), or whether yet-uncharacterized enzymes may be implicated. The results of this study raise the interesting possibility that such non-canonical mechanisms for AA release may contribute to eicosanoid production during viral infections more generally.
Amongst orthopoxviruses, the uncommon ability of MVA to activate canonical NF-κB signaling via p65/RelA is presumed to be a significant contributing factor in determining the immune response to infection. Evidence for the involvement of NF-κB in the induction of COX-2 expression and the subsequent production of PGE2 has been described in several systems (Steer et al., 2003; Yamamoto et al., 1995). However, our experiments in which MVA was compared to MVA/K1L, a recombinant virus which does not induce IκBα degradation or nuclear translocation of p65/RelA in infected cells, revealed that canonical NF-κB signaling via p65/RelA is not required for the liberation of AA, the accumulation of COX-2, or PGE2 production by MVA-infected C3HA cells. Although the ability to activate canonical NF-κB signaling may be a crucial determinant of many biological responses to MVA infection, it appears not to be important for the PG response. In addition, it has recently been shown that MVA infection activates double-stranded RNA-activated protein kinase (PKR), and that PKR activation is inhibited by expression of K1L (Lynch et al., 2009; Willis et al., 2009). Therefore, our results for MVA/K1L also suggest that MVA-induced production of PGE2 is not mediated by the activation of PKR. A similar lack of dependence on PKR has been described previously for PGE2 production by EMCV-infected macrophages (Steer et al., 2003).
The robust production of PGE2 in response to MVA infection is particularly interesting because of the potential for widespread use of MVA as a vaccine and vaccine vector. Vaccine-induced production of PGE2 is expected to have roles in influencing the overall immune response that is generated. In particular, PGE2 signaling may contribute to the Th2 response initiated by MVA-based vaccines. Although MVA is highly regarded for its ability to induce cell-mediated immune responses, a Th2 component that drives the production of neutralizing antibodies is also generated (Earl et al., 2004; Wyatt et al., 2004). The humoral component of MVA-induced immune responses is also evident in cytokine profiles measured in mouse spleen homogenates one and two days after inoculation with MVA, which indicated elevated levels of IL-6, but not IL-12 or IFN-γ (Ramirez et al., 2000). Additionally, at high inoculum doses, MVA preferentially induced the production of IgG1, a Th2 antibody isotype, over IgG2a (Ramirez et al., 2000). These Th2-biased responses correlate with known effects of PGE2 signaling (Harris et al., 2002; Hinson et al., 1996) and suggest that manipulation of MVA-induced PGE2 production might allow customization of the immune responses generated by MVA-based vaccines. In particular, enhanced Th1 responses might be generated by an MVA-based vaccine vector that is rendered incapable of inducing PGE2 production.
Recent studies have shown that PGs can mediate various effects on vaccine-induced immune responses. In several reports, the inhibition of COX-2 in combination with various anti-cancer vaccines has been shown to significantly augment vaccine efficacy (Basu et al., 2006; Haas et al., 2006; Mukherjee et al., 2009; Zeytin et al., 2004). It is thought that COX-2 inhibition, via the concomitant decrease in PGE2 production, leads to reduced activation of indoleamine 2,3-dioxygenase and increased Th1 cytokine production, which facilitates a more effective CTL response. In contrast, following vaccination with human papillomavirus type 16 virus-like particles, COX-2 activity was shown to be essential for the generation of an optimal neutralizing antibody response and for memory B-cell expansion (Ryan et al., 2006). Likewise, COX-2 activity has recently been shown to be required for an optimal antibody response to infection with VAC (Bernard et al., 2010). Based on these results, and those of the current study, it may be hypothesized that MVA-induced PGE2 signaling contributes generally to the ability of MVA-based vaccines to generate antibody responses.
MATERIALS AND METHODS
Reagents and antibodies
All of the fetal bovine serum (FBS), cell culture media, and cell culture reagents were purchased from Gibco unless otherwise stated. The recombinant GM-CSF and IL-4 were purchased from R&D Systems. The COX-2-specific inhibitor NS-398 was purchased from Cayman Chemical Company. The specific cPLA2 inhibitor (N-{(2S,4R)-4-(Biphenyl-2-ylmethyl-isobutyl-amino)-1-[2-(2,4-difluorobenzoyl)-benzoyl]-pyrrolidin-2-ylmethyl}-3-[4-(2,4-dioxothiazolidin-5-ylidenemethyl)-phenyl]acrylamide, HCl) was purchased from Calbiochem. 4-bromo A-23187 (A23187) was purchased from Invitrogen. Phorbol 12-myristate 13-acetate (PMA) was purchased from Biomol International. The radiolabeled [5,6,8,9,11,12,14,15-3H(N)]-arachidonic acid ([3H]AA) was purchased from Perkin Elmer. The COX-2-specific rabbit polyclonal Ab was purchased from Cayman Chemical. The monoclonal antibody (mAb) specific to p65/RelA (C-20) was purchased from Santa Cruz Biotechnology. Inc. The murine mAb specific to β-actin was purchased from Sigma-Aldrich. Ascites fluid containing murine mAb (TW2.3) specific to the VAC early gene product E3L (Yuwen et al., 1993) was a generous gift from Dr. Jack Bennink (National Institutes of Health). The tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-mouse, and FITC-conjugated goat anti-rabbit antibodies were obtained from Sigma-Aldrich. The secondary antibodies used with the Odyssey Infrared Imaging System, goat anti-mouse IRDye 680 and goat anti-rabbit IRDye 800CW, were purchased from Li-Cor Biosciences.
Cell culture and generation of murine bone marrow derived DCs
C3HA murine fibroblasts (Gooding, 1979) were cultured in DMEM supplemented with 1 mM sodium pyruvate and 5% FBS. BS-C-1 cells were from the American Type Culture Collection (ATCC, CCL-26) and were cultured in DMEM supplemented with 10% FBS. THP-1 human monocytes (ATCC, TIB-202) were cultured in RPMI1640 supplemented with 0.05 mM 2-mercaptoethanol, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 10% FBS. Cell lines were maintained at 37°C in a 5% CO2 atmosphere. Bone marrow cells were harvested from 12–24 week-old female C57BL/6 mice (Charles Rivers Labs). Procedures for the use and care of mice were conducted in accordance with the National Institutes of Health guidelines, and protocols approved by Duke University’s Institutional Animal Care and Use Committee. Bone marrow cells were cultured to generate DCs, as described by Lee et al. (2005). Briefly, bone marrow progenitor cells were cultured in DC medium (RPMI 1640 supplemented with 5% heat-inactivated FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, 1 mM sodium pyruvate, 1× MEM non-essential amino acids, 0.5× MEM essential amino acids, 10 mM HEPES, 2 mM L-glutamine, 55 mM β-mercaptoethanol, and 10 ng/ml each of recombinant mouse GM-CSF and IL-4). After three days, the adherent cells were washed and replenished with fresh DC medium and cultured for three additional days. Non-adherent, immature DCs, were harvested at day six and cultured in DC medium without cytokines for all experiments.
Viruses and infections
The viruses employed in this study were MVA (ATCC VR-1566), VAC strain Western Reserve (ATCC VR-1354), and MVA/K1L, a recombinant MVA virus which contains the K1L gene from VAC strain Western Reserve in place of the disrupted K1L gene of MVA (Lynch et al., 2009). The replication-defective MVAΔudg mutants, vDG014 and vDG027, which were propagated on their helper cell line, CAN20 (Garber et al., 2009) were also used. The MVAΔudg mutants and helper cell line were kindly provided by Dr. David Garber (Emory University). Unless otherwise indicated, all virus infections were conducted at 5 PFU/cell for 1 h at 37°C in medium containing 2.5% FBS. Where indicated, virus preparations were heat-inactivated by incubation for 1 h at 55°C.
Quantitative PGE2 ELISA
The accumulation of PGE2 in cell culture supernatants was measured using specific immunoassays (Assay Designs). Briefly 2.5 × 104 C3HA cells were plated into 24-well flat bottom tissue culture plates (Corning Incorporated) and incubated overnight at 37°C to allow the cells to adhere. For THP-1 human monocytes, the cells were plated at 5 × 105 cells per well and cultured at 37°C for 48 h in media containing 10 nM PMA to allow differentiation into adherent macrophage-like cells. When DCs were used, 5 × 105 cells per well were plated, followed by incubation for 1 h at 37°C to allow the cells to adhere. The virus infections and experimental treatments were conducted as indicated, and cell supernatants were collected at various times after infection or treatment. The supernatants were briefly centrifuged to remove large debris, and the immunoassays were performed according to the manufacturer’s instructions. The absorbance was measured with a PolarStar microplate reader (BMG Labtechnologies) and the concentration of PGE2 was determined by comparison to a standard curve. In experiments conducted using the COX-2 specific inhibitor, NS-398, the cells were plated, infected, and incubated in media that were supplemented with the drug (1 μM), or the solvent control (DMSO).
Immunofluorescence microscopy
8-well glass chamber slides (Nalge Nunc International) were seeded with 2 × 104 C3HA cells in 2.5% FBS DMEM and incubated at 37°C overnight. The cells were then mock-infected or infected with MVA or MVA/K1L and incubated at 37°C for the times indicated. The cells were fixed in PBS containing 4% formaldehyde for 20 min at room temperature and then washed twice in PBS containing 3% BSA. The cells were then permeabilized with PBS containing 0.1% Triton X-100 for 5 min, washed twice in PBS containing 3% BSA, and incubated with the primary antibodies in 0.3% BSA for 1 h. The cells were then again washed twice in PBS containing 3% BSA and incubated with the secondary antibodies in PBS containing 0.3% BSA for 30 min. Subsequently, the cells were washed twice in PBS containing 3% BSA and mounted in an anti-fade slide mount containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc.). Microscopy was conducted on a Zeiss Axioskop 2 Plus and the images were captured using Spot Advanced software (Diagnostic Instruments, Inc.).
[3H]AA release assays
For each experimental condition, a total of 5 × 105 C3HA cells were seeded into T25 tissue culture flasks (Becton Dickinson) and incubated at 37°C overnight in medium containing 0.1 μCi/ml [3H]AA. After labeling, the cells were washed two times with HBSS. Fresh medium supplemented with 2.5% FBS was added to the flasks and the cells were incubated at 37°C for 2 h to allow for the spontaneous release of the radiolabel. The cells were then washed again with HBSS and infected as indicated for 1 h at 37°C. Following infection, 2.5% FBS DMEM was added to each flask to a final volume of 5.5 ml and the cultures were incubated at 37°C. At the indicated times after infection, 250 μl of the supernatant were collected from each flask and briefly centrifuged to remove debris. Liquid scintillation counting was performed on 200 μl samples of this supernatant using a Beckman Coulter model LS 5801.
Immunoblotting and quantitative immunoblotting
Monolayers of mock-infected or virus-infected C3HA cells were solubilized in lysis buffer (50 mM HEPES (pH 7.4), 1 mM EGTA, 1 mM EDTA, 0.2 mM sodium orthovanadate, 2 mM PMSF, and 0.5% SDS) and collected by scraping. The total protein concentration of the samples was determined using the Bio-Rad DC protein assay (Bio-Rad Laboratories, Inc). Equal amounts of total protein (10 μg) were loaded onto 12% or 16% polyacrylamide Tris-glycine gels and separated by electrophoresis on a Novex MiniCell System (Invitrogen Life Technologies). The proteins were transferred to Immobilon-FL PVDF membranes (Millipore) and blocked for 1 h in Odyssey Blocking Buffer (Li-Cor Biosciences). The primary antibodies were diluted in 0.1% Tween-20 Odyssey Blocking Buffer and incubated with the membrane overnight at 4°C (COX-2 and mPGES-1) or for 1 h at room temperature (β-actin). The membranes were washed extensively in PBS containing 0.1% Tween-20. Secondary antibodies specifically designed for use with the Li-Cor Odyssey system were diluted in 0.1% Tween-20 Odyssey Blocking buffer and incubated for 45 min at room temperature, followed by extensive washing with PBS containing 0.1% Tween-20. The band visualization and quantification was completed on a Li-Cor Odyssey scanning system running Odyssey 2.1 software (Li-Cor Biosciences). The integrated intensity of each specific band of interest was measured and normalized to the respective β-actin control band to account for any minor variations in total protein loads.
Acknowledgments
We would like to thank Dawn Eads and Steven Abbott for expert technical assistance, Dr. Jack Bennink (National Institutes of Health) for a gift of anti-E3L mAb, and Dr. David Garber (Emory University) for generously providing the MVAΔudg mutants and their helper cell line. This project was supported by the College of Agriculture and Life Sciences, North Carolina State University, and supported in part by U.S. Public Health Service grants 1R01 CA59032 (to SML) and 1U54 AI057157 from the National Institutes of Health to the Southeastern Regional Center of Excellence in Emerging Infections and Biodefense (SERCEB). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. AHS was partially supported by U.S. Public Health Service grant T32CA09111.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Justin J. Pollara, Email: justin.pollara@duke.edu.
April H. Spesock, Email: spesocka@medimmune.com.
David J. Pickup, Email: picku001@mc.duke.edu.
Scott M. Laster, Email: scott_laster@ncsu.edu.
References
- Antoine G, Scheiflinger F, Dorner F, Falkner FG. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244:365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- Baek SH, Kwak JY, Lee SH, Lee T, Ryu SH, Uhlinger DJ, Lambeth JD. Lipase activities of p37, the major envelope protein of vaccinia virus. J Biol Chem. 1997;272:32042–32049. doi: 10.1074/jbc.272.51.32042. [DOI] [PubMed] [Google Scholar]
- Barbour SE, Wong C, Rabah D, Kapur A, Carter AD. Mature macrophage cell lines exhibit variable responses to LPS. Mol Immunol. 1998;35:977–987. doi: 10.1016/s0161-5890(98)00070-4. [DOI] [PubMed] [Google Scholar]
- Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA, Mukherjee P. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol. 2006;177:2391–2402. doi: 10.4049/jimmunol.177.4.2391. [DOI] [PubMed] [Google Scholar]
- Bernard MP, Bancos S, Chapman TJ, Ryan EP, Treanor JJ, Rose RC, Topham DJ, Phipps RP. Chronic inhibition of cyclooxygenase-2 attenuates antibody responses against vaccinia infection. Vaccine. 2010;28:1363–1372. doi: 10.1016/j.vaccine.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- Caroll MW, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology. 1997;238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- Chang CL, Ma B, Pang X, Wu TC, Hung CF. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol Therapy. 2009;17:1365–1372. doi: 10.1038/mt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver CA, Laster SM. Adenovirus type 5 exerts multiple effects on the expression and activity of cytosolic phospholipase A2, cyclooxygenase-2, and prostaglandin synthesis. J Immunol. 2007;179:4170–4179. doi: 10.4049/jimmunol.179.6.4170. [DOI] [PubMed] [Google Scholar]
- Delaloye J, Roger T, Steiner-Tardivel Q-G, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, Pantaleo G, Esteban M, Calandra T. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathogens. 2009;5:e1000480. doi: 10.1371/journal.ppat.100048. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, Eisenberg RJ, Hartmann CJ, Jackson DL, Kulesh DA, Martinez MJ, Miller DM, Mucker EM, Shamblin JD, Zwiers SH, Huggins JW, Jahrling PB, Moss B. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- Fogel-Petrovic M, Long JA, Knight DA, Thompson PJ, Upham JW. Activated human dendritic cells express inducible cyclo-oxygenase and synthesize prostaglandin E2 but not prostaglandin D2. Immunol Cell Biol. 2004;82:47–54. doi: 10.1111/j.1440-1711.2004.01213.x. [DOI] [PubMed] [Google Scholar]
- Garber DA, O’Mara LA, Zhao J, Gangadhara S, An I, Feinberg MB. Expanding the repertoire of modified vaccinia Ankara-based vaccine vectors via genetic complementation strategies. PLoS ONE. 2009;4:e5445. doi: 10.1371/journal.pone.0005445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijon MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that Do and Do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J Biol Chem. 2000;275:20146–20156. doi: 10.1074/jbc.M908941199. [DOI] [PubMed] [Google Scholar]
- Gomez CE, Najera JL, Krupa M, Esteban M. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr Gene Ther. 2008;8:97–120. doi: 10.2174/156652308784049363. [DOI] [PubMed] [Google Scholar]
- Gooding LR. Antibody blockade of lysis by T lymphocyte effectors generated against syngeneic SV40 transformed cells. J Immunol. 1979;122:2328–2336. [PubMed] [Google Scholar]
- Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, Albelda SM. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 2006;12:214–222. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- Harizi H, Gualde N. The impact of eicosanoids on the crosstalk between innate and adaptive immunity: the key roles of dendritic cells. Tissue Antigens. 2005;65:507–514. doi: 10.1111/j.1399-0039.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Harizi H, Juzan M, Grosset C, Rashedi M, Gualde N. Dendritic cells issued in vitro from bone marrow produce PGE2 that contributes to the immunomodulation induced by antigen-presenting cells. Cell Immunol. 2001;209:19–28. doi: 10.1006/cimm.2001.1785. [DOI] [PubMed] [Google Scholar]
- Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- Harper JMM, Parsonage MT, Pelham HRB, Darby G. Heat inactivation of vaccinia virus particle-associated functions: properties of heated particles in vivo and in vitro. J Virol. 1978;26:646–659. doi: 10.1128/jvi.26.3.646-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Hinson RM, Williams JA, Shacter E. Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: possible role of cyclooxygenase-2. Proc Natl Acad Sci USA. 1996;93:4885–4890. doi: 10.1073/pnas.93.10.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez MA, Martinez-Martinez S, Punzon C, Redondo JM, Fresno M. An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J Biol Chem. 2000;275:23627–23635. doi: 10.1074/jbc.M001381200. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci. 2004;9:1977–1993. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Wingerd BA, Arakawa T, Smith WL. Cyclooxygenase-2 gene transcription in a macrophage model of inflammation. J Immunol. 2006;177:8111–8122. doi: 10.4049/jimmunol.177.11.8111. [DOI] [PubMed] [Google Scholar]
- Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65:11156–11163. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- Lehmann MH, Kastenmuller W, Kandemir JD, Florian Brandt F, Suezer Y, Sutter G. Modified vaccinia virus Ankara triggers chemotaxis of monocytes and early respiratory immigration of leukocytes by induction of CCL2 expression. J Virol. 2009;83:2540–2552. doi: 10.1128/JVI.01884-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou JT, Chen ZY, Ho LJ, Yang SP, Chang DM, Liang CC, Lai JH. Differential effects of triptolide and tetrandrine on activation of COX-2, NF-κB, and AP-1 and virus production in dengue virus-infected human lung cells. Eur J Pharmacol. 2008;589:288–298. doi: 10.1016/j.ejphar.2008.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chavan R, Feinberg MB. Dendritic cells are preferentially targeted among hematolymphocytes by modified vaccinia virus Ankara and play a key role in the induction of virus-specific T cell responses in vivo. BMC Immunol. 2008;9:15. doi: 10.1186/1471-2172-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zaman W, Kaphalia BS, Ansari GA, Garofalo RP, Casola A. RSV-induced prostaglandin E2 production occurs via cPLA2 activation: role in viral replication. Virology. 2005;343:12–24. doi: 10.1016/j.virol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Lynch HE, Ray CA, Oie KL, Pollara JJ, Petty ITD, Sadler AJ, Williams BRG, Pickup DJ. Modified vaccinia virus Ankara can activate NF-κB transcription factors through a double-stranded RNA-activated protein kinase (PKR)-dependent pathway during the early phase of virus replication. Virology. 2009;391:177–186. doi: 10.1016/j.virol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr A, Stickl H, Muller HK, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author’s transl) Zentralbl Bakteriol [B] 1978;167:375–390. [PubMed] [Google Scholar]
- Moss B, Carroll MW, Wyatt LS, Bennink JR, Hirsch VM, Goldstein S, Elkins WR, Fuerst TR, Lifson JD, Piatak M, Restifo NP, Overwijk W, Chamberlain R, Rosenberg SA, Sutter G. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Basu GD, Tinder TL, Subramani DB, Bradley JM, Arefayene M, Skaar T, De Petris G. Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J Immunol. 2009;182:216–224. [PMC free article] [PubMed] [Google Scholar]
- Murono S, Inoue H, Tanabe T, Joab I, Yoshizaki T, Furukawa M, Pagano JS. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc Natl Acad Sci USA. 2001;98:6905–6910. doi: 10.1073/pnas.121016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie KL, Pickup DJ. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-κB activation. Virology. 2001;288:175–187. doi: 10.1006/viro.2001.1090. [DOI] [PubMed] [Google Scholar]
- Palumbo GJ, Buller RML, Glasgow WC. Multigenic evasion of inflammation by poxviruses. J Virol. 1994;68:1737–1749. doi: 10.1128/jvi.68.3.1737-1749.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo GJ, Glasgow WC, Buller RML. Poxvirus-induced alteration of arachidonate metabolism. Proc Natl Acad Sci USA. 1993;90:2020–2024. doi: 10.1073/pnas.90.5.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Phipps RP, Stein SH, Roper RL. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991;12:349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JC, Gherardi MM, Esteban M. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J Virol. 2000;74:923–933. doi: 10.1128/jvi.74.2.923-933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Enquist LW. Biological interactions between herpesviruses and cyclooxygenase enzymes. Rev Med Virol. 2006;16:393–403. doi: 10.1002/rmv.519. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Sutter G. Candidate influenza vaccines based on recombinant modified vaccinia virus Ankara. Expert Rev Vaccines. 2009;8:447–454. doi: 10.1586/erv.09.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EP, Malboeuf CM, Bernard M, Rose RC, Phipps RP. Cyclooxygenase-2 inhibition attenuates antibody responses against human papillomavirus-like particles. J Immunol. 2006;177:7811–7819. doi: 10.4049/jimmunol.177.11.7811. [DOI] [PubMed] [Google Scholar]
- Sapirstein A, Bonventre JV. Specific physiological roles of cytosolic phospholipase A2 as defined by gene knockouts. Biochim Biophys Acta. 2000;1488:139–148. doi: 10.1016/s1388-1981(00)00116-5. [DOI] [PubMed] [Google Scholar]
- Savard M, Belanger C, Tremblay MJ, Dumais N, Flamand L, Borgeat P, Gosselin J. EBV suppresses prostaglandin E2 biosynthesis in human monocytes. J Immunol. 2000;164:6467–6473. doi: 10.4049/jimmunol.164.12.6467. [DOI] [PubMed] [Google Scholar]
- Seno K, Okuno T, Nishi K, Murakami Y, Watanabe F, Matsuura T, Wada M, Fujii Y, Yamada M, Ogawa T, Okada T, Hashizume H, Kii M, Hara S, Hagishita S, Nakamoto S, Yamada K, Chikazawa Y, Ueno M, Teshirogi I, Ono T, Ohtani M. Pyrrolidine inhibitors of human cytosolic phospholipase A2. J Med Chem. 2000;43:1041–1044. doi: 10.1021/jm9905155. [DOI] [PubMed] [Google Scholar]
- Shisler JL, Jin XL. The vaccinia virus K1L gene product inhibits host NF-κB activation by preventing IκB3 degradation. J Virol. 2004;78:3553–3560. doi: 10.1128/JVI.78.7.3553-3560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259:315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Steer SA, Corbett JA. The role and regulation of COX-2 during viral infection. Viral Immunol. 2003;16:447–460. doi: 10.1089/088282403771926283. [DOI] [PubMed] [Google Scholar]
- Steer SA, Moran JM, Maggi LB, Buller RML, Perlman H, Corbett JA. Regulation of cyclooxygenase-2 expression by macrophages in response to double-stranded RNA and viral infection. J Immunol. 2003;170:1070–1076. doi: 10.4049/jimmunol.170.2.1070. [DOI] [PubMed] [Google Scholar]
- Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schafer H, Holzner A. MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author’s transl) Dtsch Med Wochenschr. 1974;99:2386–2392. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Sutter G, Staib C. Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery. Curr Drug Targets Infect Disord. 2003;3:263–271. doi: 10.2174/1568005033481123. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Waris G, Siddiqui A. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: role of prostaglandin E2 in RNA replication. J Virol. 2005;79:9725–9734. doi: 10.1128/JVI.79.15.9725-9734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Willis KL, Patel S, Xiang Y, Shisler JL. The effect of the vaccinia K1 protein on the PKR-eIF23 pathway in RK13 and HeLa cells. Virology. 2009;394:73–81. doi: 10.1016/j.virol.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci USA. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor κB and nuclear factor-interleukin-6 in the tumor necrosis factor 3-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- Yuwen H, Cox JH, Yewdell JW, Bennink JR, Moss B. Nuclear localization of a double-stranded RNA-binding protein encoded by the vaccinia virus E3L gene. Virology. 1993;195:732–744. doi: 10.1006/viro.1993.1424. [DOI] [PubMed] [Google Scholar]
- Zeytin HE, Patel AC, Rogers CJ, Canter D, Hursting SD, Schlom J, Greiner JW. Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA. Tg/MIN mice. Cancer Res. 2004;64:3668–3678. doi: 10.1158/0008-5472.CAN-03-3878. [DOI] [PubMed] [Google Scholar]