Abstract
A bio-inspired investigation of the reactions of substrates of type 1 with VOF3 and PIFA [phenyliodine(III) bis(trifluoroacetate)] led to a collection of colchicine-like compounds 2–5 and related systems. Biological evaluation revealed that some of the synthesized products had significant cytotoxic properties against the colon cancer cell line HT-29.
Keywords: bio-inspired synthesis, colchicine-like compounds, natural products, oxidative coupling, anti-cancer agents
The continuous unraveling of nature’s molecular diversity has led to an impressive number of medications for the treatment of disease.1,2 Natural products have served directly as therapeutics or lead compounds for the discovery of clinically used drugs3 as well as myriad of biological tools.4,5 Important pharmaceuticals originating from natural sources are used in various capacities, including as pain killers, anti-infectives, immuno-suppressants, and anti-cancer drugs.
Colchicine, a natural product used for the treatment of acute gout disease, has been the focus of numerous investigations as a potential anti-cancer drug.6 Its potent cytotoxicity (IC50 = 0.008 µM against HT-29, human colon adenocarcinoma)7 makes colchicine an intriguing chemotherapeutic candidate. However, a lack of selectivity has prevented it, thus far, from being an approved medication for cancer patients. As part of our program of creating molecular diversity as inspired by natural products and their biosyntheses, we investigated a number of synthetic pathways to colchicine-like and related compounds. Herein we report the synthesis of a library of bio-inspired compounds, and their biological evaluation as cytotoxic agents.
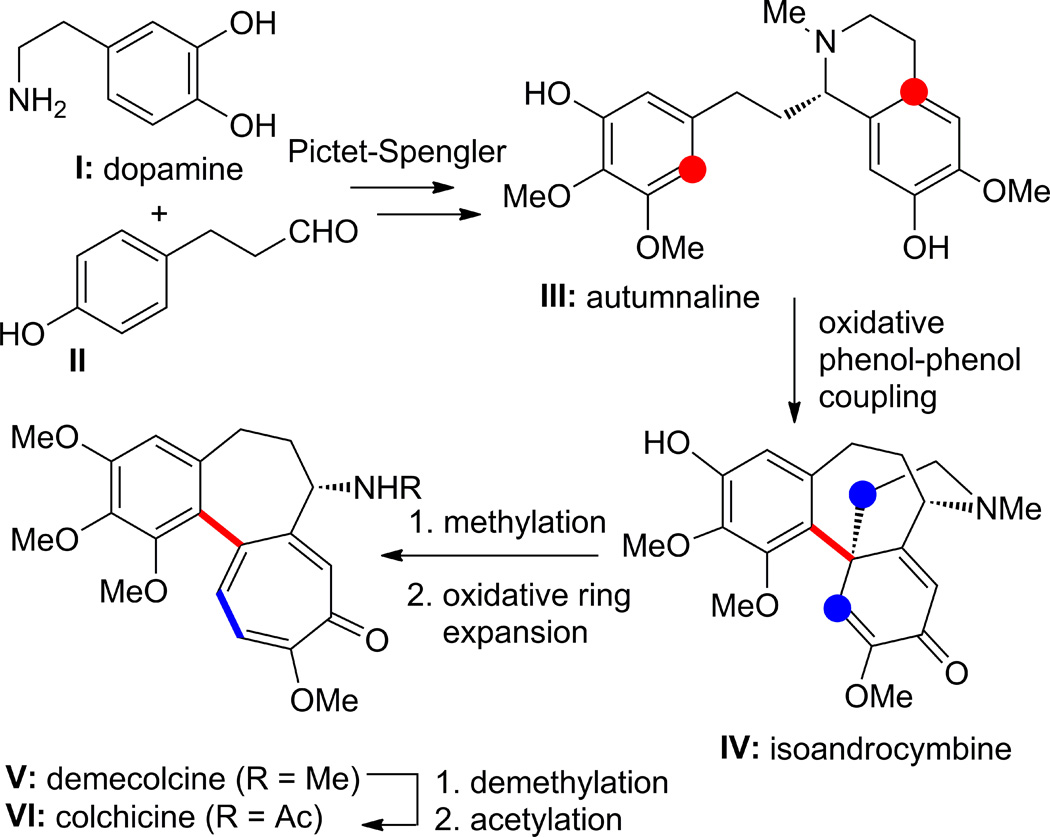
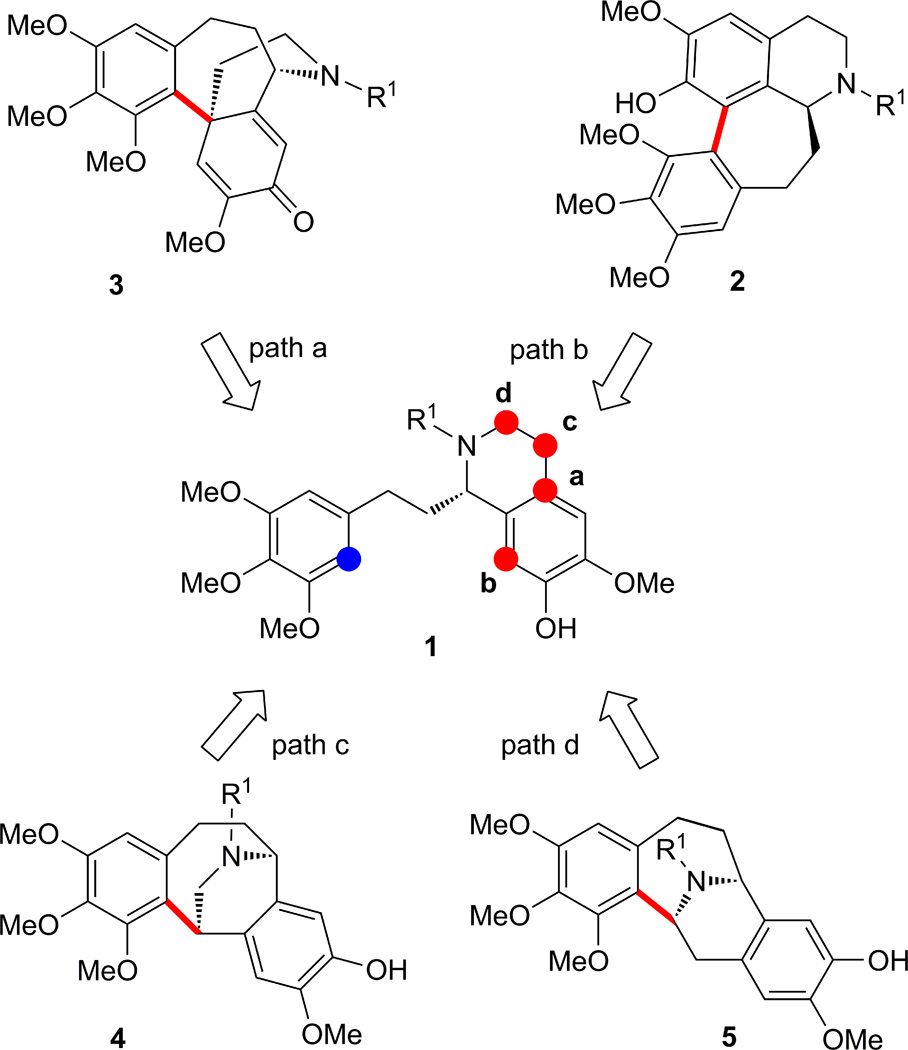
Scheme 1 summarizes the postulated biosynthetic pathway leading from dopamine (I) and phenol aldehyde (II) to demecolcine (V) and thence colchicine (VI) via (S)-autumnaline (III) and (S)-isoandrocymbine (IV). The first two enzymatic transformations of (I + II to III) and (III to IV) are well understood as a Pictet-Spengler and an oxidative phenol-phenol coupling, respectively.8 However, the conversion of isoandrocymbine to demecolcine and thence colchicine (IV to V and VI) is more complex and rather speculative (see Scheme 1).8 These complexities are reflected in the fact that a biomimetic total synthesis of colchicine still has not been reported.6 Thus, while the non-enzymatic conversion of (I + II to III) has been demonstrated in the laboratory, not all the subsequent steps have been reported as yet by chemical means.9 Our studies were, therefore, focused on finding conditions to realize useful pathways for the formation of colchicine-like structures and related scaffolds starting from substrates 1 (Figure 1), which are derivatives of autumnaline (III). The four possible tetracyclic structural motifs 2–5 were expected to be formed oxidatively from 1, as shown in Figure 1 (see color code).
Scheme 1.
Postulated biosynthesis of demecolcine (V) and colchicine (VI) from dopamine (I) and phenol aldehyde (II). Colors indicate atoms joining to form the corresponding C–C bond. The complex skeletal rearrangement from IV to V or VI can be found in Ref. 8.
Figure 1.
Possible oxidative pathways depicted in retrosynthetic format to compounds 2–5 from substrates 1.
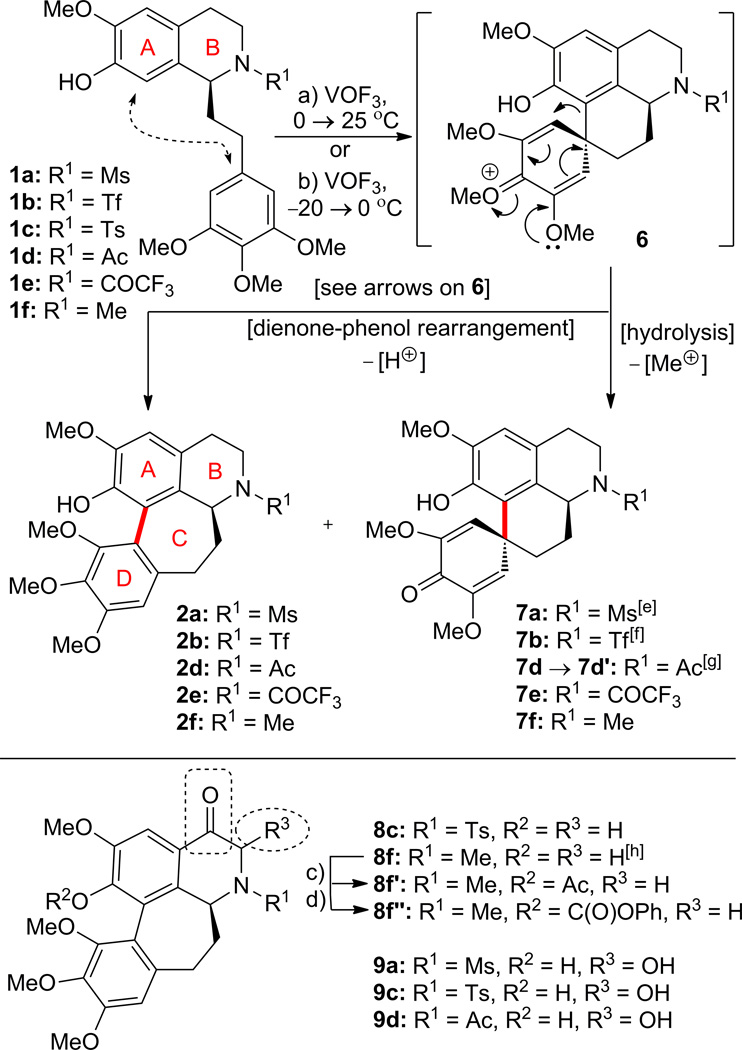
Scheme 2 summarizes the results of our investigations of the reaction of the readily available substrates 1a–f (synthesized by standard methods; see Supplementary data)8,10 with VOF3–TFA–TFAA, an approach previously reported by Kupchan and co-workers.10d,11 At the outset of our work, the potential of this reaction to produce novel molecular diversity was still considered to be high because of the limited number of products and biological data reported by Kupchan et al.10d,11 Indeed, under two different sets of conditions [a) or b)] a variety of additional products were obtained as shown in Scheme 2. Thus, under conditions a) [VOF3, TFA–TFAA, CH2Cl2, 0–25 °C] several new compounds were isolated: 2a (22% yield from 1a); 8c (7% yield) plus 9c (6% yield) from 1c; 2d (17% yield) plus 7d′ (5% yield) plus 9d (7% yield) from 1d; 2e (46% yield)10d,11 plus 11a (27% yield) from 1e; and 2f (< 2% yield) plus 7f (23% yield)10d,12 plus 8f (< 2% yield) from 1f. At lower temperatures, conditions b) [−20 → 0 °C], the VOF3 reaction yielded compounds 2a (22% yield) plus 9a (7% yield) plus 7a (< 2% yield, this compound was obtained in 17% yield under different conditions, see Scheme 2) from 1a; 2b (34% yield) plus 7b (7% yield, this compound was obtained in 19% yield under different conditions, see Scheme 2) from 1b; and 7e (11% yield) from 1e.10d Since we were primarily interested in generating molecular diversity rather than any particular compound, no further attempts were made to improve the selectivity of this reaction.
Scheme 2.
Reactions of compounds 1a–f with vanadyl trifluoride (VOF3). Synthesis of compounds 2a,b,d–f, 7a,b,d′,e,f, 8c,f,f′,f″ and 9a,c,d. Reagents and conditions: a) VOF3, TFA–TFAA (10:1), CH2Cl2, 0 to 25 °C, 0.5–3 h, 1a → 2a (22%); 1c → 8c (7%) plus 9c (6%); 1d → 2d (17%) plus 7d′ (5%) plus 9d (7%); 1e → 2e (46%) plus 11a (27%); 1f → 2f (< 2%) plus 7f (23%) plus 8f (< 2%); b) VOF3, TFA–TFAA (10:1), CH2Cl2, −20 to 0 °C, 0.5–1 h, 1a → 2a (22%) plus 9a (7%) plus 7a (< 2%); 1b → 2b (34%) plus 7b (7%); 1e → 7e (11%); c) AcCl, Et3N, CH2Cl2, 0 °C, 0.5 h, 8f′ (50%); d) PhOC(O)Cl, Et3N, CH2Cl2, 0 °C, 0.5 h, 8f″ (58%); [e] 1a → 7a (17%), −10 °C, 0.3 h; [f] 1b → 7b (19%), −30 to −10 °C, 0.8 h; [g] 7d → 7d′ through C-ring expansion (see Supplementary data); [h] 8f can be formed from 1f with PIFA in refluxing benzene or via a skeletal rearrangement from 3f (Scheme 4) in the presence of a Lewis acid (BF3•Et2O in CH2Cl2) or a protic acid (p-TsOH in AcOH) (see Supplementary data). TFA = 2,2,2-trifluoroacetic acid; TFAA = trifluoroacetic anhydride.
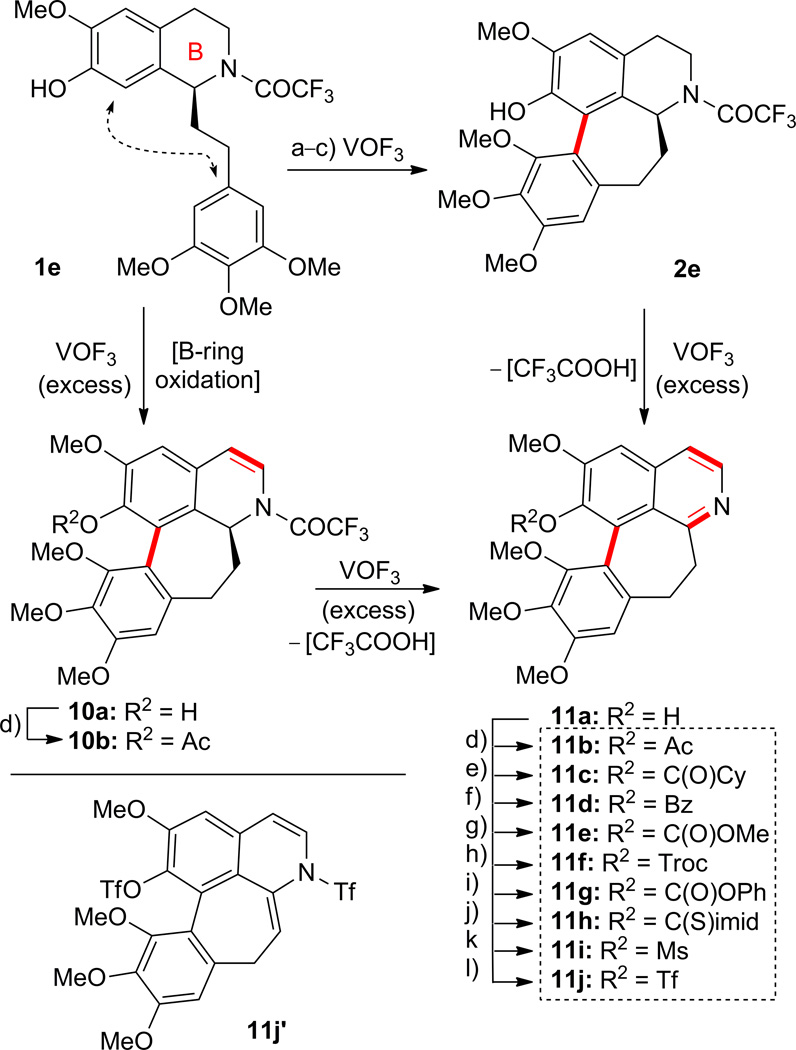
Hoping to synthesize additional novel products from substrate 1e, we explored its reaction with VOF3 under three additional sets of conditions [a), b) and c), Scheme 3]. Thus, treatment of 1e with VOF3 [TFA–TFAA–CH2Cl2, 10:1:100, conditions a)] at 0–25 °C for 3 h furnished scaffolds 10a (9% yield) and 11a (17% yield). Carrying out the same reaction in neat TFA–TFAA [75:1, conditions b)] led to the isolation of 2e in 46% yield and 11a in 27 % yield. Modifying these conditions further [TFA–TFAA–CH2Cl2 10:1:150 for 16 h, conditions c)] afforded 11a in 49% yield. Derivatives 11b–11j were prepared from 11a by standard procedures as summarized in Scheme 3. Compounds 10b and 11j′ were also formed as shown in Scheme 3, the latter as a major byproduct of the reaction of 11a with trifluoromethanesulfonic anhydride (Tf2O).
Scheme 3.
Reaction of compound 1e with vanadyl trifluoride (VOF3). Synthesis of compounds 2e, 10a,b, 11a–j,j′. Reagents and conditions: a) VOF3, TFA–TFAA–CH2Cl2 (10:1:100), 0 to 25 °C, 3 h, 1e → 10a (9%) plus 11a (17%); b) VOF3, TFA–TFAA–CH2Cl2 (75:1:0), 0 to 25 °C, 6 h, 1e → 2e (46%) plus 11a (27%); c) VOF3, TFA–TFAA–CH2Cl2 (10:1:150), 0 to 25 °C, 16 h, 1e → 11a (49%); d) AcCl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11b (64%); e) CyC(O)Cl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11c (88%); f) PhC(O)Cl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11d (94%); g) MeOC(O)Cl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11e (54%); h) 2,2,2-trichloroethyl chloroformate, Et3N, CH2Cl2, 0 °C, 0.5 h, 11f (77%); i) PhOC(O)Cl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11g (56%); j) 1,1′-thiocarbonyldiimidazole, Et3N, CH2Cl2, 25 °C, 1 h, 11h (39%); k) MsCl, Et3N, CH2Cl2, 0 °C, 0.5 h, 11i (74%); l) Tf2O, Et3N, CH2Cl2, −78 °C, 0.5 h, 11j (37%) plus 11j′ (51%). TFA = 2,2,2-trifluoroacetic acid; TFAA = trifluoroacetic anhydride; Cy = cyclohexyl; Bz = benzoyl; Troc = 2,2,2-trichloroethoxycarbonyl; C(S)imid = thionoimidazolide.
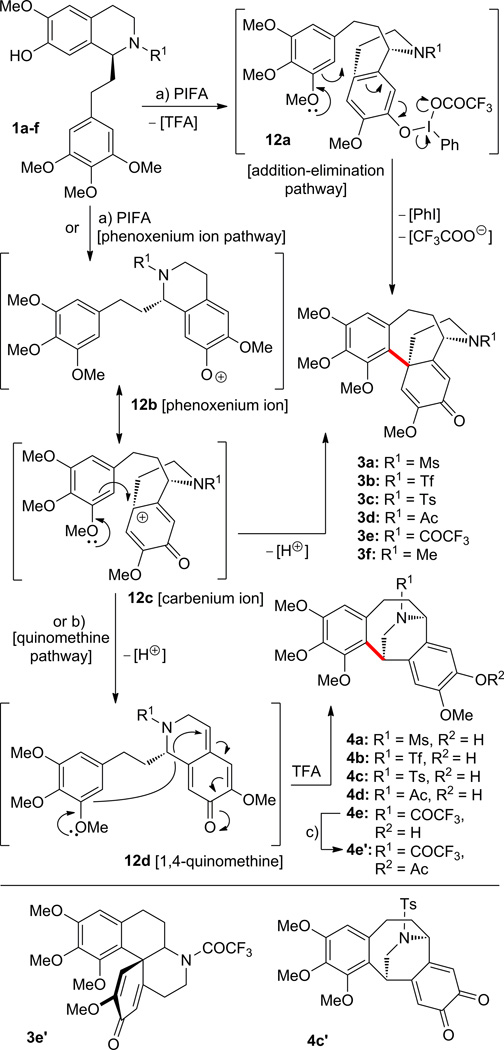
We then examined the reaction of substrates 1a–f with phenyliodine(III) bis(trifluoroacetate) (PIFA)13 from which a number of new molecular scaffolds were isolated as shown in Scheme 4. Thus, exposure of tetrahydroisoquinolines 1a–f to PIFA in CH2Cl2 at 0 → 25 °C led to products 3a (13% yield) from 1a; 3b (35% yield) from 1b; 3c (14% yield) plus o-quinone 4c′ (9% yield) from 1c; 3d (5% yield)9 plus 4d (7% yield) from 1d; 3e (8% yield)10d,11 plus 3e′ (6% yield) plus 4e (< 2% yield) from 1e; and 3f (25% yield) from 1f.8,9,12,14 Changing the solvent from CH2Cl2 to benzene and heating at 80 °C, the PIFA reaction with 1a–f mostly favors the formation of motif 4, producing 4a (12% yield) from 1a; 4b (< 2% yield) from 1b, among a complex mixture of other products; 4c (22% yield) from 1c; 4d (< 2% yield) from 1d; and 4e (10% yield) plus 3e (< 2% yield) plus 3e′ (< 2% yield) from 1e. Derivative 4e′ (83% yield) was prepared from 4e as indicated in Scheme 4. The formation of products 3a–f is presumed to proceed via either the addition-elimination pathway13d,e (intermediate 12a), or the phenoxenium ion pathway13d,e (phenoxenium ion 12b and its resonance counterpart - carbenium ion 12c) as demonstrated in Scheme 4. The generation of compounds 4a–e is assumed to proceed through fleeting intermediates 12b, 12c and 12d (1,4-quinomethine) (Scheme 4).
Scheme 4.
Reaction of compounds 1a–f with phenyliodine(III) bis(trifluoroacetate) (PIFA). Synthesis of compounds 3a–f,e′ and 4a–e,c′,e′. Reagents and conditions: a) PIFA, CH2Cl2, 0 to 25 °C, 0.5–2 h, 1a → 3a (13%); 1b → 3b (35%); 1c → 3c (14%) plus 4c′ (9%); 1d → 3d (5%) plus 4d (7%); 1e → 3e (8%) plus 3e′ (6%) plus 4e (< 2%); 1f → 3f (25%); b) PIFA, benzene, 80 °C, 0.5–1 h, 1a → 4a (12%), 2.4 h; 1b → 4b (< 2%); 1c → 4c (22%), 0.5 h; 1d → 4d (< 2%); 1e → 4e (10%) plus 3e (< 2%) plus 3e′ (< 2%); c) AcCl, Et3N, CH2Cl2, 0 °C, 0.5 h, 4e′ (83%). TFA = 2,2,2-trifluoroacetic acid.
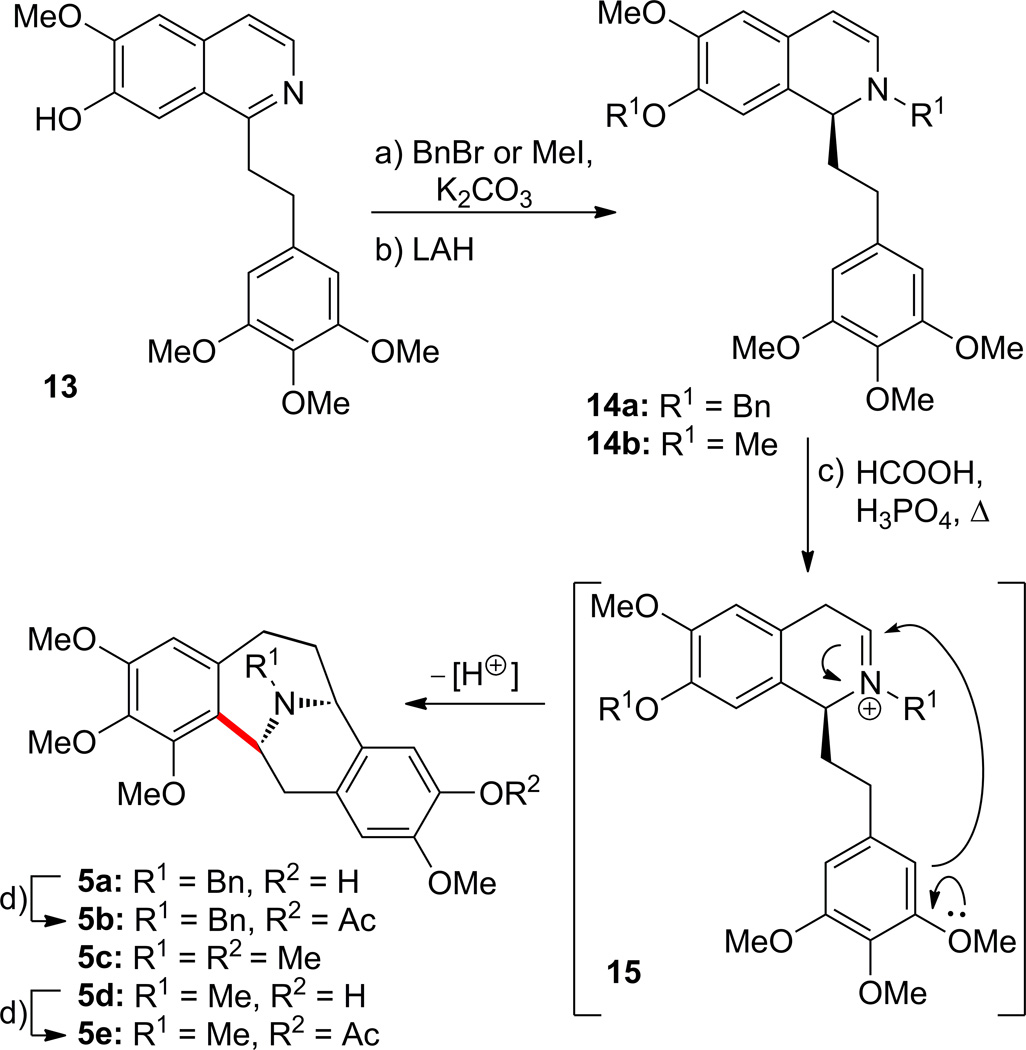
Compounds 5a–e were prepared from isoquinoline 1315 as summarized in Scheme 5. Thus, benzylation or methylation of 13 (K2CO3, BnBr or MeI) and subsequent reduction with lithium aluminum hydride (LAH) gave rather labile derivative 14a or 14b. Heating the latter compounds in the presence of HCOOH–H3PO4 15 led, through presumed iminium species 15, to 5a (19% overall yield) from 13, 5c (38% overall yield) plus 5d (14% overall yield) from 13. Acetylation of 5a and 5d led to derivatives 5b (80% yield) and 5e (70% yield), respectively.
Scheme 5.
Synthesis of compounds 5a–e. Reagents and conditions: a) BnBr or MeI, K2CO3, acetone, reflux, 2 h; b) LiAlH4, DME, 0 °C, 1 h; c) HCOOH–H3PO4 (2:1), 100 °C, 7–20 h, 13 → 5a (19%); 13 → 5c (38%) plus 5d (14%); d) AcCl, Et3N, CH2Cl2, 0 °C, 0.5 h, 5b (80%); 5e (70%). Bn = benzyl; DME = 1,2-dimethoxyethane.
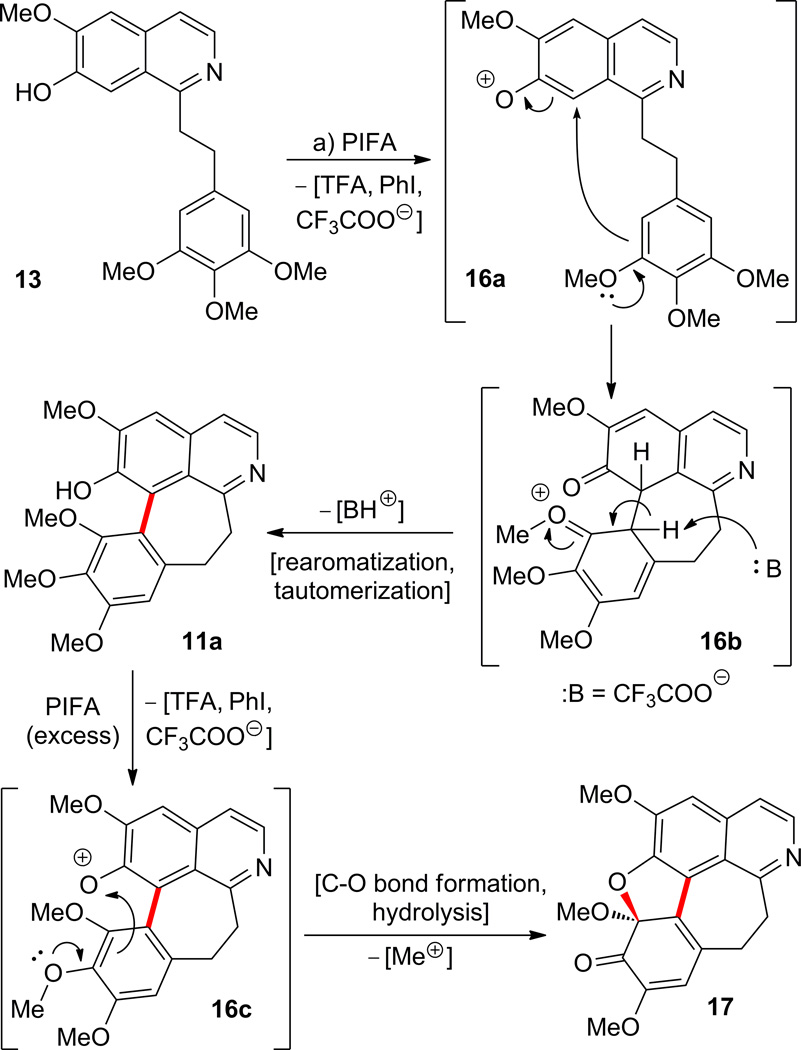
Scheme 6 summarizes the reaction of isoquinoline 13 with PIFA leading, first to the previously mentioned (see Scheme 3) tetracyclic product 11a (11% yield), and thence to pentacyclic compound 17 (7% yield from 13). This process is believed to proceed through intermediates 16a, 16b, and 16c as depicted in Scheme 6.
Scheme 6.
Reaction of isoquinoline 13 with PIFA. Preparation of compound 17 and proposed mechanism of its formation from 13 via 11a. Reagents and conditions: a) PIFA, CH2Cl2, 0 to 25 °C, 1 h, 11a (11%) plus 17 (7%). TFA = 2,2,2-trifluoroacetic acid.
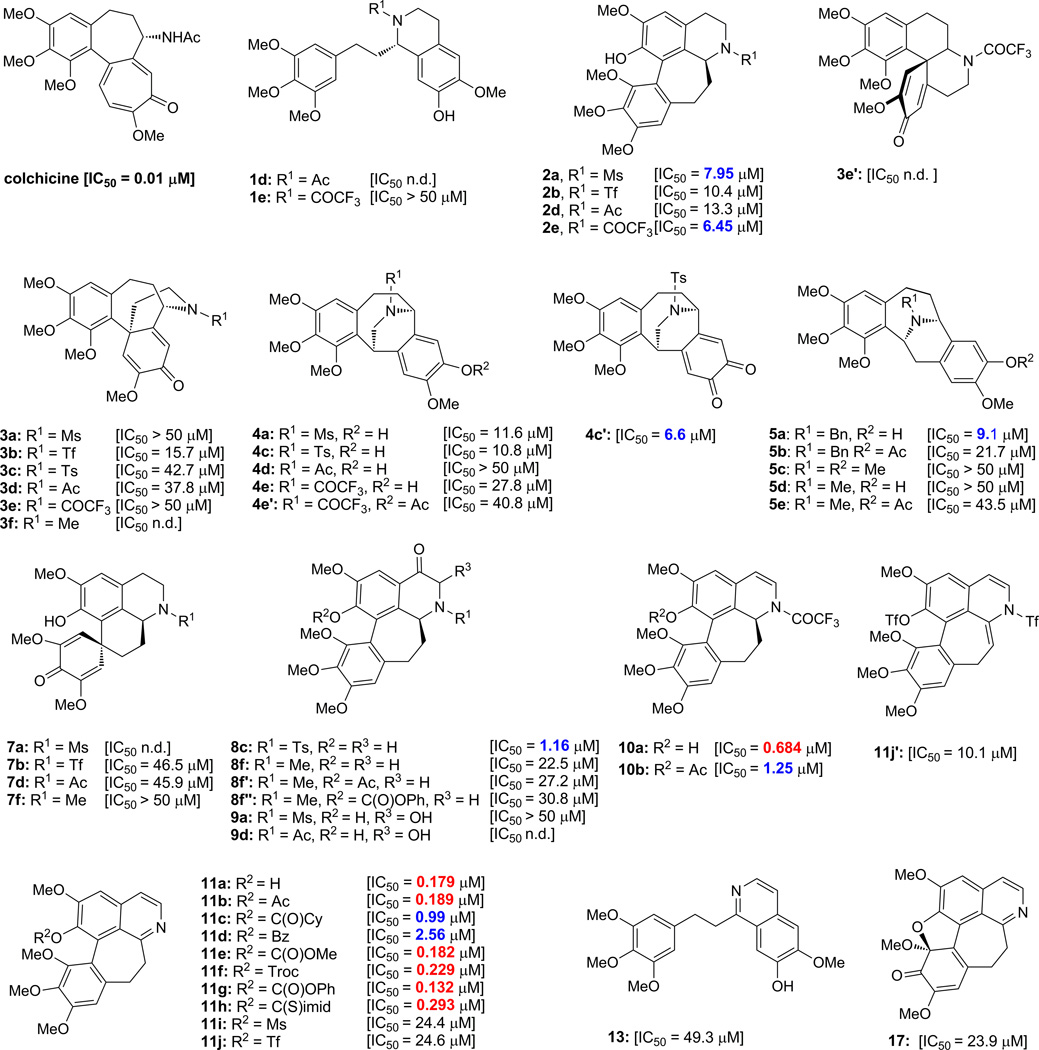
Colchicine exerts its cytotoxic activity through tubulin binding that disturbs the tubulin-microtubule equilibrium.6 As we mentioned before, colchicine is highly potent against the colorectal adenocarcinoma cell line HT-29. The synthesized colchicinoid and related compounds were, therefore, assayed for cytotoxicity against HT-29 cancer cells using the MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. The so obtained IC50 values are shown in Figure 2, together with that found for colchicine (IC50 ~ 0.01 µM) for comparison. Compounds 10a, 11a, 11b, 11e, 11f, 11g, and 11h exhibited the highest cytotoxic activity in this assay (IC50 values = 0.132–0.684 µM, in red). Compounds 2a, 2e, 4c′, 5a, 8c, 10b, 11c, and 11d exhibited lower potencies (IC50 values = 0.99–9.10 µM, in blue), while the rest of the compounds showed either low activity (IC50 values < 50 µM, in black) or their activity was not determined (n.d., in black).
Figure 2.
Cytotoxicity of synthesized compounds against HT-29 cancer cells.a
a HT-29 cells were grown in RPMI-1640 media supplemented with 10% newborn calf serum (NCS), and maintained at 37 °C with 5% CO2 and 95% air. Cells were plated in a 96-well plate (5 × 103 cells/well) 24 h prior to incubation with the indicated compound. The media was then replaced with fresh media containing varying concentrations of the indicated compound. Following a 96-hour incubation, MTT (5 mg/mL, 25 µL/well) was added and incubation was continued at 37 °C for 1–2 h (or until formazan crystals were visible in control wells). Media was carefully aspirated and DMSO was added (200 µL/well) to dissolve the purple MTT-formazan crystals. The absorbance of the dissolved formazan was quantified at 570 nm using UV-Vis plate reader and cell viability was determined as a fraction of absorbance relative to untreated control wells. Each experiment was performed in triplicate and individual data points are presented as averages +/− standard deviation. Figure 2 shows examples of compounds with high (IC50 < 1 µM; red), medium (IC50 < 10 µM; blue), low (IC50 < 50 µM; black) and negligible (IC50 more than 50 µM or n.d. = not determined) anti-HT-29 activity. Bn = benzyl; Bz = benzoyl; Cy = cyclohexyl; Troc = 2,2,2-trichloroethoxycarbonyl; C(S)imid = thionoimidazolide; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
The bio-inspired investigation of the reactions of autumnaline derivatives 1a–f led to a series of novel colchicine-related compounds. Biological evaluation of these compounds identified several agents with significant cytotoxicity against HT-29 cancer cells. Among them, the most potent ones were compounds 11a, 11b and 11e–11h. These results suggest these molecular scaffolds as leads for further studies aiming at new biological tools and potential therapies for the treatment of cancer.
Supplementary Material
Acknowledgments
We thank Drs. D. H. Huang and L. Pasternack for NMR spectroscopic assistance, Dr. G. Siuzdak for mass spectrometric assistance and Dr. E. Hamel for helpful discussions. Financial support for this work was provided by the Skaggs Institute for Research and the National Institutes of Health, USA (grant AI055475). We are grateful to the NSF for a predoctoral fellowship (to V. C.), Merck for a postdoctoral fellowship (to R. A. V.), and NIH for a Pathway to Independence Award (K99EB011530; to K. P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this with article can be found online, at doi:10.1016/j.bmcl.2012.xx.xxx.
References and notes
- 1.Nicolaou KC, Montagnon T. Molecules that Changed the World. Weinheim: Wiley-VCH; 2008. pp. 1–366. [Google Scholar]
- 2.(a) Ganis P, Avitabile G, Mechinski W, Shaffner CP. J. Am. Chem. Soc. 1971;93:4560. doi: 10.1021/ja00747a037. [DOI] [PubMed] [Google Scholar]; (b) Lee MD, Dunne TS, Siegel MM, Chang CC, Morton GO, Borders DB. J. Am. Chem. Soc. 1987;109:3464. [Google Scholar]; (c) Smith AL, Nicolaou KC. J. Med. Chem. 1996;39:2103. doi: 10.1021/jm9600398. [DOI] [PubMed] [Google Scholar]; (d) Oberlies NH, Kroll DJ. J. Nat. Prod. 2004;67:129. doi: 10.1021/np030498t. [DOI] [PubMed] [Google Scholar]; (e) Nicolaou KC, Chen JS, Dalby SM. Bioorg. Med. Chem. 2009;17:2290. doi: 10.1016/j.bmc.2008.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Kuznetsov G, Towle MJ, Cheng H, Kawamura T, TenDyke K, Liu D, Kishi Y, Yu MJ, Littlefield BA. Cancer Res. 2004;64:5760. doi: 10.1158/0008-5472.CAN-04-1169. [DOI] [PubMed] [Google Scholar]; (b) Kim D-S, Dong C-G, Kim JT, Guo H, Kishi Y. J. Am. Chem. Soc. 2009;131:15636. doi: 10.1021/ja9058475. [DOI] [PubMed] [Google Scholar]; (c) Towle MJ, Salvato KA, Wels BF, Aalfs KK, Zheng W, Seletsky BM, Zhu X, Lewis BM, Kishi Y, Yu MJ, Littlefield BA. Cancer Res. 2011;71:496. doi: 10.1158/0008-5472.CAN-10-1874. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaou KC, Sanchini S, Sarlah D, Lu G, Wu TR, Nomura DK, Cravatt BF, Cubitt B, de la Torre JC, Hessell AJ, Burton DR. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6715. doi: 10.1073/pnas.1015258108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Cragg GM, Newman DJ, Snader KM. J. Nat. Prod. 1997;60:52. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]; (b) Newman DJ, Cragg GM. J. Nat. Prod. 2007;70:461. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 6.Graening T, Schmalz H-G. Angew. Chem. Int. Ed. 2004;43:3230. doi: 10.1002/anie.200300615. [DOI] [PubMed] [Google Scholar]
- 7.(a) Alali FQ, El-Elimat T, Li C, Qandil A, Alkofahi A, Tawaha K, Burgess JP, Nakanishi Y, Kroll DJ, Navarro HA, Falkinham JO, Wani MC, Oberlies NH. J. Nat. Prod. 2005;68:173. doi: 10.1021/np0496587. [DOI] [PubMed] [Google Scholar]; (b) Al-Mahmoud MS, Alali FQ, Tawaha K, Qasaymeh RM. Nat. Prod. Res. 2006;20:153. doi: 10.1080/14786410500046224. [DOI] [PubMed] [Google Scholar]; (c) Alali FQ, Tawaha K, El-Elimat T, Qasaymeh R, Li C, Burgess J, Nakanishi Y, Kroll DJ, Wani MC, Oberlies NH. Nat. Prod. Res. 2006;20:558. doi: 10.1080/14786410500183381. [DOI] [PubMed] [Google Scholar]; (d) Yasobu N, Kitajima M, Kogure N, Shishido Y, Matsuzaki T, Nagaoka M, Takayama H. Med. Chem. Lett. 2011;2:348. doi: 10.1021/ml100287y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Leete E, Nemeth PE. J. Am. Chem. Soc. 1960;82:6055. [Google Scholar]; (b) Barker AC, Battersby AR, McDonald E, Ramage R, Clements JH. J. Chem. Soc. Chem. Commun. 1967:390. [Google Scholar]; (c) Battersby AR, McDonald E, Stachulski AV. J. Chem. Soc. Perkin Trans. I. 1983:3053. [Google Scholar]; (d) Herbert RB, Kattah AE, Knagg E. Tetrahedron. 1990;46:7119. [Google Scholar]; (e) Nasreen A, Rueffer M, Zenk MH. Tetrahedron Lett. 1996;37:8161. [Google Scholar]; (f) Maier UH, Zenk MH. Tetrahedron Lett. 1997;38:7357. [Google Scholar]; (g) Nasreen A, Gundlach H, Zenk MH. Phytochemistry. 1997;46:107. [Google Scholar]; (h) McDonald E, Ramage R, Woodhouse RN, Underhill EW, Wetter LR, Battersby AR. J. Chem. Soc. Perkin Trans. I. 1998:2979. [Google Scholar]
- 9.Ogasawara H, Tasaki S, Mutoh F, Hara H, Nishikawa H, Tanaka M, Hoshino O. Heterocycles. 1994;37:1657. [Google Scholar]
- 10.(a) Brossi A, V.-Burik J, Teitel S. Helv. Chim. Acta. 1968;51:1965. doi: 10.1002/hlca.19680510816. [DOI] [PubMed] [Google Scholar]; (b) Battersby AR, Herbert RB, Pijewska L, Santavy F, Sedmera P. J. Chem. Soc. Perkin Trans. I. 1972:1736. doi: 10.1039/p19720001736. [DOI] [PubMed] [Google Scholar]; (c) Hoshino O, Toshioka T, Ohyama K, Umezawa B. Chem. Pharm. Bull. 1974;22:1307. [Google Scholar]; (d) Kupchan SM, Dhingra OP, Kim C-K. J. Org. Chem. 1978;43:4076. doi: 10.1021/jo00395a025. [DOI] [PubMed] [Google Scholar]; (e) Taylor EC, Andrade JG, Rall GJH, McKillop A. J. Am. Chem. Soc. 1980;102:6513. [Google Scholar]; (f) Seo JW, Srisook E, Son HJ, Hwang O, Cha Y-N, Chi DY. Eur. J. Med. Chem. 2008;43:1160. doi: 10.1016/j.ejmech.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 11.(a) Kupchan SM, Liepa AJ. J. Am. Chem. Soc. 1973;95:4062. doi: 10.1021/ja00793a047. [DOI] [PubMed] [Google Scholar]; (b) Kupchan SM, Dhingra OP, Kim C-K, Kameswaran V. J. Org. Chem. 1976;41:4049. [Google Scholar]; (c) Kupchan SM, Dhingra OP, Kim C-K, Kameswaran V. J. Org. Chem. 1978;43:2521. doi: 10.1021/jo00395a025. [DOI] [PubMed] [Google Scholar]; (d) Kupchan SM, Dhingra OP, Kim C-K. J. Org. Chem. 1978;43:4464. doi: 10.1021/jo00395a025. [DOI] [PubMed] [Google Scholar]
- 12.(a) Kametani T, Koizumi M, Fukumoto K. J. Org. Chem. 1971;36:3729. [Google Scholar]; (b) Battersby AR, Bradbury RB, Herbert RB, Munro MHG, Ramage R. J. Chem. Soc. Perkin Trans. I. 1974:1394. doi: 10.1039/p19740001394. [DOI] [PubMed] [Google Scholar]; (c) Hara H, Hoshino O, Umezawa B, Iitaka Y. J. Chem. Soc. Perkin Trans. I. 1979:2657. [Google Scholar]
- 13.(a) Kita Y, Takada T, Gyoten M, Tohma H, Zenk MH, Eichhorn J. J. Org. Chem. 1996;61:5857. [Google Scholar]; (b) Kita Y, Arisawa M, Gyoten M, Nakajima M, Hamada R, Tohma H, Takada T. J. Org. Chem. 1998;63:6625. [Google Scholar]; (c) Pelish HE, Westwood NJ, Feng Y, Kirchhausen T, Shair MD. J. Am. Chem. Soc. 2001;123:6740. doi: 10.1021/ja016093h. [DOI] [PubMed] [Google Scholar]; (d) Zhdankin VV, Stang PJ. Chem. Rev. 2002;102:2523. doi: 10.1021/cr010003+. [DOI] [PubMed] [Google Scholar]; (e) Yu Z, Ju X, Wang J, Yu W. Synthesis. 2011;6:860. [Google Scholar]
- 14.(a) Kametani T, Koizumi M, Fukumoto K. J. Chem. Soc. (C) 1971:1792. [Google Scholar]; (b) Kametani T, Kohno T, Charubala R, Fukumoto K. Tetrahedron. 1972;28:3227. [Google Scholar]; (c) Schwartz MA, Rose BF, Vishnuvajjala B. J. Am. Chem. Soc. 1973;95:612. [Google Scholar]; (d) Hara H, Hoshino O, Umezawa B. Heterocycles. 1976;5:213. [Google Scholar]; (e) Kametani T, Ihara M, Takemura M, Satoh Y, Terasawa H, Ohta Y, Fukumoto K, Takahashi K. J. Am. Chem. Soc. 1977;99:3805. doi: 10.1021/ja00453a047. [DOI] [PubMed] [Google Scholar]; (f) Hara H, Hoshino O, Ishige T, Umezawa B. Chem. Pharm. Bull. 1981;29:1083. [Google Scholar]; (g) Sedmera P, Husek A, Simanek V. Acta Univ. Palacki. Olomue. (Olomoue), Fac. Med. 1991;129:297. [PubMed] [Google Scholar]
- 15.(a) Stermitz FR, Williams DK. J. Org. Chem. 1973;38:2099. doi: 10.1021/jo00951a033. [DOI] [PubMed] [Google Scholar]; (b) Pabuccuoglu V, Hesse M. Heterocycles. 1997;45:1751. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.