Abstract
Objective
There are multiple reasons why females who inject drugs may be more likely to become infected with HIV than males who inject drugs. Where this is the case, special HIV prevention programs for females would be needed.
Design
International systematic review and meta-analysis of studies across 14 countries.
Methods
Countries with high seroprevalence (>20%) HIV epidemics among persons who inject drugs (PWID) were identified from the Reference Group to the UN on HIV and Injecting Drug Use. Systematic literature reviews collected data on HIV prevalence by gender for these countries. Non-parametric and parametric tests along with meta-analytic techniques examined heterogeneity and differences in odds ratios (OR) across studies.
Results
Data were abstracted from 117 studies in 14 countries; total sample size N=128,745. The mean weighted OR for HIV prevalence among females to males was 1.18 [95% CI 1.10–1.26], with high heterogeneity among studies (I2 = 70.7%). There was a Gaussian distribution of the log ORs across studies in the sample.
Conclusion
There was a significantly higher HIV prevalence among females compared to males who inject drugs in high seroprevalence settings, but the effect size is extremely modest. The high level of heterogeneity and the Gaussian distribution suggest multiple causes of differences in HIV prevalence between females and males, with a specific difference determined by local factors. Greater understanding of factors that may protect females from HIV infection may provide insights into more effective HIV prevention for both females and males who inject drugs.
Keywords: Substance Abuse, Intravenous, Drug users statistics/numerical data, Male, Female, HIV Infections/Epidemiology, Prevalence, Sex Factors/Characteristics
1. Introduction
Gender disparities in risk for HIV infection are of considerable concern in many different countries (Madkan et al., 2006; UNAIDS, 2004; UNODC, 2006b), with females who inject drugs (FWID) often at increased risk for HIV infection compared to males who inject drugs (MWID). Studies conducted in nine European countries documented greater HIV prevalence among FWID compared to MWID (EMCDDA, 2006). In sub-Saharan Africa, 40% of HIV infections in 1985 were diagnosed in females; by 2002, 60% of HIV infections were among females (DeLay, 2004). Globally, nearly 50% of HIV infections in the last five years have been diagnosed among females (United Nations Population Fund, 2005).
FWID often face significant stigma, leading to lower participation in drug treatment, needle/syringe exchange programs (NSP), and other harm reduction services (Network, 2010; Razani et al., 2007; Simmonds and Coomber, 2009). In Dhaka Bangladesh, nearly all NSP participants are male, with harm reduction services tailored toward MWID with little attention toward FWID (Azim et al., 2008).
A Russian survey among MWID found that 21% abused their FWID partners due to the female’s drug addictions (Gorshkova ID, 2003); unfortunately, services for abused women are rarely tailored for FWID (Network, 2010). A 2003 Vancouver study reported 19% of MWID had a history of sexual violence compared to 68% among FWID.
FWID usually depend on male partners for drugs and injections, leading to elevated drug and equipment sharing (UNODC, 2006a). An Iran study among IDU couples found that males admitted their female partners often needed help injecting and relied exclusively on them to acquire drugs and injecting equipment (Razani et al., 2007).
Many FWID participate in commercial sex work (CSW) to fund their drug habit, (Benotsch et al., 2004; Cleland et al., 2007; Lowndes CM, 2002), ranging from 7% in France to 83% in the Netherlands (Gollub et al., 1998; Renwick et al., 2002). Condom use is very infrequent; a China study reported condom use as low as 6% among regular/casual partners and less than 25% among clients (Lau et al., 2005). Females are biologically more susceptible to sexual transmission of HIV and often have higher prevalence of STI infection, such as HSV-2, which increases the probability of HIV infection.
The potential higher risk for HIV among females raises the issue of general versus targeted HIV prevention programs for FWID. Should HIV prevention efforts be aimed at PWID populations as a whole, with large-scale programs possibly achieving a community-level protective effect (Des Jarlais et al., 2005a)? Or if FWID are at higher risk and not likely to be reached by general programs, are prevention programs specifically targeted to females required? Specifically targeted programs may have higher costs per person served than general programs, but they may be quite cost effective in averting infections among females. This issue becomes of particular importance in resource limited settings, where implementation of programs aimed specifically at FWID may reduce resources available for HIV prevention in the injecting community as whole.
The question of whether females who inject are more likely to be infected with HIV compared to males who inject is, however, an empirical question. Data on differences in HIV infection between the two genders can be utilized for scarce-resource allocation decisions. In this study, we conducted an international systematic review and meta-analysis to assess differences in HIV prevalence among females and males who inject drugs in high seroprevalence areas.
2. Methods
As the same odds ratio (OR) is of greater public health importance in a setting of high HIV prevalence versus low prevalence, we restricted our study to areas that at one time had greater than 20% HIV prevalence among PWID. Countries with high seroprevalence (>20%) HIV epidemics among PWID were identified from the Reference Group to the UN on HIV and Injecting Drug Use (Mathers et al., 2008). The countries identified and included in this review are Argentina, Brazil, China, Estonia, France, Italy, the Netherlands, Puerto Rico, Russia, Scotland, Spain, Ukraine and Vietnam. New York City USA, was also included because very high prevalence levels occurred among IDUs there and the city served as the epicenter for the HIV epidemic among PWID in the northeastern part of the US (CDUHR, 1999). Nepal and Indonesia were excluded from the review due to lack of reliable data on HIV prevalence by gender among their PWID populations. We utilized New York City instead of the entire US because the New York City/Northeast corridor has been the primary region for HIV infection among PWID in the US (meeting the criterion for high prevalence) (CDC, 1984).
Participants were recruited from a variety of different locations including NSP locations and other harm reduction services, through community outreach, and through various types of peer referrals, including respondent driven sampling. Sampling drug users is often quite difficult, as participants may be reluctant to participate in research studies due to legal issues or social stigma, and in many instances, the size of this population is unknown and cannot be adequately measured (Magnani et al., 2005; Watters J., 1989).
2.1 Search Methodology
Studies were selected from several sources including PubMed, EMBASE, NLM Gateway, conference abstracts from International AIDS Society conferences, and government reports published by UNAIDS and UNGASS. Systematic literature searches were conducted to identify potentially eligible articles from journals and government/country reports. In addition, we also searched conference abstracts and references from review articles regarding injecting populations in any of the countries selected for inclusion.
In order for a study to be eligible for inclusion, the authors had to report HIV prevalence among PWID by gender, verified by HIV testing; the sample had to be made up of at least 90% PWID (who may or may not be currently injecting drugs). Studies that used self-report to assess prevalence were excluded; we also excluded studies that had fewer than 5 females in the entire sample. One of the major advantages of meta-analysis is the ability to appropriately combine reports with small samples. However, extremely small samples of key subpopulations (females who inject drugs in this case) raise concern not only because of the statistical uncertainty, but also because of the likelihood that an extremely small sample for a key subpopulation will not represent the diversity within that group.
Our search included reports published from January 1985 (when HIV antibody testing became generally available) through June, 2011. We recognize that there may be considerable variation in HIV infection among PWID in different parts of the same country, particularly for large, diverse countries. In these large countries, we attempted to obtain data from as many locations as possible, focusing on large cities and locations where PWID are located. Table 1 gives the breakdown of terminology used to search for eligible studies. The same search terms were utilized for all databases (EMBASE, PubMed, NLM, etc.).
Table 1.
Search Terms used for Retrieval of Eligible Citations
| (HIV Infections/prevention and control[MeSH] OR HIV[MeSH] OR “HIV Infections”[Mesh] OR “HIV Seropositivity”[Mesh] OR “HIV Seroprevalence”[Mesh] OR hiv[tw] OR hiv-1[tw] OR hiv-2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immune deficiency virus[tw] OR human immuno-deficiency virus[tw] OR human immune-deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndromes[tw] OR acquired immune deficiency syndrome[tw] OR acquired immuno-deficiency syndrome[tw] OR acquired immune-deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw]))) |
| AND (“Substance Abuse, Intravenous”[Mesh] OR “Injection Drug Use”[TIAB] OR “IDU” [TIAB] OR “Injectors”[TIAB] OR “Intravenous Drug Use”[TIAB] or “Intravenous Drug Abuse”[TIAB] OR “Injection Drug Abuse”[TIAB]) |
| AND (“Argentina”[Mesh] OR “Brazil”[Mesh] OR “China” [Mesh] OR “Estonia”[Mesh] OR “Indonesia”[Mesh] OR “Italy”[Mesh] OR “New York City”[Mesh] OR “Netherlands”[Mesh] OR “Puerto Rico” [Mesh] AND “Russia”[Mesh] OR “Scotland”[Mesh] OR “Spain”[Mesh] OR “Ukraine”[Mesh] OR “Vietnam”[Mesh] OR “France”[Mesh] OR “Argentina”[TIAB] OR “Brazil”[TIAB] OR “China” [TIAB] OR “Estonia”[TIAB] OR “Indonesia”[TIAB] OR “Italy”[TIAB] OR “New York City”[TIAB] OR “NYC”[TIAB] OR “Netherlands”[TIAB] OR “Puerto Rico”[TIAB] OR “Russia”[TIAB] OR “Scotland”[TIAB] OR “Spain”[TIAB] OR “Ukraine”[TIAB] OR “Vietnam”[TIAB] OR “France”[TIAB]) |
| AND (“Female”[Mesh] AND “Male”[Mesh])* |
Note that search was performed with last modified ((“Female”[Mesh] AND “Male”[Mesh]) phrase included and excluded
We occasionally found multiple reports from the same parent research project. We excluded all duplicate reports from the same authors that utilized identical data, that is, reports with the same sample size, the same dates of data collection and the same recruitment sites. There were, however, examples of multiple reports from the same research project where the data were “similar” but not identical. For example, consider a cohort study with one published report of the baseline data and then a second published report with 2-year follow-up data. Should these two reports be considered as reports on the “same” subjects? Clearly subjects who seroconverted during the follow-up period should not be considered the “same” as they were at baseline. Using only one of the two reports would mean discarding either the baseline data or the data on seroconversions, and there is no obvious criteria for deciding which report to use. For serial cross-sectional research projects, the “similarity” problem was usually overlapping data collection periods in different reports. For example, one report might contain data collected from 1990 to 2000 and a second report might contain data collected from 1995 to 2002. Again, selecting only one report to use would mean discarding data, and there is no obvious basis for selecting which report to use and which to discard.
Statistically, the “similarity/non-independence” of data in different reports from the same parent research project might be considered as a problem of an interclass correlation between the two reports. If individual-level data had been available, it would be possible to calculate the interclass correlation coefficient, and to adjust (reduce) the effective total sample size. As individual level data were not available for any of the reports, we considered multiple but not identical reports from the same parent research project as separate studies, and then examined how adjusting for interclass correlations might have affected the total effective sample size for the meta-analysis (specifically the weighted pooled OR for female:male HIV prevalence) (Gleser L., 2009).
2.2 Data Analysis
Data on HIV prevalence for females and for males were abstracted from each eligible study, converted into female:male HIV prevalence odds ratios (ORs) and then transformed into natural logarithm odds ratios (log ORs). All analyses were conducted with the log ORs. Presentation of the results used either the log ORs or conversions from log ORs back to ORs. Forest plots were used to report female:male HIV prevalence log ORs with 95% confidence intervals. Funnel plots and the Egger’s test were used to assess possible publication bias in the located studies, and I2 was used to assess heterogeneity among the log ORs. Weighting of the log ORs was done using random effects. STATA 11 (College Station, TX USA) (StataCorp LP., 2009) was used for analysis.
3. Results
3.1 Search Results
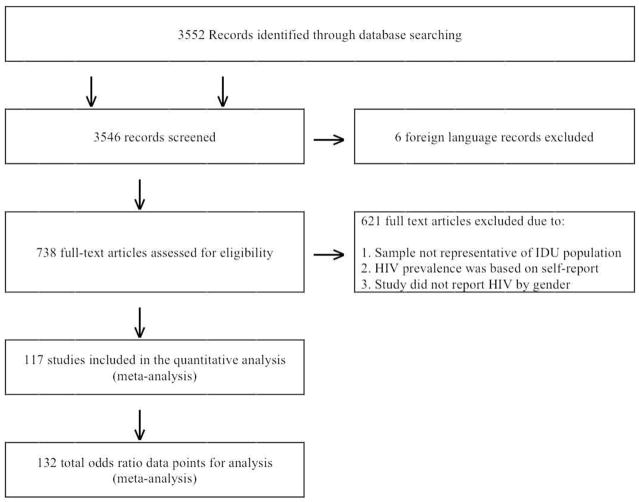
Figure 1 shows the PRISMA diagram (Liberati et al., 2009; Moher et al., 2009) for the searching and screening that led to the final number of studies included in this review. Searching identified 3552 article titles. Six papers in languages other than English that could not be obtained were eliminated. We screened 3546 abstracts against the inclusion criteria and retrieved 738 full text articles. Of the articles and reports retrieved, 117 met all criteria for inclusion and were coded for our review (these studies are presented in Table 2). These 117 articles provided a total of 132 female:male HIV prevalence odds ratio comparisons from 14 different countries. (Some studies presented data separately for two or more different samples in the same article.) The included studies contained 128,745 subjects. The primary reasons for exclusion of abstracts or full text articles included: the sample came from an HIV medical service, i.e. sample was HIV positive, the HIV data were based on self report rather than laboratory testing, or the study did not report HIV prevalence by gender. When appropriate, we contacted authors that did not report HIV prevalence by gender, in order to obtain this information directly from the primary author of the paper.
Figure 1.
Prisma diagram of eligible studies in review
Table 2.
Summary of Included Studies
|
Argentina (Estimated number of IDU: 65,829) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Weissenbacher 2003 (Weissenbacher et al., 2003) | 174 | 2000 | 2001 | Hospital Clinic | 137 | 37 | 0.460 | 0.378 | 0.713 |
| Diaz 1988 (Diaz et al., 2001) | 99 | 1986 | 1987 | Street-recruited | 89 | 10 | 0.404 | 0.500 | 1.472 |
|
Brazil (Estimated number of IDU: 800,000) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Peixinho 1990 (Peixinho et al., 1990) | 188 | 1986 | 1987 | Drug Treatment | 170 | 18 | 0.153 | 0.222 | 1.580 |
| Telles 1994 (Telles PR, 1992) | 123 | 1989 | 1992 | Drug Treatment | 103 | 20 | 0.359 | 0.250 | 0.595 |
| Mesquita 2001 (Mesquita et al., 2001) | 457 | 1991 | 1999 | Drug Treatment | 315 | 142 | 0.530 | 0.710 | 2.171 |
| de Carvalho 1996 (de Carvalho et al., 1996) | 214 | 1991 | 1992 | Drug Treatment | 125 | 89 | 0.590 | 0.670 | 1.411 |
| AL Kritski 1992 (Kritski AL, 1992) | 58 | 1992 | 1992 | Drug Treatment | 51 | 7 | 0.353 | 0.286 | 0.733 |
| Guimarães 2001 (Guimaraes et al., 2001) | 175 | 1994 | 1997 | Drug Treatment | 147 | 28 | 0.252 | 0.321 | 1.408 |
| Dourado 1999 (Dourado et al., 1999) | 216 | 1994 | 1996 | Street Recruitment | 177 | 39 | 0.441 | 0.744 | 3.681 |
| Cintra 2006 (Cintra et al., 2006) | 855 | 2000 | 2001 | Syringe Exchange | 709 | 146 | 0.360 | 0.390 | 1.137 |
| Caiaffa 2006 (Caiaffa et al., 2006) | 857 | 2000 | 2001 | Syringe Exchange | 710 | 147 | N/A | N/A | 1.150 |
| Teixeira 2004 (Teixeira et al., 2004) | 608 | 1999 | 2001 | Street Recruitment | 494 | 114 | 0.069 | 0.070 | 1.021 |
|
China (Estimated number of IDU: 2,350,000) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Zheng 1994 (Zheng et al., 1994) | 282 | 1992 | 1992 | Street Recruitment | 276 | 6 | 0.496 | 0.333 | 0.507 |
| Zhang 2002 (Zhang et al., 2002) | 97 | 2000 | 2000 | Drug Treatment | 94 | 3 | 0.702 | 0.667 | 0.848 |
| Zhang 2002 (Zhang et al., 2002) | 96 | 2000 | 2000 | Drug Treatment | 52 | 44 | 0.654 | 0.841 | 2.798 |
| Zhang 2007 (Zhang et al., 2007a) | 781 | 2002 | 2002 | Street Recruitment | 698 | 83 | 0.310 | 0.140 | 0.362 |
| Zhang 2007 (Zhang et al., 2007b) | 508 | 2002 | 2002 | Street Recruitment | 442 | 66 | 0.080 | 0.080 | 1.000 |
| Ruan 2004 (Ruan et al., 2004) | 379 | 2002 | 2002 | Street Recruitment | 313 | 66 | 0.121 | 0.076 | 0.598 |
| Hao 2008 (Hao C., 2008) | 333 | 2002 | 2006 | Street Recruitment | 272 | 61 | 0.121 | 0.076 | 0.594 |
| Yin 2007 (Yin et al., 2007) | 314 | 2004 | 2004 | Street Recruitment | 269 | 45 | 0.197 | 0.067 | 0.293 |
| Jia 2008 (Jia et al., 2008) | 682 | 2004 | 2005 | Street Recruitment | 560 | 122 | 0.554 | 0.344 | 0.423 |
| Zhang 2008 (Zhang et al., 2008) | 383 | 2005 | 2005 | Street Recruitment | 339 | 44 | 0.360 | 0.455 | 1.484 |
| Jia 2010 (Jia et al., 2008) | 740 | 2008 | 2008 | Detoxification Unit | 679 | 61 | 0.046 | 0.049 | 1.081 |
| Zhou 2011 (Zhou et al., 2011) | 403 | 2009 | 2009 | Methadone Clinic | 399 | 4 | 0.336 | 0.500 | 1.976 |
|
France (Estimated number of IDU: 122,000) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Helal 1995 (Helal et al., 1995) | 147 | 1993 | 1993 | HIV Testing Center | 109 | 38 | 0.101 | 0.027 | 0.247 |
|
Netherlands (Estimated number of IDU: 17,700) (EMCDDA, 2010)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Van den Hoek 1988 (van den Hoek et al., 1988) | 251 | 1985 | 1987 | Methadone Clinic | 142 | 109 | 0.380 | 0.310 | 0.733 |
| Van den Hoek 1989 (van den Hoek et al., 1989) | 263 | 1985 | 1988 | Methadone Clinic | 140 | 123 | 0.360 | 0.300 | 0.762 |
| Spijkerman 1996 (Spijkerman et al., 1996) | 758 | 1986 | 1994 | Methadone Clinic | 430 | 328 | 0.321 | 0.302 | 0.915 |
| Van den Hoek 1990 (van den Hoek et al., 1990) | 243 | 1989 | 1999 | Methadone Clinic | 130 | 113 | 0.360 | 0.430 | 1.341 |
| van der Snoek 2000 (van der Snoek et al., 2000) | 70 | 1993 | 1993 | STD Clinic | 24 | 46 | 0.125 | 0.152 | 1.256 |
| Wiessing 1995 (Wiessing et al., 1995) | 340 | 1994 | 1994 | Methadone Clinic | 259 | 81 | 0.104 | 0.074 | 0.687 |
| IM de Boer 2004 (de Boer et al., 2004) | 419 | 1994 | 2002 | Methadone Clinic | 326 | 93 | 0.089 | 0.140 | 1.666 |
| van der Snoek 2000 (van der Snoek et al., 2000) | 64 | 1998 | 1998 | STD Clinic | 32 | 32 | 0.031 | 0.031 | 1.000 |
|
Ukraine (Estimated number of IDU: 375,000) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Booth 2006 (Booth et al., 2006) | 774 | 2004 | 2004 | Street Recruitment | 610 | 164 | 0.221 | 0.311 | 1.591 |
| Booth 2007 (Booth et al., 2007) | 1557 | 2004 | 2006 | Street Recruitment | 1182 | 375 | 0.320 | 0.400 | 1.417 |
| Dumchev 2009 (Dumchev et al., 2009) | 315 | 2005 | 2005 | Street Recruitment | 258 | 57 | 0.140 | 0.141 | 1.008 |
| Pohorila 2010 (Pohorila, 2010) | 3962 | 2007 | 2009 | Street Recruitment | 3036 | 926 | 0.205 | 0.250 | 1.293 |
| Taran 2010 (Taran et al., 2011) | 3711 | 2008 | 2008 | Street Recruitment | 2768 | 943 | N/A | N/A | 1.600 |
|
Puerto Rico (Estimated number of IDU: 29,130) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Robles 1992 (Robles et al., 1994) | 1637 | 1989 | 1990 | Street Recruitment | 1308 | 329 | 0.489 | 0.416 | 0.740 |
| Rodriguez 1993 (Marrero Rodriguez et al., 1993) | 255 | 1989 | 1990 | Street Recruitment | 184 | 71 | 0.228 | 0.254 | 1.150 |
| Robles 1994 (Robles et al., 1994) | 342 | 1990 | 1991 | Detoxification Clinic | 290 | 52 | 0.287 | 0.34 | 1.280 |
| Deren 2001 (Deren et al., 2001) | 290 | 1992 | 1999 | Street Recruitment | 249 | 41 | 0.22 | 0.22 | 1.01 |
|
Italy (Estimated number of IDU: 326,000) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Serraino 1992 (Serraino et al., 1992) | 349 | 1984 | 1988 | Drug Treatment | 247 | 102 | 0.450 | 0.320 | 0.575 |
| Serraino 1991 (Serraino et al., 1991) | 581 | 1984 | 1988 | Drug Treatment | 434 | 147 | 0.410 | 0.327 | 0.697 |
| Sabbatani 2005 (Sabbatani, 2005) | 1214 | 1984 | 2002 | Drug Treatment | 916 | 298 | 0.459 | 0.608 | 1.828 |
| Romano 1992 (Romano et al., 1992) | 812 | 1985 | 1990 | Methadone Clinic | 678 | 134 | 0.490 | 0.664 | 2.061 |
| Rezza 1994 (Rezza et al., 1994) | 8602 | 1986 | 1987 | Drug Treatment | 7066 | 1536 | 0.234 | 0.355 | 1.801 |
| Zaccarelli 1990 (Zaccarelli et al., 1990) | 1180 | 1986 | 1989 | Drug Treatment | 925 | 255 | 0.357 | 0.455 | 1.505 |
| De Rosa 2007 (De Rosa et al., 2007) | 263 | 1986 | 1999 | Infectious Dis. Clinic | 202 | 61 | 0.356 | 0.459 | 1.532 |
| Sasse 1989 (Sasse et al., 1989) | 1175 | 1987 | 1987 | Drug Treatment | 875 | 179 | 0.365 | 0.403 | 1.178 |
| Farci 1992 (Farci et al., 1992) | 145 | 1987 | 1987 | AIDS Surv. Program | 102 | 43 | 0.637 | 0.558 | 0.719 |
| Salmaso 1991 (Salmaso et al., 1991) | 1027 | 1988 | 1988 | Drug Treatment | 811 | 216 | 0.379 | 0.380 | 1.004 |
| Rezza 1993 (Rezza et al., 1993) | 11829 | 1990 | 1990 | Drug Treatment | 9694 | 2135 | 0.199 | 0.235 | 1.235 |
| Rezza 1993 (Rezza et al., 1993) | 13233 | 1991 | 1991 | Drug Treatment | 11113 | 2120 | 0.159 | 0.202 | 1.342 |
| Boschini 1996 (Boschini et al., 1996) | 4236 | 1991 | 1994 | Drug Rehab Center | 3321 | 915 | 0.365 | 0.244 | 0.559 |
| Turrina 2001 (Turrina et al., 2001) | 178 | 1993 | 1993 | Methadone Clinic | 119 | 59 | 0.723 | 0.695 | 0.874 |
| Lugoboni 2002 (Lugoboni et al., 2002) | 486 | 1994 | 2000 | Drug Treatment | 401 | 85 | 0.032 | 0.047 | 1.492 |
| Quaglio 2006 (Quaglio et al., 2006) | 1091 | 2002 | 2002 | Drug Treatment | 920 | 171 | 0.116 | 0.170 | 0.641 |
|
New York City (Estimated number of IDU: 105,000)(NYC National HIV Behavioral Surveillance Team, 2009)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Des Jarlais 1989 (Des Jarlais et al., 1989) | 287 | 1984 | 1984 | Drug Treatment | 214 | 73 | 0.486 | 0.589 | 1.516 |
| Des Jarlais 1994 (Des Jarlais et al., 1994) | 141 | 1984 | 1984 | Drug Treatment | 93 | 48 | 0.548 | 0.563 | 1.059 |
| Grieco 1989 (Grieco et al., 1989) | 199 | 1984 | 1987 | Drug Treatment | 136 | 63 | 0.338 | 0.286 | 0.785 |
| Burkett 1990 (Burkett and Brown, 1990) | 374 | 1985 | 1985 | Drug Treatment | 260 | 114 | 0.538 | 0.596 | 1.267 |
| Banks 1991 (Banks et al., 1991) | 284 | 1986 | 1986 | Drug Treatment | 170 | 114 | 0.547 | 0.544 | 0.987 |
| Nemoto 1991 (Nemoto et al., 1991) | 254 | 1986 | 1986 | Drug Treatment | 156 | 98 | 0.596 | 0.633 | 1.169 |
| Burkett 1990 (Burkett and Brown, 1990) | 253 | 1986 | 1986 | Drug Treatment | 156 | 97 | 0.590 | 0.649 | 1.289 |
| Burkett 1990 (Burkett and Brown, 1990) | 221 | 1987 | 1987 | Drug Treatment | 143 | 78 | 0.629 | 0.538 | 0.687 |
| Des Jarlais 1989 (Des Jarlais et al., 1989) | 135 | 1987 | 1987 | STD Clinic | 97 | 38 | 0.443 | 0.421 | 0.913 |
| Das Gupta 1995 (Dasgupta, 1995) | 278 | 1987 | 1988 | Street Recruitment | 190 | 88 | 0.526 | 0.489 | 0.860 |
| El Sadr 1992 (el-Sadr et al., 1992) | 223 | 1988 | 1989 | Drug Treatment | 147 | 76 | 0.578 | 0.513 | 0.769 |
| Chiasson 1991 (Chiasson et al., 1991) | 292 | 1988 | 1990 | STD Clinic | 206 | 86 | 0.471 | 0.477 | 1.024 |
| Stricof 1991 (Stricof et al., 1991) | 60 | 1988 | 1989 | Homeless Shelter | 44 | 16 | 0.159 | 0.125 | 0.756 |
| Cournos 1994 (Cournos et al., 1994) | 73 | 1989 | 1991 | Psychiatric Hospital | 54 | 19 | 0.148 | 0.263 | 2.054 |
| Des Jarlais 1994 (Des Jarlais et al., 1994) | 974 | 1990 | 1992 | Drug Treatment | 770 | 204 | 0.519 | 0.471 | 0.822 |
| Des Jarlais 2010 (Des Jarlais et al., 2010) | 261 | 1990 | 1994 | Drug Treatment | 181 | 80 | 0.165 | 0.310 | 2.274 |
| Des Jarlais 2009 (Des Jarlais et al., 2009a) | 1203 | 1990 | 1994 | Drug Treatment | 982 | 221 | 0.490 | 0.480 | 0.961 |
| Des Jarlais 1999 (Des Jarlais et al., 1999) | 3375 | 1990 | 1996 | Detoxification Clinic | 2609 | 766 | 0.025 | 0.112 | 4.873 |
| Des Jarlais 2005 (Des Jarlais et al., 2005b) | 480 | 1990 | 2001 | Drug Treatment | 390 | 90 | 0.250 | 0.200 | 0.750 |
| Neaigus 1996 (Neaigus et al., 1996) | 174 | 1991 | 1993 | Street Recruitment | 99 | 75 | 0.152 | 0.360 | 3.150 |
| Kottiri 2002 (Kottiri et al., 2002) | 662 | 1991 | 1993 | Street Recruitment | 470 | 192 | N/A | N/A | 1.050 |
| Jose 1993 (Jose et al., 1993) | 660 | 1991 | 1993 | Street Recruitment | 475 | 185 | 0.492 | 0.619 | 1.678 |
| Des Jarlais 2009 (Des Jarlais et al., 2009a) | 1109 | 1995 | 2008 | Drug Treatment | 839 | 270 | 0.050 | 0.090 | 1.879 |
| Des Jarlais 2010 (Des Jarlais et al., 2010) | 1153 | 1995 | 2008 | Drug Treatment | 877 | 276 | 0.047 | 0.090 | 2.005 |
| Diaz 2001 (Diaz et al., 2001) | 156 | 1997 | 1999 | Street Recruitment | 112 | 44 | 0.063 | 0.227 | 4.412 |
| Frajzyngier 2007 (Frajzyngier et al., 2007) | 249 | 1999 | 2003 | Street Recruitment | 164 | 85 | 0.019 | 0.013 | 0.680 |
| Neaigus 2007 (Neaigus et al., 2007) | 259 | 1999 | 2003 | Street Recruitment | 176 | 83 | 0.023 | 0.036 | 1.612 |
| Des Jarlais 2007 (Des Jarlais et al., 2007b) | 1891 | 2000 | 2004 | Street Recruitment | 1532 | 359 | 0.140 | 0.160 | 1.170 |
| Des Jarlais 2007 (Des Jarlais et al., 2007a) | 229 | 2001 | 2004 | Drug Treatment | 178 | 51 | 0.170 | 0.080 | 0.425 |
| Des Jarlais 2007 (Des Jarlais et al., 2007a) | 1725 | 2001 | 2004 | Street Recruitment | 1392 | 333 | 0.130 | 0.140 | 1.089 |
| Des Jarlais 2007 (Des Jarlais et al., 2007b) | 333 | 2004 | 2004 | Drug Treatment | 256 | 77 | 0.230 | 0.350 | 1.803 |
| Des Jarlais 2009 (Des Jarlais et al., 2009b) | 363 | 2005 | 2007 | Drug Treatment | 301 | 62 | 0.150 | 0.270 | 2.096 |
|
Scotland (Estimated number of IDU: 27,357) (King et al., 2009)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| McIntyre 2001 (McIntyre et al., 2001) | 217 | 1993 | 1993 | SCIEH Records | 164 | 53 | 0.049 | 0.038 | 0.765 |
| McIntyre 2001 (McIntyre et al., 2001) | 411 | 1995 | 1996 | SCIEH Records | 318 | 93 | 0.028 | 0.011 | 0.373 |
| McIntyre 2001 (McIntyre et al., 2001) | 174 | 1997 | 1997 | SCIEH Records | 125 | 49 | 0.056 | 0.041 | 0.717 |
| Ronald 1993 (Ronald et al., 1993) | 320 | 1982 | 1993 | Street Recruitment | 223 | 97 | 0.534 | 0.546 | 1.053 |
|
Spain (Estimated number of IDU: 83,972) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Ribera 1998 (Ribera et al., 1998) | 283 | 1984 | 1995 | Hospital Recruitment | 224 | 59 | 0.777 | 0.712 | 0.710 |
| Muga 2007 (Muga et al., 2007) | 490 | 1987 | 1991 | Drug Treatment | 409 | 101 | 0.7 | 0.75 | 1.29 |
| Hernandez-Aguado 1993 (Hernandez-Aguado and Bolumar, 1993) | 2687 | 1987 | 1991 | Drug Treatment | 2015 | 672 | 0.500 | 0.490 | 0.961 |
| Hernandez-Aguado 1999 (Hernandez-Aguado et al., 1999) | 7130 | 1987 | 1996 | HIV Testing Center | 5488 | 1642 | 0.428 | 0.472 | 1.194 |
| Rebagliato 1995 (Rebagliato et al., 1995) | 4131 | 1987 | 1992 | HIV Testing Center | 3151 | 978 | 0.475 | 0.514 | 1.170 |
| Muga 1997 (Muga et al., 1997) | 386 | 1987 | 1990 | Drug Treatment | 311 | 75 | 0.685 | 0.667 | 0.921 |
| Muga 2003 (Muga et al., 2003) | 1111 | 1987 | 2001 | Hospital Based | 903 | 208 | 0.576 | 0.654 | 1.391 |
| Rivas 2010 (Rivas et al., 2010) | 1223 | 1987 | 2006 | Drug Treatment | 982 | 241 | 0.418 | 0.544 | 1.661 |
| Muga 2006 (Muga et al., 2006) | 452 | 1987 | 1989 | Detoxification Unit | 363 | 89 | 0.713 | 0.742 | 1.152 |
| Hurtado 2008 (Hurtado Navarro et al., 2008) | 5948 | 1988 | 2005 | Drug Treatment | 4612 | 1336 | 0.410 | 0.460 | 1.226 |
| Bolao 1995 (Bolao and Ramon, 1995) | 60 | 1988 | 1988 | Detoxification Unit | 49 | 11 | 0.735 | 0.727 | 0.963 |
| Bolao 1995 (Bolao and Ramon, 1995) | 101 | 1989 | 1989 | Detoxification Unit | 88 | 13 | 0.727 | 0.692 | 0.844 |
| Muga 2006 (Muga et al., 2006) | 560 | 1990 | 1992 | Detoxification Unit | 457 | 103 | 0.637 | 0.680 | 1.210 |
| Bolao 1995 (Bolao and Ramon, 1995) | 88 | 1990 | 1990 | Detoxification Unit | 69 | 19 | 0.696 | 0.684 | 0.948 |
| Portu 2002 (Portu et al., 2002) | 1131 | 1991 | 1999 | Drug Treatment | 857 | 274 | 0.467 | 0.478 | 1.047 |
| Bolao 1995 (Bolao and Ramon, 1995) | 91 | 1991 | 1991 | Detoxification Unit | 68 | 23 | 0.632 | 0.652 | 1.090 |
| Muga 2007 (Muga et al., 2007) | 393 | 1992 | 1996 | Drug Treatment | 681 | 170 | 0.63 | 0.710 | 1.44 |
| Muga 1990 (Muga et al., 1990) | 864 | 1992 | 1992 | Drug Treatment | 758 | 106 | 0.507 | 0.462 | 0.837 |
| Bolao 1995 (Bolao and Ramon, 1995) | 105 | 1992 | 1992 | Detoxification Unit | 90 | 15 | 0.500 | 0.533 | 1.143 |
| Muga 2006 (Muga et al., 2006) | 525 | 1993 | 1995 | Detoxification Unit | 416 | 109 | 0.452 | 0.578 | 1.660 |
| Bolao 1995 (Bolao and Ramon, 1995) | 98 | 1993 | 1993 | Detoxification Unit | 77 | 22 | 0.468 | 0.667 | 2.278 |
| Secretaria 1999 [centers, 1999 #136] | 1718 | 1996 | 1996 | STD Clinic | 1255 | 463 | 0.186 | 0.175 | 0.925 |
| Muga 2006 (Muga et al., 2006) | 395 | 1996 | 1998 | Detoxification Unit | 330 | 65 | 0.373 | 0.508 | 1.736 |
| Muga 2007 (Muga et al., 2007) | 298 | 1997 | 2004 | Drug Treatment | 776 | 183 | 0.548 | 0.650 | 1.53 |
| Muga 2006 (Muga et al., 2006) | 287 | 1999 | 2001 | Detoxification Unit | 238 | 49 | 0.387 | 0.510 | 1.653 |
| Vallejo 2008 (Vallejo et al., 2008) | 460 | 2001 | 2003 | Street Recruitment | 346 | 114 | 0.386 | 0.333 | 0.794 |
| Barrio 2007 (Barrio et al., 2007) | 621 | 2001 | 2003 | Street Recruitment | 460 | 161 | 0.241 | 0.304 | 1.376 |
|
Estonia (Estimated number of IDU: 13,801) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Uuskula 2007 (Uuskula et al., 2007) | 159 | 2004 | 2004 | Syringe Exchange | 134 | 25 | 0.560 | 0.560 | 1.001 |
| Platt 2006 (Platt et al., 2006) | 350 | 2005 | 2005 | Street Recruitment | 291 | 59 | 0.533 | 0.593 | 1.277 |
| Uuskula 2010 (Uuskula et al., 2010) | 350 | 2007 | 2007 | Street Recruitment | 294 | 56 | 0.534 | 0.643 | 1.572 |
|
Russia (Estimated number of IDU: 1,825,000) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Platt 2008 (Platt et al., 2008) | 234 | 2001 | 2004 | Street Recruitment | 153 | 81 | 0.309 | 0.487 | 2.123 |
| Rhodes 2002 (Rhodes et al., 2002) | 415 | 2001 | 2001 | Street Recruitment | 264 | 151 | 0.527 | 0.609 | 1.402 |
| Shaboltas 2006 (Shaboltas et al., 2006) | 898 | 2002 | 2002 | Street Recruitment | 639 | 259 | 0.301 | 0.301 | 1.003 |
| Platt 2005 (Platt et al., 2005) | 423 | 2003 | 2003 | Street Recruitment | 268 | 155 | 0.527 | 0.594 | 1.310 |
| Rhodes 2006 (Rhodes et al., 2006) | 403 | 2003 | 2003 | Street Recruitment | 268 | 134 | 0.134 | 0.142 | 1.070 |
| Rhodes 2006 (Rhodes et al., 2006) | 477 | 2003 | 2003 | Street Recruitment | 361 | 117 | 0.025 | 0.034 | 1.373 |
| Rhodes 2006 (Rhodes et al., 2006) | 499 | 2003 | 2003 | Street Recruitment | 343 | 153 | 0.090 | 0.085 | 0.939 |
| Gyarmathy 2010 (Gyarmathy et al., 2011) | 535 | 2004 | 2008 | Street Recruitment | 349 | 186 | 0.381 | 0.371 | 0.958 |
| Niccolai 2010 (Niccolai et al., 2010) | 387 | 2005 | 2006 | Street Recruitment | 286 | 101 | 0.480 | 0.560 | 1.379 |
| Abdala 2010 (Abdala et al., 2010) | 331 | 2005 | 2008 | Street Recruitment | 243 | 88 | 0.247 | 0.341 | 1.578 |
|
Vietnam (Estimated number of IDU: 135,305) (Mathers et al., 2008)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Citation | Sample Size | Years of Data Collection | Recruitment Location | Male | Female | HIV Prevalence Male | HIV Prevalence Female | Odds Ratio | |
| Quan 2009 (Quan et al., 2009) | 309 | 2000 | 2004 | Street Recruitment | 299 | 10 | 0.428 | 0.300 | 0.573 |
We examined studies from the same area in order to identify multiple reports from the same research project that contained “similar” but not “identical” data, as mentioned above in the methods section. While authors often did not provide the level of detail we would have liked, we were able to identify 8 pairs of reports and one group of 4 reports where the “data similarity” problem was apparent. (Either reports from the same cohort study or reports from the same serial cross-sectional study.) These 20 reports contained 14,649 subjects. If we had been able to calculate and adjust for interclass correlations, the maximum effect would have been on the order of reducing the effective sample size for these studies by approximately half (from 14,649 subjects to 7,325 subjects). Given the total sample size of 128,745 subjects across all studies, this reduction in the effective sample size would not have affected the statistical analyses.
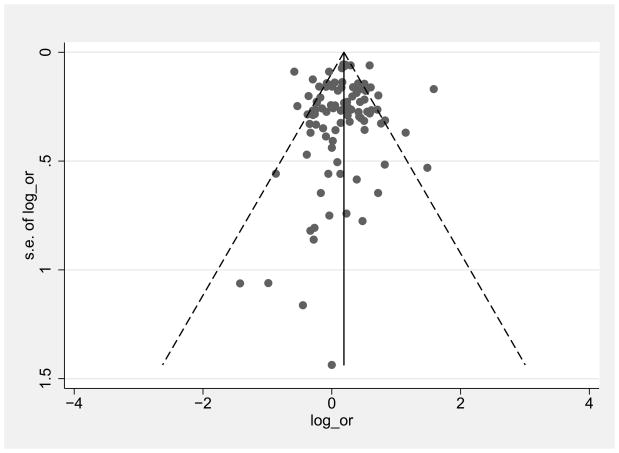
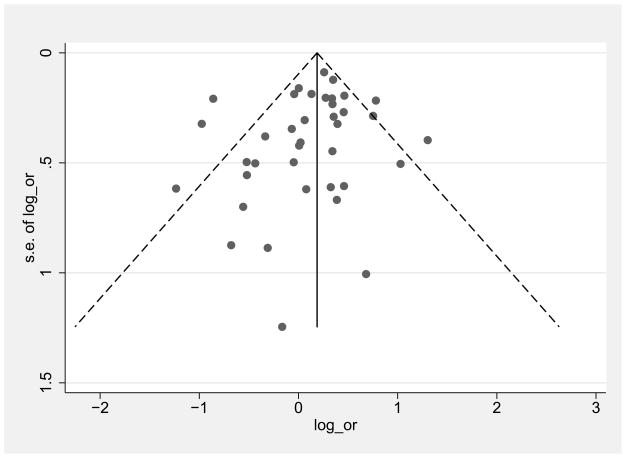
3.2 Potential publication bias
Figure 2 shows funnel plots for low/middle income countries and high income countries of female:male log OR comparisons graphed by effect size (log OR) on the x axis and precision (standard error of the log OR) on the y axis. The plots are roughly symmetrical with no obvious gaps in any quadrant, suggesting a lack of publication bias. The Egger’s test for publication bias was not significant for either the low/middle countries (p=0.3) or high income countries (p=0.4)
Figure 2.
Funnel plots of female/male HIV log odds ratio (OR) in high income countries
3.3 Heterogeneity of the ORs
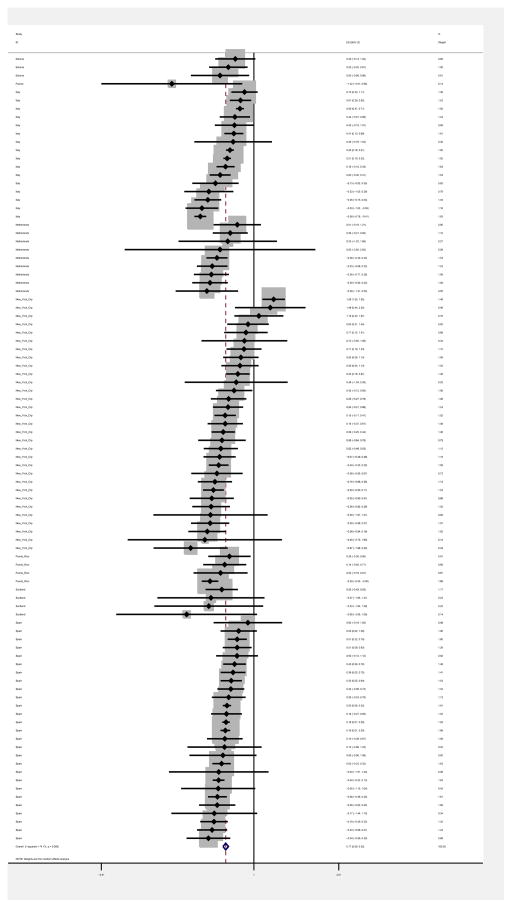
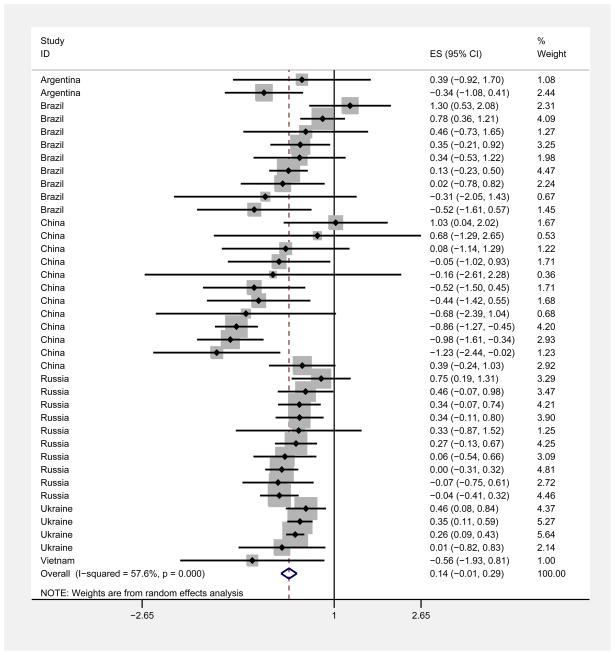
There was a great amount of heterogeneity among log ORs for female:male HIV prevalence in the studies (I2 = 70.7%, p<0.0001). The heterogeneity was somewhat high for studies among low/middle income countries (I2 = 57.7%, p<0.0001), and quite high for high income countries (I2 =74.1%, p<0.0001). Note that an I2 >50% is usually considered to be a high level of heterogeneity (Schroll et al., 2011). The range in the ORs was also quite substantial, with an absolute range of 0.25 to 4.87, and an interquartile range of 0.84 to 1.51.
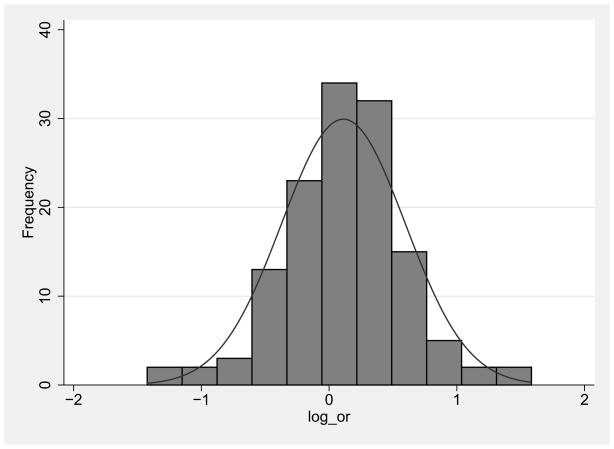
3.4 Distribution of the log ORs
Figure 3 shows the distribution of the log ORs for female:male HIV prevalence for all of the included comparisons. The log ORs are on the x-axis and the number of studies in each band is on the y-axis. The width of the bands is approximately .25 logs and generated by Stata 11 (College Station, TX; StataCorp LP., 2009). The OR distribution approximates a Gaussian (normal) distribution, and the interaction of kurtosis x skewness was not significantly different from a Gaussian distribution.
Figure 3.
Funnel plots of female/male HIV log odds ratio (OR) in low/middle income countries
3.5 HIV prevalence among FWID compared to male MWID
Pooling all studies, there was a slightly higher prevalence of HIV among females compared to males (weighted pooled OR = 1.18, 95% CI: 1.10, 1.26). Figures 4 and 5 are forest plots with log ORs, 95% confidence intervals, and weights for low/middle and high income countries. The weighted pooled OR was similar among low/middle income countries (OR = 1.15, 95% CI: 0.99, 1.34) and high income countries (OR =1.18, 95% CI: 1.10, 1.28).
Figure 4.
Gaussian distribution of log odds ratios (OR)
Figure 5.
Forest plot of female/male HIV log odds ratio (OR) in high income countries
We examined the log ORs as a function of the proportion of female PWID in each study. The slope of the regression line for log OR as a function of the percentage of females in the sample was not significantly different from 0 (beta = 0.4, p=0.5), indicating that there was no relationship between the percentage of females in the different studies and the log OR for female:male HIV prevalence.
We compared the weighted mean OR for female:male HIV prevalence for reports in which subjects were recruited from healthcare settings (substance use treatment programs, detoxification programs, hospitals, clinics) against the weighted mean OR for reports in which subjects were recruited from community settings (street outreach, peer referral, venue-based sampling, targeted sampling). In the 6 reports that included both types of recruitment settings typically did not present HIV prevalence by gender for each type of recruitment setting, so we did not include these reports in the comparison. There was no difference in the weighted mean female:male ORs for healthcare setting recruitment (OR = 1.19, 95% CI: 1.09, 1.30) and community setting recruitment (OR = 1.20, 95% CI: 1.10, 1.27).
3.6 Studies with extreme value log ORs
We examined the studies with the 10 highest (Bolao and Ramon, 1995; Des Jarlais et al., 2009b; Des Jarlais et al., 2010; Des Jarlais et al., 1999; Diaz et al., 2001; Dourado et al., 1999; Mesquita et al., 2001; Neaigus et al., 1996; Platt et al., 2008; Zhang et al., 2002) and 10 lowest log ORs (Boschini et al., 1996; Des Jarlais et al., 2007a; Helal et al., 1995; Jia et al., 2008; McIntyre et al., 2001; Quan et al., 2009; Serraino et al., 1992; Yin et al., 2007; Zhang et al., 2007a; Zheng et al., 1994) to possibly identify factors associated with extreme values. The potential factors examined included sexual behavior, use of non-injected drugs such as crack cocaine, participation in commercial sex work, male-with-male sexual behavior, stigmatization of females and access to services.
In the 10 studies with the highest female:male log ORs, sexual transmission of HIV appeared to be the most likely reason for the high female:male ORs in these studies, as the authors of all 10 studies suggested factors related to sexual transmission (including sex work, crack use, heterosexual sex with a person who inject drugs, and syphilis) as possible explanations of the high female:male ORs. In none of the 10 studies with the lowest female:male ORs, did the authors propose explanations for low female:male log ORs, other than small numbers of females in the samples.
4. Discussion
Gender disparities in HIV/AIDS have been of great concern in many different countries (Madkan et al., 2006; UNAIDS, 2004; UNODC, 2006b). To our knowledge, this is the first systematic review to assess female:male differences in HIV infection among PWID. This review was restricted to countries that have experienced high seroprevalence epidemics among PWID (seroprevalence reached 20% or higher). Determining whether the findings from high seroprevalence areas also hold for low to moderate seroprevalence countries would require additional research. However, as the same odds ratio would represent a greater absolute difference in female:male HIV prevalence in a high prevalence setting than in a low prevalence setting, we believe it was appropriate to examine the high prevalence settings first.
The review generated a number of unexpected findings.
First, there was very great variation in the female:male HIV prevalence odds ratios across the different studies. The I2 for all studies combined was 70.7%, and the inter-quartile range among the ORs was 0.84 to 1.51.
Second, in the pooled analysis, there was a very modest (though statistically significant) effect of females having higher HIV prevalence than males. The weighted mean OR was 1.18 (95% CI 1.10 to 1.26). Thus, if HIV prevalence was 40% among males in an “average” study, it would be 44% among females in that study. The various reasons as to why females may be more likely to be infected with HIV noted in the introduction do not appear to have dominant effects in the studies from high seroprevalence areas in this review.
We did examine several potential correlates of greater female:male disparity in HIV prevalence, including: national income, the percentage of females in the study sample, and recruitment setting. These analyses were based on hypotheses: 1) that FWID in low/middle income countries might face greater stigmatization, and that this greater stigmatization would lead to larger female:male disparities in HIV prevalence; 2) that studies that had great difficulties in recruiting females might have ended up with biased samples of females, and 3) females may have greater difficulties in obtaining substance use treatment and thus treatment program samples would have biased samples. We did not find significant differences in any of these analyses. This does not mean that females do not face greater stigmatization, are equally likely to participate in research studies, or do not have greater difficulties in obtaining substance use treatment. Rather it appears that these factors do not create large and consistent female:male differences in HIV prevalence in high seroprevalence settings.
The approximately Gaussion distribution of the log ORs suggests that the female:male differences in HIV prevalence are a complex phenomena, determined by a large number of causal factors, without any single factor being dominant (Gooman N.R, 1963; Houghton et al., 1985; Wald et al., 1999).
4.1 Limitations
This systematic review and meta-analysis has a number of limitations that should be noted. First, as in any systematic review and meta-analysis, we were limited by the quality of the original studies that we reviewed. In particular, we could not “correct” any of the problems that the original study might have encountered in trying to recruit female subjects. We did exclude reports that had fewer than 5 females out of concern that an extremely small sample of females would fail to represent the diversity among females who inject drugs in that location. This limit was a compromise between the potential lack of representativeness in a very small sample and the general systematic review principle of utilizing all available data.
Second, we searched for and reviewed studies from countries in which HIV had reached 20% or more among PWID (Mathers et al., 2008). Determining whether the findings from the analyses presented here also apply to low and moderate seroprevalence settings would require additional research. However, the total number of subjects in the studies reviewed here was 128,745, and our use of high HIV prevalence countries means that we were using data representing the great number of HIV seropositive people who inject drugs throughout the world. Note that Russia and China, which have among the largest populations of people who inject drugs of any countries in the world (Mathers et al., 2008) were included in our analyses.
Third, as discussed in the methods section, we did eliminate multiple reports of exactly the same data from “parent” research studies, but included multiple reports with “similar” but not identical data. It would have required individual level data to calculate interclass correlations to adjust for this non-independence of studies with “similar” data. However, as noted in the results section, there were only a modest number of multiple reports with similar data, and adjusting for interclass correlations would not have meaningfully changed our total effective sample size or affected the statistical significance level of any of the results.
4.2 Implications for HIV prevention and treatment
The great heterogeneity among the studies reviewed here suggests that “know your local epidemic” is likely to be the starting point for effective HIV prevention for both males and females who inject drugs.
The very modest difference in female:male HIV prevalence among all studies combined indicates that current HIV prevention programs do not consistently lead to large differences in HIV infection among females compared to males who inject drugs. This certainly should be seen as encouraging in areas where effective prevention programs have been implemented and as further reason to implement effective programs in areas where they have not yet been implemented. While again noting the importance of “know your local epidemic,” the very modest difference in the female:male prevalence ratios suggests that interventions to reduce drug injecting related HIV transmission in the population as a whole, e.g. large-scale needle/syringe access programs, are effective for both males and females, without specific targeting by gender. As many females who inject share injection equipment with male partners, protecting males from injecting related HIV infection would also protect females. It would be important, however, to avoid barriers to female participation in HIV prevention and care programs. Implementing HIV prevention programs that reach a large proportion of the local PWID population at a low cost per person reached would be particularly important in resource-limited settings.
In all 10 of the studies with the highest ORs for female:male HIV prevalence, the authors suggested that sexual transmission was the reason for the difference. Thus, special programs to reduce HIV risk for females should be implemented in settings with high rates of sexual transmission of HIV among PWID and should focus on sexual transmission. Screening and treatment for sexually transmitted diseases would be an example of an intervention focused on sexual transmission and very likely to have benefits for females.
The overall modest difference in HIV prevalence among females and males should not be interpreted as females having equal access to either treatment for HIV infection or for drug dependence. It is quite likely that females who inject drugs face considerable barriers in accessing these services.
Finally, as noted in the introduction, there are many hypotheses as to why FWID may be more likely to become infected with HIV than MWID. There seems to be a lack of research into factors through which FWID might be protected against HIV infection. Note that none of the authors of the 10 studies with the lowest ORs suggested reasons why females had lower HIV prevalence than males in the study. Identification of factors that protect females might provide insights into more effective HIV prevention for both females and males who inject drugs.
Figure 6.
Forest plot of female/male HIV log odds ratio (OR) in low/middle income countries
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIH Grant R01 AI 083035-02; NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
D. Des Jarlais and H. Hagan designed the study and wrote the protocol. J. Feelemyer and S. Modi managed the literature searches and summaries of previous related work. K. Arasteh undertook the statistical analysis, and author D. Des Jarlais wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors have no conflicts of interest with respect to the submitted manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdala N, White E, Toussova OV, Krasnoselskikh TV, Verevochkin S, Kozlov AP, Heimer R. Comparing sexual risks and patterns of alcohol and drug use between injection drug users (IDUs) and non-IDUs who report sexual partnerships with IDUs in St. Petersburg, Russia. BMC Public Health. 2010;10:676. doi: 10.1186/1471-2458-10-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim T, Rahman M, Alam MS, Chowdhury IA, Khan R, Reza M, Chowdhury EI, Hanifuddin M, Rahman AS. Bangladesh moves from being a low-prevalence nation for HIV to one with a concentrated epidemic in injecting drug users. Int J STD AIDS. 2008;19:327–331. doi: 10.1258/ijsa.2007.007269. [DOI] [PubMed] [Google Scholar]
- Banks SE, Brown LS, Jr, Ajuluchukwu D. Sexual behaviors and HIV infection in intravenous drug users in New York City. J Addict Dis. 1991;10:15–23. doi: 10.1300/J069v10n03_03. [DOI] [PubMed] [Google Scholar]
- Barrio G, De La Fuente L, Toro C, Brugal TM, Soriano V, Gonzalez F, Bravo MJ, Vallejo F, Silva TC. Prevalence of HIV infection among young adult injecting and non-injecting heroin users in Spain in the era of harm reduction programmes: gender differences and other related factors. Epidemiol Infect. 2007;135:592–603. doi: 10.1017/S0950268806007266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benotsch EG, Somlai AM, Pinkerton SD, Kelly JA, Ostrovski D, Gore-Felton C, Kozlov AP. Drug use and sexual risk behaviours among female Russian IDUs who exchange sex for money or drugs. Int J STD AIDS. 2004;15:343–347. doi: 10.1177/095646240401500514. [DOI] [PubMed] [Google Scholar]
- Bolao F, Ramon JM. Trends of prevalence of HIV infection in a cohort of parenteral drug users. Med Clin (Barc) 1995;105:396. [PubMed] [Google Scholar]
- Booth RE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Predictors of HIV sero-status among drug injectors at three Ukraine sites. AIDS. 2006;20:2217–2223. doi: 10.1097/QAD.0b013e328010e019. [DOI] [PubMed] [Google Scholar]
- Booth RE, Lehman WE, Brewster JT, Sinitsyna L, Dvoryak S. Gender differences in sex risk behaviors among Ukraine injection drug users. J Acquir Immune Defic Syndr. 2007;46:112–117. doi: 10.1097/QAI.0b013e318141f965. [DOI] [PubMed] [Google Scholar]
- Boschini A, Smacchia C, Di Fine M, Schiesari A, Ballarini P, Arlotti M, Gabrielli C, Castellani G, Genova M, Pantani P, Lepri AC, Rezza G. Community-acquired pneumonia in a cohort of former injection drug users with and without human immunodeficiency virus infection: incidence, etiologies, and clinical aspects. Clin Infect Dis. 1996;23:107–113. doi: 10.1093/clinids/23.1.107. [DOI] [PubMed] [Google Scholar]
- Burkett W, Brown LS., Jr Death, AIDS morbidity, and HIV seroprevalence in New York City intravenous drug abusers. J Natl Med Assoc. 1990;82:777–780. [PMC free article] [PubMed] [Google Scholar]
- Caiaffa WT, Bastos FI, Freitas LL, Mingoti SA, Proietti FA, Carneiro-Proietti AB, Gandolfi D, Doneda D. The contribution of two Brazilian multi-center studies to the assessment of HIV and HCV infection and prevention strategies among injecting drug users: the AjUDE-Brasil I and II Projects. Cad Saude Publica. 2006;22:771–782. doi: 10.1590/s0102-311x2006000400016. [DOI] [PubMed] [Google Scholar]
- CDC; CDC. MMWR Weekly. CDC; Atlanta: 1984. Update: Acquired Immunodeficiency Syndrome (AIDS) -- United States; pp. 337–339. [PubMed] [Google Scholar]
- CDUHR; CDUHR. CDUHR News. Center for Drug Use and HIV Research; New York: 1999. The HIV-drug use epidemic in New York City: entering the fourth decade; p. 8. [Google Scholar]
- Chiasson MA, Stoneburner RL, Hildebrandt DS, Ewing WE, Telzak EE, Jaffe HW. Heterosexual transmission of HIV-1 associated with the use of smokable freebase cocaine (crack) AIDS. 1991;5:1121–1126. doi: 10.1097/00002030-199109000-00011. [DOI] [PubMed] [Google Scholar]
- Cintra AM, Caiaffa WT, Mingoti SA. Characteristics of male and female injecting drug users of the AjUDE-Brasil II Project. Cad Saude Publica. 2006;22:791–802. doi: 10.1590/s0102-311x2006000400018. [DOI] [PubMed] [Google Scholar]
- Cleland CM, Des Jarlais DC, Perlis TE, Stimson G, Poznyak V. HIV risk behaviors among female IDUs in developing and transitional countries. BMC Public Health. 2007;7:271. doi: 10.1186/1471-2458-7-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournos F, Horwath E, Guido JR, McKinnon K, Hopkins N. HIV-1 infection at two public psychiatric hospitals in New York City. AIDS Care. 1994;6:443–452. doi: 10.1080/09540129408258659. [DOI] [PubMed] [Google Scholar]
- Dasgupta SF, Samuel R, Jose Benny, Alan Neaigus, et al. Using retrospective behavioral data to determine HIV risk factors among street-recruited drug injectors. J Drug Issues. 1995;25:161–171. [Google Scholar]
- de Boer IM, Op de Coul EL, Beuker RJ, de Zwart O, Al Taqatqa W, van de Laar MJ. Trends in HIV prevalence and risk behaviour among injecting drug users in Rotterdam, 1994–2002. Ned Tijdschr Geneeskd. 2004;148:2325–2330. [PubMed] [Google Scholar]
- de Carvalho HB, Mesquita F, Massad E, Bueno RC, Lopes GT, Ruiz MA, Burattini MN. HIV and infections of similar transmission patterns in a drug injectors community of Santos, Brazil. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:84–92. doi: 10.1097/00042560-199605010-00012. [DOI] [PubMed] [Google Scholar]
- De Rosa FG, Cicalini S, Canta F, Audagnotto S, Cecchi E, Di Perri G. Infective endocarditis in intravenous drug users from Italy: the increasing importance in HIV-infected patients. Infection. 2007;35:154–160. doi: 10.1007/s15010-007-5125-0. [DOI] [PubMed] [Google Scholar]
- DeLay P. Gender and monitoring the response to HIV/AIDS pandemic. Emerg Infect Dis. 2004;10:1979–1983. doi: 10.3201/eid1011.040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deren S, Robles R, Andia J, Colon HM, Kang SY, Perlis T. Trends in HIV seroprevalence and needle sharing among Puerto Rican drug injectors in Puerto Rico and New York: 1992–1999. J Acquir Immune Defic Syndr. 2001;26:164–169. doi: 10.1097/00042560-200102010-00009. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, Hagan H, McKnight C, Perlman DC, Friedman SR. Persistence and change in disparities in HIV infection among injection drug users in New York City after large-scale syringe exchange programs. Am J Public Health. 2009a;99(Suppl 2):S445–451. doi: 10.2105/AJPH.2008.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman D, Friedman SR. Using hepatitis C virus and herpes simplex virus-2 to track HIV among injecting drug users in New York City. Drug Alcohol Depend. 2009b;101:88–91. doi: 10.1016/j.drugalcdep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman DC, Torian LV, Beatice S, Semaan S, Friedman SR. HIV infection during limited versus combined HIV prevention programs for IDUs in New York City: the importance of transmission behaviors. Drug Alcohol Depend. 2010;109:154–160. doi: 10.1016/j.drugalcdep.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, Perlis T, Hagan H, Abdul-Quader A, Heckathorn DD, McKnight C, Bramson H, Nemeth C, Torian LV, Friedman SR. Convergence of HIV seroprevalence among injecting and non-injecting drug users in New York City. AIDS. 2007a;21:231–235. doi: 10.1097/QAD.0b013e3280114a15. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, Perlis T, Hagan H, Heckathorn DD, McKnight C, Bramson H, Friedman SR. The transition from injection to non-injection drug use: long-term outcomes among heroin and cocaine users in New York City. Addiction. 2007b;102:778–785. doi: 10.1111/j.1360-0443.2007.01764.x. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Friedman SR, Novick DM, Sotheran JL, Thomas P, Yancovitz SR, Mildvan D, Weber J, Kreek MJ, Maslansky R. HIV-1 infection among intravenous drug users in Manhattan, New York City, from 1977 through 1987. JAMA. 1989;261:1008–1012. doi: 10.1001/jama.261.7.1008. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Friedman SR, Perlis T, Chapman TF, Sotheran JL, Paone D, Monterroso E, Neaigus A. Risk behavior and HIV infection among new drug injectors in the era of AIDS in New York City. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:67–72. doi: 10.1097/00042560-199901010-00010. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Friedman SR, Sotheran JL, Wenston J, Marmor M, Yancovitz SR, Frank B, Beatrice S, Mildvan D. Continuity and change within an HIV epidemic. Injecting drug users in New York City, 1984 through 1992. JAMA. 1994;271:121–127. [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Beatrice S, Milliken J, Mildvan D, Yancovitz S, Friedman SR. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am J Public Health. 2005a;95:1439–1444. doi: 10.2105/AJPH.2003.036517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Hagan H, Beatrice S, Smith L, Wethers J, Milliken J, Mildvan D, Yancovitz S, Friedman SR. Reductions in hepatitis C virus and HIV infections among injecting drug users in New York City, 1990–2001. AIDS. 2005b;19(Suppl 3):S20–25. doi: 10.1097/01.aids.0000192066.86410.8c. [DOI] [PubMed] [Google Scholar]
- Diaz T, Vlahov D, Greenberg B, Cuevas Y, Garfein R. Sexual orientation and HIV infection prevalence among young Latino injection drug users in Harlem. J Womens Health Gend Based Med. 2001;10:371–380. doi: 10.1089/152460901750269698. [DOI] [PubMed] [Google Scholar]
- Dourado I, Andrade T, Carpenter CL, Galvao-Castro B. Risk factors for human T cell lymphotropic virus type I among injecting drug users in northeast Brazil: possibly greater efficiency of male to female transmission. Mem Inst Oswaldo Cruz. 1999;94:13–18. doi: 10.1590/s0074-02761999000100006. [DOI] [PubMed] [Google Scholar]
- Dumchev KV, Soldyshev R, Qian HZ, Zezyulin OO, Chandler SD, Slobodyanyuk P, Moroz L, Schumacher JE. HIV and hepatitis C virus infections among hanka injection drug users in central Ukraine: a cross-sectional survey. Harm Reduct J. 2009;6:23. doi: 10.1186/1477-7517-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sadr W, Goetz RR, Sorrell S, Joseph M, Ehrhardt A, Gorman JM. Clinical and laboratory correlates of human immunodeficiency virus infection in a cohort of intravenous drug users from New York, NY. Arch Intern Med. 1992;152:1653–1659. [PubMed] [Google Scholar]
- EMCDDA; EMCDDA. Annual Report 2006: the state of the drugs problem in Europe. 2006. European Monitoring Centre for Drugs and Drug Addiction. [Google Scholar]
- EMCDDA; EMCDDA. Situation Summary for the Netherlands. The European Monitoring Centre for Drugs and Drug Addiction; Portugal: 2010. [Google Scholar]
- Farci P, Novick DM, Orgiana G, Coiana A, Lai ME, Mandas A, Strazzera A, Marongiu F, Chessa L, Lusso P, et al. Epidemiology of infection with HIV-1 in Sardinia: a multicentre prospective study. Eur J Epidemiol. 1992;8:132–135. doi: 10.1007/BF03334988. [DOI] [PubMed] [Google Scholar]
- Frajzyngier V, Neaigus A, Gyarmathy VA, Miller M, Friedman SR. Gender differences in injection risk behaviors at the first injection episode. Drug Alcohol Depend. 2007;89:145–152. doi: 10.1016/j.drugalcdep.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Gleser L, OI . Stochastically Dependent Effect Sizes. In: Cooper H, HL, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. Russel Sage Foundation; New York: [Google Scholar]
- Gollub EL, Rey D, Obadia Y, Moatti JP. Gender differences in risk behaviors among HIV+ persons with an IDU history. The link between partner characteristics and women’s higher drug-sex risks The Manif 2000 Study Group. Sex Transm Dis. 1998;25:483–488. doi: 10.1097/00007435-199810000-00008. [DOI] [PubMed] [Google Scholar]
- Gooman NR. Statistical Analysis Based on a Certain Multivariate Complex Gaussian Distribution (An Introduction) Ann Math Stat. 1963;34:152–177. [Google Scholar]
- Gorshkova ID, SI . Violence against women in Russian families. Proceedings of the University; Lomonosov, Women’s Soviet: 2003. [Google Scholar]
- Grieco MH, Reddy MM, Fusillo CA, Sorrell SJ, Buimovici-Klein E, Gindi EJ, Brown DK, Saxinger WC, Weiss SH, Flaster ER. Cross-sectional study of immunologic abnormalities in intravenous drug abusers on methadone maintenance in New York City. AIDS. 1989;3:235–237. doi: 10.1097/00002030-198904000-00007. [DOI] [PubMed] [Google Scholar]
- Guimaraes ML, Bastos FI, Telles PR, Galvao-Castro B, Diaz RS, Bongertz V, Morgado MG. Retrovirus infections in a sample of injecting drug users in Rio de Janeiro City, Brazil: prevalence of HIV-1 subtypes, and co-infection with HTLV-I/II. J Clin Virol. 2001;21:143–151. doi: 10.1016/s1386-6532(01)00158-5. [DOI] [PubMed] [Google Scholar]
- Gyarmathy VA, Li N, Tobin KE, Hoffman IF, Sokolov N, Levchenko J, Batluk J, Kozlov AA, Kozlov AP, Latkin CA. Unprotected sex in heterosexual partnerships of injecting drug users in St. Petersburg, Russia. AIDS Behav. 2011;15:58–64. doi: 10.1007/s10461-010-9721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C, QGM, Qian HZ, Ruan YH, Zhu JL, Yin L, Chen KL, Liang H, Xing H, Hong KX, Shao YM. HIV Incidence Among Injection Drug Users in Southwestern China: A 4-year Follow-up Cohort Study. Proceedings of the International AIDS Conference; 2008. [DOI] [PubMed] [Google Scholar]
- Helal H, Momas I, Pretet S, Marsal L, Poinsard R. HIV prevalence and risk behaviour among intravenous drug users attending HIV counselling and testing centres in Paris. Addiction. 1995;90:1627–1633. doi: 10.1046/j.1360-0443.1995.901216275.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Aguado I, Avino MJ, Perez-Hoyos S, Gonzalez-Aracil J, Ruiz-Perez I, Torrella A, Garcia de la Hera M, Belda F, Fernandez E, Santos C, Trullen J, Fenosa A. Human immunodeficiency virus (HIV) infection in parenteral drug users: evolution of the epidemic over 10 years. Valencian Epidemiology and Prevention of HIV Disease Study Group. Int J Epidemiol. 1999;28:335–340. doi: 10.1093/ije/28.2.335. [DOI] [PubMed] [Google Scholar]
- Hernandez-Aguado I, Bolumar F. Determinants of HIV-1 infection in intravenous drug users in Valencia, Spain, 1987–1991. Int J Epidemiol. 1993;22:537–542. doi: 10.1093/ije/22.3.537. [DOI] [PubMed] [Google Scholar]
- Houghton A, Khurana A, Seco FJ. Fluctuation-driven first-order phase transition, below four dimensions, in the random-field ising model with a Gaussian random-field distribution. Phys Rev Lett. 1985;55:856. doi: 10.1103/PhysRevLett.55.856. [DOI] [PubMed] [Google Scholar]
- Hurtado Navarro I, Alastrue I, Del Amo J, Santos C, Ferreros I, Tasa T, Perez-Hoyos S. Differences between women and men in serial HIV prevalence and incidence trends. Eur J Epidemiol. 2008;23:435–440. doi: 10.1007/s10654-008-9246-2. [DOI] [PubMed] [Google Scholar]
- Jia Y, Lu F, Zeng G, Sun X, Xiao Y, Lu L, Liu W, Ni M, Qu S, Li C, Liu J, Wu P, Vermund SH. Predictors of HIV infection and prevalence for syphilis infection among injection drug users in China: community-based surveys along major drug trafficking routes. Harm Reduct J. 2008;5:29. doi: 10.1186/1477-7517-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose B, Friedman SR, Neaigus A, Curtis R, Grund JP, Goldstein MF, Ward TP, Des Jarlais DC. Syringe-mediated drug-sharing (backloading): a new risk factor for HIV among injecting drug users. AIDS. 1993;7:1653–1660. doi: 10.1097/00002030-199312000-00017. [DOI] [PubMed] [Google Scholar]
- King R, Bird SM, Hay G, Hutchinson SJ. Estimating current injectors in Scotland and their drug-related death rate by sex, region and age-group via Bayesian capture--recapture methods. Stat Methods Med Res. 2009;18:341–359. doi: 10.1177/0962280208094701. [DOI] [PubMed] [Google Scholar]
- Kottiri BJ, Friedman SR, Neaigus A, Curtis R, Des Jarlais DC. Risk networks and racial/ethnic differences in the prevalence of HIV infection among injection drug users. J Acquir Immune Defic Syndr. 2002;30:95–104. doi: 10.1097/00042560-200205010-00013. [DOI] [PubMed] [Google Scholar]
- Lau JT, Feng T, Lin X, Wang Q, Tsui HY. Needle sharing and sex-related risk behaviours among drug users in Shenzhen, a city in Guangdong, southern China. AIDS Care. 2005;17:166–181. doi: 10.1080/09540120512331325662. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lowndes CM, RT, Judd A, Mikhailova L, Sarang A, Rylkov A, Tichonov M, Platt L. Female injection drug users who practise sex work in Togliatti City, Russian Federation: HIV prevalence and risk behaviour. Int Conf AIDS 2002 [Google Scholar]
- Lugoboni F, Quaglio G, Mezzelani P, Lechi A. No positive tests for syphilis in 6 years of observation among heroin drug users in north-eastern Italy. Addiction. 2002;97:104–105. doi: 10.1046/j.1360-0443.2002.0050j.x. [DOI] [PubMed] [Google Scholar]
- Madkan VK, Giancola AA, Sra KK, Tyring SK. Sex differences in the transmission, prevention, and disease manifestations of sexually transmitted diseases. Arch Dermatol. 2006;142:365–370. doi: 10.1001/archderm.142.3.365. [DOI] [PubMed] [Google Scholar]
- Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS. 2005;19(Suppl 2):S67–72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- Marrero Rodriguez CA, Robles RR, Colon HM, Freeman DH, Matos TD, Reyes JC. HIV risk behaviors and HIV seropositivity among young injection drug users. P R Health Sci J. 1993;12:7–12. [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- McIntyre PG, Hill DA, Appleyard K, Taylor A, Hutchinson S, Goldberg DJ. Prevalence of antibodies to hepatitis C virus, HIV and human T-cell leukaemia/lymphoma viruses in injecting drug users in Tayside, Scotland, 1993–7. Epidemiol Infect. 2001;126:97–101. doi: 10.1017/s0950268801005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita F, Kral A, Reingold A, Bueno R, Trigueiros D, Araujo PJ. Trends of HIV infection among injection drug users in Brazil in the 1990s: the impact of changes in patterns of drug use. J Acquir Immune Defic Syndr. 2001;28:298–302. doi: 10.1097/00042560-200111010-00016. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Muga R, Langohr K, Tor J, Sanvisens A, Serra I, Rey-Joly C, Munoz A. Survival of HIV-infected injection drug users (IDUs) in the highly active antiretroviral therapy era, relative to sex- and age-specific survival of HIV-uninfected IDUs. Clin Infect Dis. 2007;45:370–376. doi: 10.1086/519385. [DOI] [PubMed] [Google Scholar]
- Muga R, Roca J, Tor J, Pigem C, Rodriguez R, Egea JM, Vlahov D, Munoz A. Syphilis in injecting drug users: clues for high-risk sexual behaviour in female IDUs. Int J STD AIDS. 1997;8:225–228. doi: 10.1258/0956462971919967. [DOI] [PubMed] [Google Scholar]
- Muga R, Sanvisens A, Bolao F, Tor J, Santesmases J, Pujol R, Tural C, Langohr K, Rey-Joly C, Munoz A. Significant reductions of HIV prevalence but not of hepatitis C virus infections in injection drug users from metropolitan Barcelona: 1987–2001. Drug Alcohol Depend. 2006;82(Suppl 1):S29–33. doi: 10.1016/s0376-8716(06)80005-0. [DOI] [PubMed] [Google Scholar]
- Muga R, Sanvisens A, Egea JM, Tor J, Rey-Joly C. Trends in human immunodeficiency virus infection among drug users in a detoxification unit. Clin Infect Dis. 2003;37(Suppl 5):S404–409. doi: 10.1086/377550. [DOI] [PubMed] [Google Scholar]
- Muga R, Tor J, Llibre JM, Soriano V, Rey-Joly C, Foz M. Risk factors for HIV-1 infection in parenteral drug users. AIDS. 1990;4:259–260. [PubMed] [Google Scholar]
- Neaigus A, Friedman SR, Jose B, Goldstein MF, Curtis R, Ildefonso G, Des Jarlais DC. High-risk personal networks and syringe sharing as risk factors for HIV infection among new drug injectors. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:499–509. doi: 10.1097/00042560-199604150-00011. [DOI] [PubMed] [Google Scholar]
- Neaigus A, Gyarmathy VA, Miller M, Frajzyngier V, Zhao M, Friedman SR, Des Jarlais DC. Injecting and sexual risk correlates of HBV and HCV seroprevalence among new drug injectors. Drug Alcohol Depend. 2007;89:234–243. doi: 10.1016/j.drugalcdep.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto T, Foster K, Sr, Brown LS., Jr Effect of psychological factors on risk behavior of human immunodeficiency virus (HIV) infection among intravenous drug users (IVDUs) Int J Addict. 1991;26:441–456. doi: 10.3109/10826089109058896. [DOI] [PubMed] [Google Scholar]
- Network, E.H.R.; EHRN. Eurasian Harm Reduction Network (EHRN) about Special Groups. Lithuania: 2010. [Google Scholar]
- Niccolai LM, Toussova OV, Verevochkin SV, Barbour R, Heimer R, Kozlov AP. High HIV prevalence, suboptimal HIV testing, and low knowledge of HIV-positive serostatus among injection drug users in St. Petersburg, Russia. AIDS Behav. 2010;14:932–941. doi: 10.1007/s10461-008-9469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYC National HIV Behavioral Surveillance Team; NYC Health Department. 2009 National HIV Behavioral Surveillance Study. NYC Health, CDC, NDRI, NYU; New York City: 2009. HIV Risk and Prevalence among New York City Injection Drug Users. [Google Scholar]
- Peixinho ZF, Mendes NF, Longo IM, Moura NC, Hernandez HJ, Lacouture CL, Dines I, Coscina AL, Gonzaga AL, Giraldes PR. Seroepidemiological studies of HIV-1 infection in large Brazilian cities. Nat Immun Cell Growth Regul. 1990;9:133–136. [PubMed] [Google Scholar]
- Platt L, Bobrova N, Rhodes T, Uuskula A, Parry JV, Ruutel K, Talu A, Abel K, Rajaleid K, Judd A. High HIV prevalence among injecting drug users in Estonia: implications for understanding the risk environment. AIDS. 2006;20:2120–2123. doi: 10.1097/01.aids.0000247586.23696.20. [DOI] [PubMed] [Google Scholar]
- Platt L, Rhodes T, Hickman M, Mikhailova L, Lisetsky K, Sarang A, Lewis K, Parry J. Changes in HIV prevalence and risk among new injecting drug users in a Russian city of high HIV prevalence. J Acquir Immune Defic Syndr. 2008;47:623–631. doi: 10.1097/QAI.0b013e318165dbf7. [DOI] [PubMed] [Google Scholar]
- Platt L, Rhodes T, Lowndes CM, Madden P, Sarang A, Mikhailova L, Renton A, Pevzner Y, Sullivan K, Khutorskoy M. Impact of gender and sex work on sexual and injecting risk behaviors and their association with HIV positivity among injecting drug users in an HIV epidemic in Togliatti City, Russian Federation. Sex Transm Dis. 2005;32:605–612. doi: 10.1097/01.olq.0000175391.13947.f7. [DOI] [PubMed] [Google Scholar]
- Pohorila NB, Taran Y, Kolodiy I, Diyeva T. Behavior monitoring and HIV-infection prevalence among injection drug users. The Global Fund; Kyiv: 2010. Analytical Report Based on Results of Linked Survey. [Google Scholar]
- Portu JJ, Aldamiz-Etxebarria M, Agud JM, Arevalo JM, Almaraz MJ, Ayensa C. Tuberculin skin testing in intravenous drug users: differences between HIV-seropositive and HIV-seronegative subjects. Addict Biol. 2002;7:235–241. doi: 10.1080/135562102200120460. [DOI] [PubMed] [Google Scholar]
- Quaglio G, Lugoboni F, Pattaro C, Montanari L, Lechi A, Mezzelani P, Des Jarlais DC. Patients in long-term maintenance therapy for drug use in Italy: analysis of some parameters of social integration and serological status for infectious diseases in a cohort of 1091 patients. BMC Public Health. 2006;6:216. doi: 10.1186/1471-2458-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan VM, Go VF, Nam le V, Bergenstrom A, Thuoc NP, Zenilman J, Latkin C, Celentano DD. Risks for HIV, HBV, and HCV infections among male injection drug users in northern Vietnam: a case-control study. AIDS Care. 2009;21:7–16. doi: 10.1080/09540120802017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani N, Mohraz M, Kheirandish P, Malekinejad M, Malekafzali H, Mokri A, McFarland W, Rutherford G. HIV risk behavior among injection drug users in Tehran, Iran. Addiction. 2007;102:1472–1482. doi: 10.1111/j.1360-0443.2007.01914.x. [DOI] [PubMed] [Google Scholar]
- Rebagliato M, Avino MJ, Hernandez-Aguado I, Ruiz I, Perez-Hoyos S, Bolumar F, Ferrer L. Trends in incidence and prevalence of HIV-1 infection in intravenous drug users in Valencia, Spain. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:297–301. doi: 10.1097/00042560-199503010-00012. [DOI] [PubMed] [Google Scholar]
- Renwick N, Dukers NH, Weverling GJ, Sheldon JA, Schulz TF, Prins M, Coutinho RA, Goudsmit J. Risk factors for human herpesvirus 8 infection in a cohort of drug users in the Netherlands, 1985–1996. J Infect Dis. 2002;185:1808–1812. doi: 10.1086/340817. [DOI] [PubMed] [Google Scholar]
- Rezza G, De Rose A, Dorrucci M, Arpino C, Serafin I. Declining prevalence of HIV infection among injecting drug users entering drug treatment in Italy: 1990–1991. Eur J Epidemiol. 1993;9:663–666. doi: 10.1007/BF00211443. [DOI] [PubMed] [Google Scholar]
- Rezza G, Nicolosi A, Zaccarelli M, Sagliocca L, Nespoli M, Gattari P, Spizzichino L, Ippolito G, Lazzarin A. Understanding the dynamics of the HIV epidemic among Italian intravenous drug users: a cross-sectional versus a longitudinal approach. J Acquir Immune Defic Syndr. 1994;7:500–503. [PubMed] [Google Scholar]
- Rhodes T, Lowndes C, Judd A, Mikhailova LA, Sarang A, Rylkov A, Tichonov M, Lewis K, Ulyanova N, Alpatova T, Karavashkin V, Khutorskoy M, Hickman M, Parry JV, Renton A. Explosive spread and high prevalence of HIV infection among injecting drug users in Togliatti City, Russia. AIDS. 2002;16:F25–31. doi: 10.1097/00002030-200209060-00002. [DOI] [PubMed] [Google Scholar]
- Rhodes T, Platt L, Maximova S, Koshkina E, Latishevskaya N, Hickman M, Renton A, Bobrova N, McDonald T, Parry JV. Prevalence of HIV, hepatitis C and syphilis among injecting drug users in Russia: a multi-city study. Addiction. 2006;101:252–266. doi: 10.1111/j.1360-0443.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- Ribera E, Miro JM, Cortes E, Cruceta A, Merce J, Marco F, Planes A, Pare JC, Moreno A, Ocana I, Gatell JM, Pahissa A. Influence of human immunodeficiency virus 1 infection and degree of immunosuppression in the clinical characteristics and outcome of infective endocarditis in intravenous drug users. Arch Intern Med. 1998;158:2043–2050. doi: 10.1001/archinte.158.18.2043. [DOI] [PubMed] [Google Scholar]
- Rivas I, Martinez E, Sanvisens A, Bolao F, Tor J, Torrens M, Pujol R, Fuster D, Rey-Joly C, Munoz A, Muga R. Hepatitis B virus serum profiles in injection drug users and rates of immunization over time in Barcelona: 1987–2006. Drug Alcohol Depend. 2010;110:234–239. doi: 10.1016/j.drugalcdep.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Robles RR, Colon HM, Diaz N, Cancel LI, MacGowan R, Cole GE, Allen DM. Behavioural risk factors and HIV infection of injection drug users at detoxification clinics in Puerto Rico. Int J Epidemiol. 1994;23:595–601. doi: 10.1093/ije/23.3.595. [DOI] [PubMed] [Google Scholar]
- Romano N, Vitale F, Alesi DR, Bonura F, La Licata R, Intonazzo V, Dardanoni G, Mammina C. The changing pattern of human immunodeficiency virus type 1 infection in intravenous drug users. Results of a six-year seroprevalence study in Palermo, Italy. Am J Epidemiol. 1992;135:1189–1196. doi: 10.1093/oxfordjournals.aje.a116225. [DOI] [PubMed] [Google Scholar]
- Ronald PJ, Robertson JR, Wyld R, Weightman R. Heterosexual transmission of HIV in injecting drug users. BMJ. 1993;307:1184–1185. doi: 10.1136/bmj.307.6913.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YH, Hong KX, Liu SZ, He YX, Zhou F, Qin GM, Chen KL, Xing H, Chen JP, Shao YM. Community-based survey of HCV and HIV coinfection in injection drug abusers in Sichuan Province of China. World J Gastroenterol. 2004;10:1589–1593. doi: 10.3748/wjg.v10.i11.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatani SA, Manfredi D, Chiodo RF. Reduction of fatality events in a cohort of drug addicts in the metropolitan area of Bologna, Italy. J Appl Res Clin Exp Therapeutics. 2005;5:585–597. [Google Scholar]
- Salmaso S, Conti S, Sasse H. Drug use and HIV-1 infection: report from the Second Italian Multicenter Study. J Acquir Immune Defic Syndr. 1991;4:607–613. [PubMed] [Google Scholar]
- Sasse H, Salmaso S, Conti S. Risk behaviors for HIV-1 infection in Italian drug users: report from a multicenter study. First Drug User Multicenter Study Group. J Acquir Immune Defic Syndr. 1989;2:486–496. [PubMed] [Google Scholar]
- Schroll JB, Moustgaard R, Gotzsche PC. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med Res Methodol. 2011;11:22. doi: 10.1186/1471-2288-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraino D, Franceschi S, Vaccher E, Diodato S, Errante D, Crosato I, Guarneri S, Tirelli U. Condom use and sexual habits of heterosexual intravenous drug users in northern Italy. Eur J Epidemiol. 1992;8:723–729. doi: 10.1007/BF00145390. [DOI] [PubMed] [Google Scholar]
- Serraino D, Franceschi S, Vaccher E, Diodato S, Santini GF, La Vecchia C, Hayes R, Tirelli U. Risk factors for human immunodeficiency virus infection in 581 intravenous drug users, northeast Italy, 1984–1988. The AIDS and Related Syndromes Study Group. Int J Epidemiol. 1991;20:264–270. doi: 10.1093/ije/20.1.264. [DOI] [PubMed] [Google Scholar]
- Shaboltas AV, Toussova OV, Hoffman IF, Heimer R, Verevochkin SV, Ryder RW, Khoshnood K, Perdue T, Masse BR, Kozlov AP. HIV prevalence, sociodemographic, and behavioral correlates and recruitment methods among injection drug users in St. Petersburg, Russia. J Acquir Immune Defic Syndr. 2006;41:657–663. doi: 10.1097/01.qai.0000220166.56866.22. [DOI] [PubMed] [Google Scholar]
- Simmonds L, Coomber R. Injecting drug users: a stigmatised and stigmatising population. Int J Drug Policy. 2009;20:121–130. doi: 10.1016/j.drugpo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Spijkerman IJ, van Ameijden EJ, Mientjes GH, Coutinho RA, van den Hoek A. Human immunodeficiency virus infection and other risk factors for skin abscesses and endocarditis among injection drug users. J Clin Epidemiol. 1996;49:1149–1154. doi: 10.1016/0895-4356(96)00180-1. [DOI] [PubMed] [Google Scholar]
- StataCorp LP. Stata Statistical Software: Release 11. StataCorp; College Station Texas: 2009. [Google Scholar]
- Stricof RL, Kennedy JT, Nattell TC, Weisfuse IB, Novick LF. HIV seroprevalence in a facility for runaway and homeless adolescents. Am J Public Health. 1991;81(Suppl):50–53. doi: 10.2105/ajph.81.suppl.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taran YS, Johnston LG, Pohorila NB, Saliuk TO. Correlates of HIV risk among injecting drug users in sixteen Ukrainian cities. AIDS Behav. 2011;15:65–74. doi: 10.1007/s10461-010-9817-6. [DOI] [PubMed] [Google Scholar]
- Teixeira SL, Bastos FI, Telles PR, Hacker MA, Brigido LF, de FOCA, Bongertz V, Morgado MG. HIV-1 infection among injection and ex-injection drug users from Rio de Janeiro, Brazil: prevalence, estimated incidence and genetic diversity. J Clin Virol. 2004;31:221–226. doi: 10.1016/j.jcv.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Telles PR, BF, Lima ES, Friedman SR, Des Jarlais DC. HIV-1 Epidemiology Among IDUs in Rio de Janeiro, Brazil. Proceedings of the International Conference on AIDS.1992. [Google Scholar]
- Turrina C, Fiorazzo A, Turano A, Cacciani P, Regini C, Castelli F, Sacchetti E. Depressive disorders and personality variables in HIV positive and negative intravenous drug-users. J Affect Disord. 2001;65:45–53. doi: 10.1016/s0165-0327(00)00269-x. [DOI] [PubMed] [Google Scholar]
- UNAIDS; UNAIDS. Number of women living with HIV increases in each region of the world. Geneva: 2004. [Google Scholar]
- United Nations Population Fund; Fund, U.N.P. State of World Population 2005. UNPF; New York: 2005. Reproductive Health: A Measure of Equity. [Google Scholar]
- UNODC; UNODC. 2006 HIV/AIDS prevention and care for female injecting drug users. Vienna: 2006a. [Google Scholar]
- UNODC; UNODC. HIV/AIDS prevention and care for female injecting drug users. UNODC; Vienna: 2006b. [Google Scholar]
- Uuskula A, McMahon JM, Raag M, Silm S, Ruutel K, Talu A, Abel-Ollo K, Ahas R, Des Jarlais DC. Emergent properties of HIV risk among injection drug users in Tallinn, Estonia: synthesis of individual and neighbourhood-level factors. Sex Transm Infect. 2010;86(Suppl 3):iii79–84. doi: 10.1136/sti.2009.040212. [DOI] [PubMed] [Google Scholar]
- Uuskula A, McNutt LA, Dehovitz J, Fischer K, Heimer R. High prevalence of blood-borne virus infections and high-risk behaviour among injecting drug users in Tallinn, Estonia. Int J STD AIDS. 2007;18:41–46. doi: 10.1258/095646207779949907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo F, Toro C, de la Fuente L, Brugal MT, Soriano V, Silva TC, Bravo MJ, Ballesta R, Barrio G. Prevalence of and risk factors for hepatitis B virus infection among street-recruited young injection and non-injection heroin users in Barcelona, Madrid and Seville. Eur Addict Res. 2008;14:116–124. doi: 10.1159/000130415. [DOI] [PubMed] [Google Scholar]
- van den Hoek A, van Haastrecht HJ, Coutinho RA. Heterosexual behaviour of intravenous drug users in Amsterdam: implications for the AIDS epidemic. AIDS. 1990;4:449–453. doi: 10.1097/00002030-199005000-00011. [DOI] [PubMed] [Google Scholar]
- van den Hoek JA, Coutinho RA, van Haastrecht HJ, van Zadelhoff AW, Goudsmit J. Prevalence and risk factors of HIV infections among drug users and drug-using prostitutes in Amsterdam. AIDS. 1988;2:55–60. doi: 10.1097/00002030-198802000-00010. [DOI] [PubMed] [Google Scholar]
- van den Hoek JA, van Haastrecht HJ, Coutinho RA. Risk reduction among intravenous drug users in Amsterdam under the influence of AIDS. Am J Public Health. 1989;79:1355–1357. doi: 10.2105/ajph.79.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Snoek EM, Chin ALRA, de Ridder MA, Willems PW, Verkooyen RP, van der Meijden WI. Prevalence of sexually transmitted diseases (STD) and HIV-infection among attendees of STD Outpatient Clinic, Dijkzigt University Hospital in Rotterdam; comparative analysis of years 1993 and 1998. Ned Tijdschr Geneeskd. 2000;144:1351–1355. [PubMed] [Google Scholar]
- Wald NJ, Hackshaw AK, Frost CD. When can a risk factor be used as a worthwhile screening test? BMJ. 1999;319:1562–1565. doi: 10.1136/bmj.319.7224.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JBP. Targeted sampling: options for the study of hidden populations. Soc Prob. 1989;36:416–430. [Google Scholar]
- Weissenbacher M, Rossi D, Radulich G, Sosa-Estani S, Vila M, Vivas E, Avila MM, Cuchi P, Rey J, Peralta LM. High seroprevalence of bloodborne viruses among street-recruited injection drug users from Buenos Aires, Argentina. Clin Infect Dis. 2003;37(Suppl 5):S348–352. doi: 10.1086/377560. [DOI] [PubMed] [Google Scholar]
- Wiessing LG, Houweling H, Meulders WA, Cerda E, Jansen M, Sprenger MJ. Prevalence of HIV infection among drug users in Zuid-Limburg. Ned Tijdschr Geneeskd. 1995;139:1936–1940. [PubMed] [Google Scholar]
- Yin L, Qin G, Qian HZ, Zhu Y, Hu W, Zhang L, Chen K, Wang Y, Liu S, Zhou F, Xing H, Ruan Y, Wang N, Shao Y. Continued spread of HIV among injecting drug users in southern Sichuan Province, China. Harm Reduct J. 2007;4:6. doi: 10.1186/1477-7517-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccarelli M, Rezza G, Girardi E, Puro V, Pezzotti P, Lelli V, Narciso P. Monitoring HIV trends in injecting drug users: an Italian experience. AIDS. 1990;4:1007–1010. doi: 10.1097/00002030-199010000-00010. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang R, Xia X, Qin S, Dai J, Zhang Z, Peng Z, Wei T, Liu H, Pu D, Luo J, Takebe Y, Ben K. High prevalence of HIV-1 and hepatitis C virus coinfection among injection drug users in the southeastern region of Yunnan, China. J Acquir Immune Defic Syndr. 2002;29:191–196. doi: 10.1097/00042560-200202010-00014. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhu J, Rui B, Zhang Y, Yin L, Ruan Y, Qian HZ, Shao Y. High HIV risk among Uigur minority ethnic drug users in northwestern China. Trop Med Int Health. 2008;13:814–817. doi: 10.1111/j.1365-3156.2008.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shan H, Trizzino J, Ruan Y, Beauchamp G, Masse B, Ma J, Gu Y, He Y, Rui B, Wang J, Poundstone K, Jiang Y, Brooks Jackson J, Shao Y. Demographic characteristics and risk behaviors associated with HIV positive injecting drug users in Xinjiang, China. J Infect. 2007a;54:285–290. doi: 10.1016/j.jinf.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shan H, Trizzino J, Ruan Y, Beauchamp G, Masse B, Ma J, Rui B, Wang J, Liu M, Wang Y, He Y, Poundstone K, Jiang Y, Jackson JB, Shao Y. HIV incidence, retention rate, and baseline predictors of HIV incidence and retention in a prospective cohort study of injection drug users in Xinjiang, China. Int J Infect Dis. 2007b;11:318–323. doi: 10.1016/j.ijid.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Zheng X, Tian C, Choi KH, Zhang J, Cheng H, Yang X, Li D, Lin J, Qu S, Sun X, et al. Injecting drug use and HIV infection in southwest China. AIDS. 1994;8:1141–1147. doi: 10.1097/00002030-199408000-00017. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Liu FL, Yao ZH, Duo L, Li H, Sun Y, Zheng YT. Comparison of HIV-, HBV-, HCV- and co-infection prevalence between Chinese and Burmese intravenous drug users of the China-Myanmar border region. PLoS One. 2011;6:e16349. doi: 10.1371/journal.pone.0016349. [DOI] [PMC free article] [PubMed] [Google Scholar]