Abstract
Hypoxic preconditioning of stem cells and neural progenitor cells has been tested for promoting cell survival after transplantation. The present investigation examined the hypothesis that hypoxic preconditioning of bone marrow mesenchymal stem cells (BMSCs) could not only enhance their survival but also reinforce regenerative properties of these cells. BMSCs from eGFP engineered rats or pre-labeled with BrdU were pre-treated with normoxia (20% O2, N-BMSCs) or sublethal hypoxia (0.5% O2. H-BMSCs). The hypoxia exposure up-regulated HIF-1α and trophic/growth factors in BMSCs, including brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), vascular endothelial growth factor (VEGF) and its receptor FIK-1, erythropoietin (EPO) and its receptor EPOR, stromal derived factor-1 (SDF-1) and its CXC chemokine receptor 4 (CXCR4). Meanwhile, many pro-inflammatory cytokines/chemokines were downregulated in H-BMSCs. N-BMSCs or H-BMSCs were intravenously injected into adult rats 24 hrs after 90-min middle cerebral artery occlusion. Comparing to N-BMSCs, transplantation of H-BMSCs showed greater effect of suppressing microglia activity in the brain. Significantly more NeuN-positive and Glut1-positive cells were seen in the ischemic core and peri-infarct regions of the animals received H-BMSC transplantation than that received N-BMSCs. Some NeuN-positive and Glut-1-positive cells showed eGFP or BrdU immunoflourescent reactivity, suggesting differentiation from exogenous BMSCs into neuronal and vascular endothelial cells. In Rota-rod test performed 15 days after stroke, animals received H-BMSCs showed better locomotion recovery compared with stroke control and N-BMSC groups. We suggest that hypoxic preconditioning of transplanted cells is an effective means of promoting their regenerative capability and therapeutic potential for the treatment of ischemic stroke.
Keywords: hypoxic preconditioning, bone marrow mesenchymal stem cell, transplantation, angiogenesis, neurogenesis
Introduction
Stroke is a leading cause of human death and disability in the United States. There are currently very few effective clinical therapies for acute ischemic stroke. Alternatively, cell-based transplantation therapy using embryonic and adult stem cells has provided promising hope to enhance tissue repair and functional recovery after stroke. Among stem cells that are candidates for transplantation in human stroke patients, bone marrow mesenchymal stem cells (BMSCs) are preferred because they are available from autologous donation therefore eliminates ethical disputes and other concerns related to graft rejection (Malgieri et al., 2010). Basic and clinical studies suggest that human BMSCs are not antigen-presenting cells and would not cause activation of the host’s immune system (Tse et al., 2003), suggesting that even allogeneic BMSCs may be used for transplantation therapies (Li et al., 2006). In addition, BMSCs have other advantages. For example, BMSCs have an inhibitory action on inflammatory responses, which exacerbates ischemic damage; and they express a variety of neurotrophic and growth factors that may show autocrine and paracrine effects after transplantation (Malgieri et al., 2010; Tse et al., 2003). Examples of those factors include glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and vascular endothelial growth factor (VEGF); all are important for brain protection and tissue regeneration (Chen et al., 2002; Kurozumi et al., 2005).
Transplantation of BMSCs promotes the repair and regeneration of nerve tissue within the central and peripheral nervous systems (Nandoe Tewarie et al., 2006). These cells are being evaluated in human clinical trials for efficacy in treating genetic bone diseases, to speed hematopoietic recovery after bone marrow transplantation and to treat severe graft-versus-host disease (GVHD) (Le Blanc et al., 2008; Singer and Caplan, 2010). In experimental stroke animal studies, compelling evidence shows that transplantation of BMSCs has promising benefits on functional recovery after ischemic stroke or traumatic injury (Li and Chopp, 2009). BMSCs can be administered either via intra-cerebral injection into a specific brain region or by intravenous/intra-arterial injection. Systemically delivered BMSCs can pass through the blood brain barrer and translocate into or “home to” the brain ischemic regions although the number of these cells are limited (Chen et al., 2001).
The mechanism by which BMSCs show functional benefits after transplantation has not been well defined and is sometimes debatable. BMSCs are self-renewable and they are multipotent cells capable of differentiating into diverse cell types (Chen et al., 2006). BMSCs may differentiate into mesodermal lineage cells such as osteoblasts, chondrocytes, adipocytes, and muscle cells but also neurons and astrocytes (Phinney and Isakova, 2005). The clinical significance of the differentiation potential of BMSCs, however, has been questioned in recent years because very few transplanted BMSCs are detected homing to and survive in the ischemic region of the brain while functional activities are still improved by the transplantation therapy (Chen et al., 2001; Hess and Borlongan, 2008). Injection of the BMSC “conditioned” media alone was shown to have functional benefits in stroke animals (Chen et al., 2002). Thus, it has been proposed and commonly accepted that the functional benefits of BMSC transplantation are due to increased trophic support from these cells (Caplan and Dennis, 2006; Hess and Borlongan, 2008). It has been further argued that, since it is the trophic factors but not BMSCs themselves that lead to the functional benefits, the survival, homing and neural differentiation of transplanted BMSCs in the ischemic tissue are not critical in BMSC stroke therapy. While we fully agree that the enhanced trophic support provides important protection for the ischemic brain and stimulates endogenous regenerative mechanisms, the appealing benefits of improving survival, migration, homing, and engraftment of transplanted cells in the neural network repair should not be underestimated.
As new combination strategies are emerging in experimental stem cell therapy, optimizing survival, migration, homing capabilities of BMSCs and promoting their neural differentiation as well as appropriate integration with host tissues remain to be important goals in the development of cell based therapy using BMSCs and other stem cells. It can be expected that improved cell survival and neural differentiation in the ischemic region, in turn, can further enforce the local trophic support and regenerative supply. Based on this idea, we and others have tested genetic modifications of transplanted cells and showed enhanced cell survival and functional recovery (Aggarwal et al.; 2010; Wei et al., 2005). Taking the advantage of a comprehensive increase in endogenous defense/regenerative genes induced by hypoxic preconditioning (HP), we recently explored and reported the preconditioning strategy in stem cell therapy (Francis, 2010; Hu et al., 2011; Hu et al., 2008; Theus et al., 2008). Using this strategy, we have shown that hypoxic preconditioning-treated embryonic stem (ES) cells and BMSCs are much more resistant to necrotic and apoptotic insults, survive much better in vitro and in the ischemic tissue, and show additional functional benefits after transplantation into the ischemic brain and heart (Francis, 2010; Hu et al., 2011; Hu et al., 2008; Theus et al., 2008). In addition, hypoxia or HP promotes neuronal differentiation of embryonic stem cells and BMSCs (Francis, 2010; Pacary et al., 2006; Theus et al., 2008).
In the present investigation, we hypothesize that the preconditioning approach in BMSC transplantation therapy can lead to a comprehensive regulation that increases trophic factor support, promotes cell survival, homing and migration, and stimulates cell differentiation needed for tissue repair. Combination of these effects will result in increased endogenous and/or exogenous neurogenesis, angiogenesis and better functional recovery after ischemic stroke. To test our hypothesis, BMSC transplantation was examined using the transient 90 min ligation of the middle cerebral artery (MCA) that causes a large infarct volume in the ipsilateral hemisphere of the adult rats (Snider et al., 2001).
Materials and Methods
Bone marrow mesenchymal stem cell cultures
Mesenchymal stem cells were harvested from 2 weeks old Wistar rats as previously described (Hu et al., 2011; Hu et al., 2008). Briefly, BMSCs were flushed out from the femoral and tibial bones of donor adult rats using a syringe and 20-gauge needle. The cells were suspended in DMEM with 10% fetal bovine serum and incubated in 95% room air and 5% carbon dioxide at 37°C for 24 hrs. The medium containing the non-adherent hematopoietic cells was then removed from the flask, and fresh medium was added to allow for selection by plastic adherence. Upon BMSC isolation, the medium was changed every 3 days, and the primary cultures were passaged at a ratio of 1:2 once the BMSCs reached 80% confluence. To confirm the cellular identity of cultured cells, BMSCs were subjected to fluorescence-activated cell sorting using CD90, CD34 and CD45 markers, and cultured cells were identified as CD90 positive and CD34/CD45 negative cells.
To facilitate cell tracking after transplantation, BMSCs were isolated from the transgenic rats expressing the enhanced green fluorescent protein (eGFP), acquired from the Rat Resource & Research Center (Columbia, MO). Alternatively, BMSCs isolated from WT rats were labeled with 10 μM sterile BrdU (Sigma-Aldrich, St. Louis, MO) for 48 hrs. In some experiments, BMSCs were also pre-labeled with 10 μM Hoechst 33342 (Molecular Probes, Carlsbad, CA) for 2 hrs prior to transplantation, which further facilitated identification of transplanted cells in brain sections.
Hypoxia protocol and normoxia control
Hypoxic treatment of cells was performed using a well characterized, finely controlled ProOx-C-chamber system (Biospherix, Redfield, NY). The O2 concentration in the chamber was controlled by the ProOx model 110 and maintained at 0.5%. For the re-oxygenation procedure, the fresh aspirated culture medium was added upon termination of hypoxia, and the cells were returned to a 37°C incubator with 20% O2 and 5% CO2 for the times required. Cells of normoxia control were subjected to the same procedures except that they were exposed to the 20% O2 during the whole duration of preparation.
Neuronal differentiation induction of BMSCs in vitro
Neuronal differentiation was induced following previous reported procedures (Woodbury et al., 2000). Briefly, isolated BMSCs were preinducted with DMEM + 20% certified FBS + 1 mM β-mercaptoethanol (BME) for 24 hrs, followed by induction with DMEM + 100 mM butylated hydroxyanisole (BHA) + 2% dimethylsulfoxide (DMSO) for 6 hrs. Maintenance media was consisted of DMEM + 100 mM BHA + 2% DMSO + 25 mM KCl + 2 mM valproic acid + 10 μM forskolin + N2 supplement. Cells were fixed for immunocytochemistry at 1 to 3 days postinduction.
Transient ischemia model of middle cerebral artery occlusion
Adult male Wistar rats (270 to 300 g) were used for ischemic stroke experiments. Transient cerebral ischemia was induced by 90-min occlusion of the right middle cerebral artery (MCA) following previous procedures (Snider et al., 2001). Briefly, rats were anesthetized with 4% isoflurane and maintained by 2% isoflurane in 70% N2O and 30% O2. To expose the right MCA, a 2-cm vertical skin incision was made midway between the right eye and ear after splitting the temporalis muscle; a 2-mm burr hole was drilled at the junction of the zygomatic arch and the squamous bone. The right MCA was ligated with a 10-0 suture under an operating microscope. Both common carotid arteries (CCAs) that had been previously isolated and freed of soft tissues and nerves were then occluded using non-traumatic aneurysm clips, resulting in right MCA territory ischemia (reduction of blood flow by 88–92%). At the end of the ischemic period, the MCA ligature and both CCA clips were released, and restoration of blood flow was confirmed visually. Free access to food and water was allowed after animals recovered from anesthesia. Physiological parameters including arterial blood pressure and pulse rate were monitored using Digi-Med™ Blood Pressure Analyzer (Micro-Med, Inc. Louisville, Kentucky). During and 2 hrs after surgery, the rectal temperature was maintained at 37.0 ± 0.5°C via an electronic temperature controller (Versa-Therm 2156, Cole-Parmer, Chicago, IL) linked to a heating lamp and homeothermic blanket control unit (Harvard Apparatus, South Natick, MA).
All animal experiments and surgical procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Emory University and followed NIH guidelines and regulations.
Intravenous administration of BMSCs
Rats were randomly divided into three experimental groups: the control group received intravenous (IV) infusion of DMEM cell culture medium; the normoxia group received IV administration of normoxia BMSCs (N-BMSC group); the hypoxia group received IV administration of hypoxia pre-conditioned BMSCs (H-BMSC group). Animal numbers were 10 to 15 in each group.
All transplantation procedures were performed under aseptic conditions. At 24 hrs after MCA occlusion, animals were subjected to isoflurane anesthesia again and IV infusion of control medium, N-BMSCs or H-BMSCs (300 μl cell-free PBS solution or solution containing 1×106; tail vein injection).
Immunofluorescence staining
For immunocytochemical staining of BMSC cultures, cells were plated on glass-bottom dishes and subjected to normoxia or hypoxia treatment. Dishes were fixed in 10% formalin in PBS for 10 min twice, then washed with PBS 3 times, followed by treatment in 0.2%Triton-100 for 5 min, and washed in PBS 3 times between each step. Slides were blocked in 2% fish gelatin (Sigma-Aldrich) at room temperature for 1 hr, and subsequently incubated with rabbit primary antibody to erythropoietin (EPO) or EPO receptor (EPOR) (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) or Von Willebrand factor (vWF) (1:400, Chemicon, Temeccula, CA) diluted in PBS overnight at 4°C. After rinsing with PBS, sections were then treated with Cy3-conjugated donkey anti-rabbit IgG (1:800, Jackson ImmunoResearch, West Grove, PA) or Alexa Fluor 488 anti-rabbit IgG (1:200, Molecular Probes, Carlsbad, CA) for 1 hr at room temperature. After washing 3 times with PBS, dishes were incubated in Hoechst 33342 (1:20000, Molecular Probes, Carlsbad, CA) for 5 min. Then the dishes were mounted, cover slipped and photographed under a florescent microscope BX51 (Olympus, Tokyo, Japan).
For immunohistochemical staining of brain sections, fresh frozen sections of 8-μm thickness were cut on a cryostat microtome (Vibratome 5040, St. Louis, MA). The slices were fixed with 10% formalin for 10 min. Primary antibodies used in this study are mouse anti-NeuN (MAB377, 1:500, Chemicon), rabbit anti-MAP2 (AB5622, 1:200, Chemicon), mouse anti-tubulin, beta III isoform (MAB1637, 1:200, Chemicon), rabbit anti-Glut1 (AB1341, 1:800, Chemicon), rat anti-BrdU (1:500, Abcom, Cambridge, MA), rat anti-GFAP (1:500, Sigma-Aldrich), rat anti-iba-1 (1:400, Abcam), and rat anti-OX-42 (1:100, Abcam). According to the manufacturer’s protocol, each primary antibody was diluted and incubated for 1 hr with secondary antibody, Cy3, Cy5 or Alexa Fluor 488. The slices were visualized and digitally photographed with a fluorescence microscope BX51 (Olympus, Tokyo, Japan) and counted at a magnification of 20X using software ImageJ (MIH, Bethesda, MD).
Western blot analysis
Whole cell lysates were collected, and the protein concentration of each sample was determined using the Bicinchoninic Acid Assay (Sigma-Aldrich). SDS-PAGE was performed using 40 μg of protein per sample on a 6 – 15% gradient gel using a Hoefer Mini-Gel system (Amersham Biosciences, Piscataway, NJ). The proteins were transferred to a PVDF membrane (BioRad, Hercules, CA) using the Hoefer Transfer Tank (Amersham Biosciences). Membranes were blocked with buffer (Tris-buffered saline containing 0.1% Tween-20 (TBS-T), pH 7.6, 7% milk) at room temperature for 2 hrs and incubated overnight at 4°C with rabbit poly clonal antibody to EPO (1: 1000, Santa Cruz Biotechnology), EPO receptor (1: 1000, Santa Cruz Biotechnology), HIF-1α (1:1000, Santa Cruz Biotechnology), GDNF (1:1000, Santa Cruz Biotechnology), BDNF (1:1000, Santa Cruz Biotechnology), SDF-1 (1:200, Santa Cruz Biotechnology) and CXCR4 (1:200, Santa Cruz Biotechnology). As a loading control, a mouse monoclonal antibody to β-actin (1:5,000, Sigma-Aldrich) was used. The blots were washed in 0.5% TBS-T and incubated with alkaline phosphatase-conjugated Goat anti-rabbit or anti-mouse IgG (Promega, Madison, WI) for 2 hrs at room temperature. Finally, membranes were washed with TBS-T followed by three washes with TBS. Signals were detected by the addition of BCIP/NBT solution (Sigma-Aldrich).
RT-PCR analysis of inflammatory factors
mRNA is isolated from cells in culture and is reversely-transcribed into cDNA. PCR is then performed on the cDNA for the various genes of interest.
Cell counting in brain sections
Cell count was performed using design-based stereology with systematic random sampling. For counting differentiated and transplanted cells in brain sections, every tenth brain section (100 μm apart) across the entire region of interest (400 μm) was analyzed, and six fields per brain section were randomly chosen, photographed under 20X magnification using a fluorescent microscope. Stereologic parameters from point counts, boundary intersections, or profiles taken from sampling frames were placed in microscopic fields in serial sections.
Motor function test
Motor behavior of rats was tested before MCA occlusion and 14 days after transplantation with control medium or BMSCs (i.e. 15 days after stroke). Rats were placed on an accelerating rotarod cylinder (Economex, Columbus In., Columbus, OH) and the length of time the animals remained on the rotarod was measured. The speed was slowly increased from 4 to 40 rpm in 5 min. The animals were trained for 3 days before MCA occlusion. The mean duration on the device was recorded with three measurements.
Statistical Analysis
Student two-tailed t test was used for comparison of two experimental groups. Multiple comparisons were done using one-way ANOVA followed by Tukey’s post hoc test for multiple pair-wise examinations. Differences were considered significant at P < 0.05. All measured values are reported in mean ± SEM.
Results
Characterization of rat BMSCs
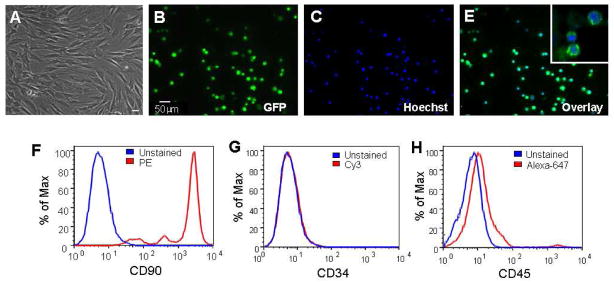
Bone marrow cells were collected from 2–3 week old rats and the adherent mesenchymal stem cells were isolated using established procedures (Hu et al., 2008). Using the same method, we have systematically characterized the properties and differentiation potential of BMSCs from mice in our investigations (Hu et al., 2011; Hu et al., 2008). Typically, cells isolated from mice and rats were either spindle or triangular-shaped (Fig. 1A). Cells isolated from the eGFP-transgenic rats exhibited green fluorescence (Fig. 1B–1E). The eGFP gene, under the control of the human ubiqutin-C promoter, expressed eGFP in 100% of cells. The surface markers of these cells were identified by flow cytometry, showing expression of CD90, but not CD34 and CD45 (Fig. 1F–1H). The expression of CD90 was consistent with characteristic surface markers of undifferentiated BMSCs (Dominici et al., 2006). The lack of expression of CD34 and CD45 suggested that the cell population was depleted of hematopoietic stem cells during sub cultivation by plastic adherence. Thus the cells employed in this investigation were regarded as BMSCs or BMSC-like cells.
Figure 1. Characterization of bone marrow cells isolated from rats.
Cells were harvested from rats and adherent mesenchymal cells were isolated. A. The phase contrast photo shows typical isolated cells in cultured dishes showing either spindle or triangular shape, consistent to the morphology of BMSCs. B to E. BMSCs in culture, showing 100% overlaps between EGFP (green) and Hoechst 33342 (blue). The insert in E is an enlarge image of GFP/Hoechst double labeling. F–H. Rat’s BMSCs were identified by fluorescence-activated cell sorting, confirming the positive expression of CD90 and negative identification of CD45 and CD34.
Hypoxic preconditioning upregulates expression of regenerative factors in rat BMSCs
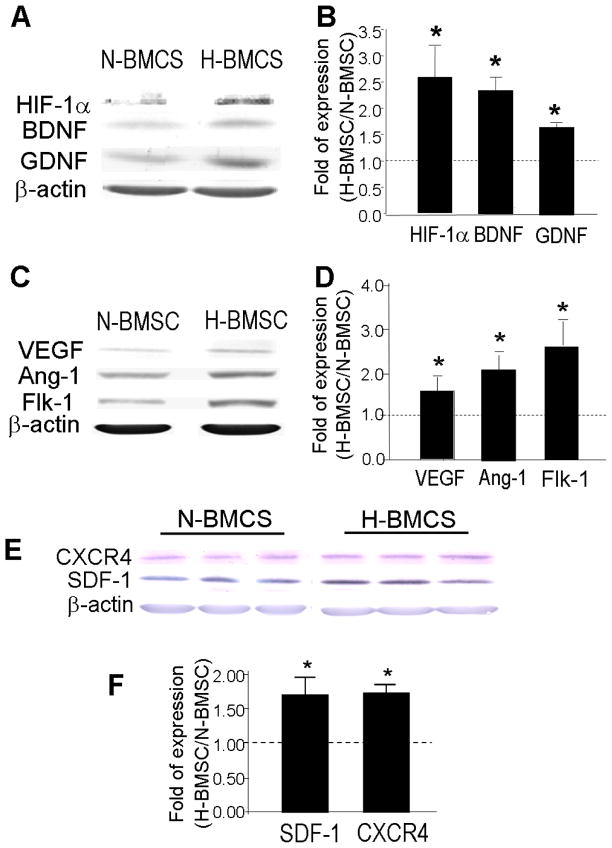
It is known that BMSCs normally exist in vivo at a low oxygen tension of ~4% (Lennon et al., 2001). We thus selected the hypoxia treatment of 0.5% O2. On the other hand, the term normoxia is adopted in this report referring to the atmospheric oxygen level of 20%. Based on previous investigations, we hypothesized that the hypoxic exposure could up-regulate protective and regenerative genes in BMSCs. BMSC cultures were exposed to normoxia (20% O2) or hypoxia (0.5% O2) for 24 to 72 hrs. This hypoxic treatment did not cause any cell death as tested using trypan blue staining (data not shown). Western blot analysis showed that BMSCs under normoxic culture condition (N-BMSCs) express a detectable basal level of HIF-1α as well as several trophic/growth factors including GDNF, BDNF, VEGF, VEGF receptor Flk-1 and angiotensin-1 (Ang-1)(Fig. 2A–2D). In BMSCs exposed to hypoxia for 24 to 48 hrs (H-BMSCs), expression of these factors was significantly elevated from the basal levels in N-BMSCs (Fig. 2A–2D). In addition, we observed that HP increased expression of chemokine SDF-1 and its receptor CXCR4 (Fig. 2E–2F), which play an important role in augmenting ischemic neovascularization in the damaged tissue (Hiasa et al., 2004; Yamaguchi et al., 2003) and in mobilizing/directing the migration of neuroblasts (Ceradini et al., 2004; Shyu et al., 2004).
Figure 2. Hypoxic preconditioning up-regulated HIF-1α and growth factors.
Western blot analysis was applied to measure expression of several hypoxia-induced genes in cultured BMSCs after hypoxia (0.5% O2, 48 hrs). A and B. Representative gel electrophoresis images and analyzed data of the protein level of HIF-1α and growth factors GDNF and BDNF in N-BMSCs and H-BMSCs. Bar graph from densitometry analysis shows the ratio (fold of increases vs. N-BMSCs). Data were corrected by loading control of mouse β-actin. The dotted line represents the baseline level of the expression in N-BMSCs. C and D. Comparisons of protein levels of VEGF, Ang-1 and Fik-1 in N-BMSCs and H-BMSCs. E and F. Original electrophoresis images and comparisons of protein levels of CXCR4 and SDF-1 in N-BMSCs and H-BMSCs. N=3 independent assays. *. P<0.05 compared with N-BMSCs.
The erythropoietic hormone EPO has been demonstrated in recent years as a neuroprotective and angiogenic factor in the brain after ischemic stroke (Keogh et al., 2007; Liu et al., 2008). Immunocytochemical staining and Western blotting showed that hypoxia and/or hypoxia plus re-oxygenation increased expression of EPO and EPOR in H-BMSCs (Fig. 3). Using Western blot, we examined the time-dependent up-regulation of EPO and EPOR. The protein level of EPO was significantly elevated after 48 hr hypoxia treatment (Fig. 3C). The increased EPO expression declined toward the basal level 24 hrs after termination of hypoxia (24 hrs re-oxygenation). EPOR levels, however, was markedly enhanced after 24 or 48 hrs hypoxia exposure (Fig. 3D). The increased EPOR levels remained for at least 48 hrs after termination of hypoxia (Fig. 3D).
Figure 3. Hypoxic preconditioning enhanced EPO and EPO receptor expression in BMSCs.
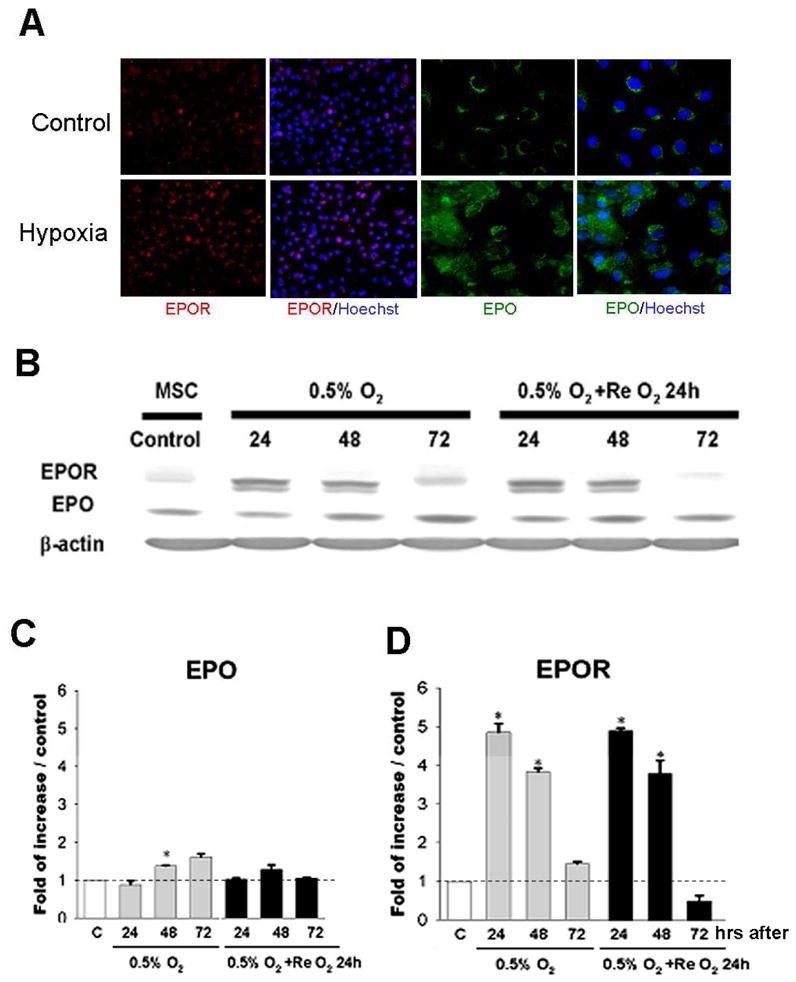
Immunocytochemical staining and Western blotting were applied to measure regulation of hypoxia on EPO/EPOR expression in BMSCs. A. Immunostaining of BMSCs show immunoreactivity of EPO (green) and EPOR (red) in N-BMSCs and H-BMSCs. Blue color represents Hoechst 33342 nuclear stain of total cells. Magnification=40, Bar=10 μm. B to D. Western blotting shows that EPO and EPO receptor expression at 24, 48, and 72 hrs after 0.5% O2 and after re-oxygenation for 24 hrs. Normalized data are expressed in the bar graph show increased EPO after 48 to 72 hr hypoxia (C) and EPOR increases after 24 to 48 hr hypoxia as well as after 24 to 48 hr reoxygenation (D). The dotted line represent the basal level of expression (N-BMSC controls). N=3 independent assays. *. P<0.05 compared with N-BMSCs.
Hypoxic preconditioning reduced inflammatory genes in BMSCs and promoted anti-inflammation effect after transplantation
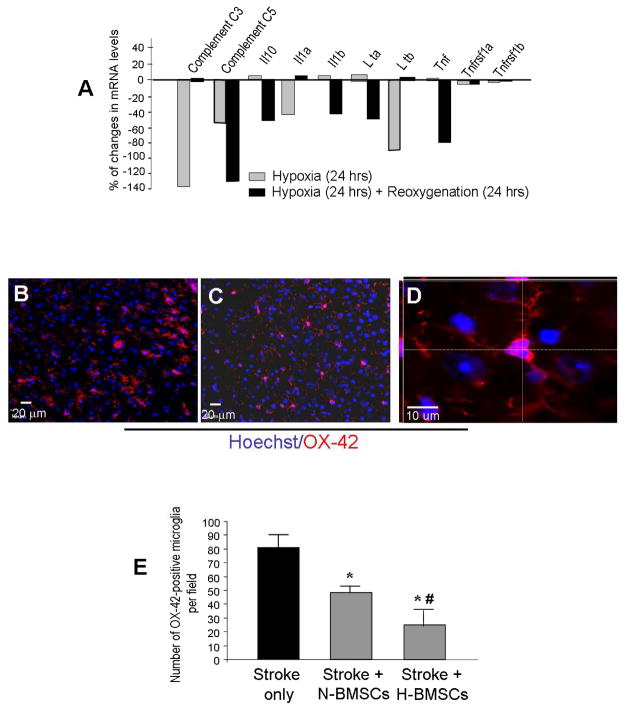
RT-PCR was applied to measure mRNA levels of inflammatory genes in N-BMSCs and H-BMSCs. The results unambiguously showed reductions of most of these genes in H-BMSCs after the hypoxia and re-oxygenation protocol (Fig. 4A and Supplementary Material), suggesting that HP-treated BMSCs could carry less inflammatory genes to the host tissue. In Supplemental Table 2, a 24-hr hypoxia treatment markedly increased OX-42 mRNA expression, but this increase was reversed after 24 hrs of re-oxygenation (Supplemental Table 2).
Figure 4. Effects of hypoxic preconditioning of BMSCs on inflammatory gene expression and inflammatory response in vitro and in vivo.
Inflammatory factor expression in BMSCs and microglial activation were measured in cultures and in the ischemia brain after N-BMSC transplantation. A. RT-PCR analysis shows a general suppression on the mRNA levels of inflammatory cytokines by the sublethal hypoxia protocol in rat BMSCs. More data of the regulation of over 50 related genes are shown in 5 Tables in the supplemental file. N=6 animals in each group. B and C. Immunohistochemical staining of brain cortex sections shows OX-42-positive cells (microglia/macrophages, red) in the ischemic cortex 4 days after ischemia (3 days after control (B) and H-BMSC administration (C)). Blue is Hoechst 33342 staining of all cells. D. The enlarged confocal image shows OX-42 and Hoechst staining of brain cells including a microglia cell that shows the near-rod cell shape and multi-dendritic morphology of an activated microglia. E. Cell counts summarized in a bar graph showing significant reduction of OX-42-positive cells in the ischemic brain received N-BMSCs and H-BMSCs. H-BMSCs showed even stronger inhibitory effect on OX-42-positive cells. *. P<0.05 compared with stroke control, #. P<0.05 compared with N-BMSC group.
It is well known that BMSCs are suppressants on T cell proliferation and inflammation in several systems or organs such as the lungs (Singer and Caplan, 2010). To understand whether BMSC transplantation show any impact on inflammation, we examined microglia cells in the ischemic cortex. One to 10 days after BMSC transplantation performed one day after ischemia, the numbers of OX-42-positive or iba-1-positive (data not shown) positive microglia were noticeably lower compared to stroke-only rats (Fig. 4B and 4C). In the immunohistochemical assay with OX-42 that is suggested to be more specific for activated microglia, the number of OX-42-positive cells after transplantation was reduced by N-BMSCs or H-BMSCs (Fig. 4E). Transplantation of H-BMSCs showed stronger effect of inhibiting OX-42-positive cells (Fig. 4E). This result supports the idea that HP pretreatment reinforces the anti-inflammation property of BMSCs.
Endothelial cell differentiation of BMSCs and enhanced angiogenesis after transplantation of BMSCs
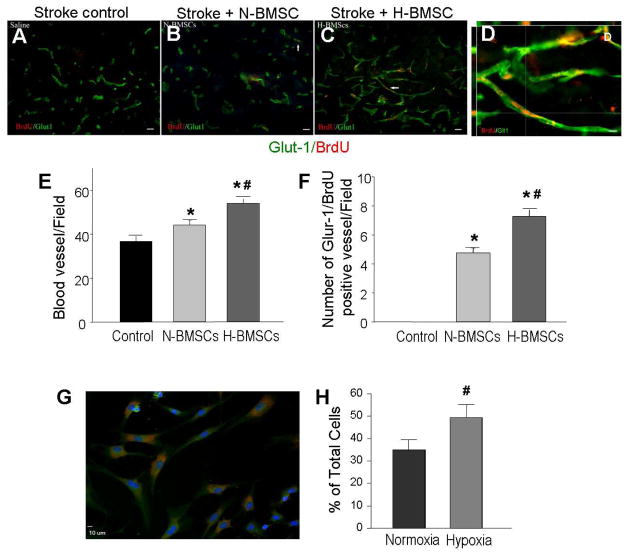
To understand whether BMSC transplantation might have an impact on angiogenesis in the post-ischemic brain, injection of H-BMSCs or N-BMSCs via the tail vein was performed 24 hrs after focal ischemia induced by 90-min ligation of MCA (Snider et al., 2001). In this experiment, BMSCs were pre-labeled with BrdU before transplantation in order to track these cells and show their proliferation in the brain. Fourteen days after transplantation, vascular endothelial cells were evaluated by Glut-1 staining in the ischemic core and penumbra regions. Although the majority of the Glut-1-positive vasculature appeared from an endogenous origin, there were some Glut-1 and BrdU double positive vessels/endothelial cells (Fig. 5D), suggesting that 1) homing of BMSCs to these regions and 2) possible endothelial cell differentiation of transplanted BMSCs (Fig. 5A – 5D). The vessel density and area of Glut-1-positive staining were significantly higher in two BMSC transplantation groups than that in the stroke control group. Between the two transplantation groups, higher vessel density and area were observed in rats that received H-BMSC transplantation (Fig. 5E and 5F).
Figure 5. Differentiation of BMSCs in vitro and angiogenesis in vivo.
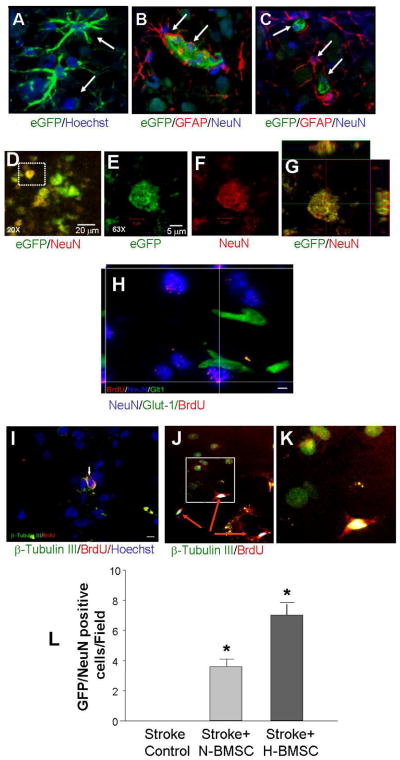
Endothelial cells and vessel-like structures were identified after BMSC transplantation. A – D. Endothelial cell differentiation in the ischemic core 14 days after BMSC transplantation. BrdU (red) shows prelabeled BMSCs and Glut-1 (green) staining shows vascular endothelial cells. Co-staining of these two dyes suggested endothelial cell differentiation of transplanted BMSCs. In the H-MSC transplantation group, more BrdU/Glut-1 double positive cells were detected compared with N-BMSC group and control group. Magnification=20, Bar=20 μm. Image D is a confocal image of BrdU (red) and Glut-1 (green) double staining. Magnification=80, Bar=10 μm. E and F. Summary of blood vessel counts. Transplantation of BMSCs increased the capillary number in penumbra. A greater increase was seen with H-BMSC transplantation compared with N-BMSCs group. In F, the number of Glut-1/BrdU double positive vessels, suggesting an angiogenic component originated from exogenous BMSCs. Rats received H-BMSCs developed more Glut-1/BrdU double positive vessels. G and H. NeuN-positive cells derived from BMSCs after 3-day neuronal differentiation. Red: NeuN staining; blue: Hoechst 33342 staining. The bar graph shows an increased percentage of NeuN-positive cells differentiated from H-BMSC compared to that from N-BMSCs. N=4 per group. *. P<0.05 vs. control group; #. P<0.05 vs. N-BMSC group.
Neuronal differentiation of BMSCs in vitro and after transplantation
Neuronal differentiation of BMSCs has been demonstrated in previous investigations (Rismanchi et al., 2003; Wislet-Gendebien et al., 2005). We observed that after 3 day neuronal induction, a large population of BMSCs became NeuN-positive cells (Fig. 5G). The number of NeuN-positive cells was significantly increased when the induction was carried out under the HP condition (Fig. 5H).
NeuN staining was also performed in brain sections to detect neuronal cells in the ischemic cortex. Fourteen days after transplantation of eGFP-BMSCs or BrdU-labeled BMSCs, we did not see much difference in the number of total NeuN-positive cells in ischemic/penumbra regions. We noticed, however, some NeuN-positive cells were also eGFP-positive or showed BrdU immunoreactivity, suggesting neuronal differentiation from transplanted BMSCs (Fig. 6). These double-positive cells could also be seen with MAP2 or β–Tubulin III staining in penumbra 14 days after H-BMSCs transplantation (Fig. 6J – 6L). More importantly, the number of NeuN/eGFP positive cells was about doubled in the ischemic/penumbra regions that received H-BMSCs when compared with N-BMSC transplantation (Fig. 6M). Within nearby vicinity, some BMSCs were co-labeled with the astrocyte marker GFAP or the endothelial cell marker Glut-1 (Fig. 6B, 6C, 6I). The close approximation of their locations around vessel-like structures implied that the neuronal and non-neuronal differentiation might benefit repair of the neurovascular unit.
Figure 6. Transplantation of BMSCs enhanced neurogenesis in the ischemic cortex.
Fourteen days after BMSC transplantation, transplantation, immunohistochemical staining was performed to identify neuronal and non-neuronal cells in the ischemic/penumbra regions using specific antibodies. A. In brain sections from rats received eGFP-BMSCs, the eGFP-BMSCs (green) were detected in the ischemic cortex. Blue is Hoechst 33342 staining of nuclei. B. Some BMSCs (17/32 counted in 2 animals; 3 sections/animal) were co-labeled with the astrocyte marker GFAP (red). C. Triple labeling of GFP-BMSCs (green) with GFAP (red) and NeuN (blue; 4/51, 2 animals, 3 sections/animal). D. Confocal imaging shows co-localization of eGFP-BMSCs (green) and NeuN (red) in penumbra. The overlay of green and red resulted in yellowish color shown in the image. E – G. Enlarged image from the frame in D, showing an eGFP/NeuN double positive cells. Side images in G show z-sections of the cell, verifying the overlap of the two markers. H. Confocal image of BrdU (red), NeuN (blue) and Glut1 (green) triple staining at high magnification. Bar=5 μm. I – K. Neuronal marker β–Tubulin III (green), BrdU (red) and Hoechst (blue) triple staining in the penumbra region. Magnification=80, Bar=5 μm. M. The number of NeuN/eGFP positive cells was significantly increased in the H-BMSC group compared to the N-BMSC group. *.P<0.05 vs. N-BMSC group.
Functional evaluation after stroke and BMSC transplantation
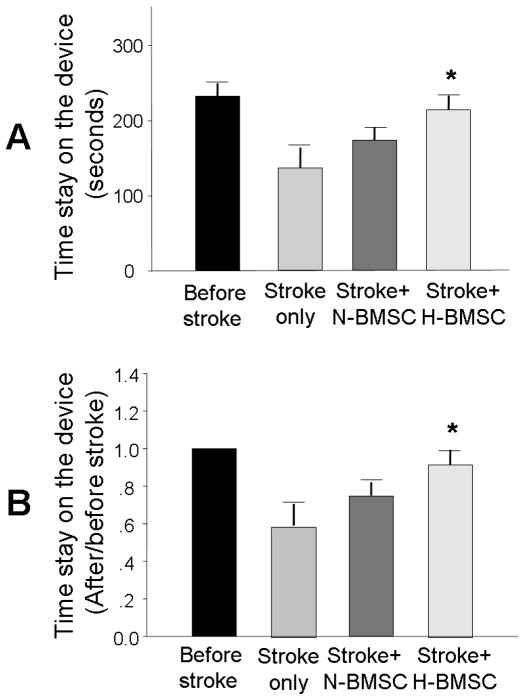
To compare functional benefits of intravenous transplantation of H-BMSCs and N-BMSCs after ischemic stroke, we assessed the motor function using the rotarod test 14 days after transplantation. Although rats that received N-BMSCs exhibited a trend of improved motor activity, the improvement was not statistically significant (Fig. 7). On the other hand, rats that received H-BMSC transplantation performed significantly better than stroke controls (Fig. 7).
Figure 7. Functional recovery after BMSC transplantation.
Rotarod functional testing before ischemia and 14 days after BMSC transplantation (i.e. 15 days after ischemia). A. Mean duration that animals could stay on the testing device; B. Percentage of mean duration on the rotarod compared with the internal baseline control (before surgery). N=10 animals each group, *. P<0.05 vs. control group.
Discussion
The present study explored the preconditioning strategy in BMSC transplantation therapy in a transient but severe ischemic stroke model of adult rats. We show that sublethal hypoxia increased expression of several trophic/growth factors in BMSCs. Supporting our previous demonstrations that HP treatment promotes survival of transplanted cells in the ischemic brain and heart (Ogle et al., 2009; Theus et al., 2008), we show here that hypoxic preconditioning has a marked effect of broadly down-regulating inflammatory genes in BMSCs. This effect creates transplantation cells with reduced expression of inflammatory factors, which should help to minimize post-ischemic inflammation and ischemic damage in the host brain. Enhanced angiogenesis and neurogenesis evolved in the ischemic brain after BMSC transplantation. Neuronal and non-neuronal differentiation of both exogenous and endogenous origins may take place in the ischemic brain after intravenous administration of BMSCs. These protective and regenerative activities ultimately lead to functional recovery in stroke animals. Significantly greater benefits are observed with transplantation of hypoxia-treated BMSCs.
Increasing evidence agrees that BMSCs are hypoimmunogenic after implantation and this unique property initiated the proposal that BMSCs can be used for the treatment of neurological disorders with an inflammatory etiology (Momin et al.). Moreover, a body of evidence suggests that, after transplantation into the injured tissues, BMSCs can suppress T cell activation and prevent expression and activation of a number of inflammatory factors (Tse et al., 2003). Allogeneic and syngeneic bone marrow stromal cell treatment after stroke in rats improved neurological recovery and enhanced reactive oligodendrocyte and astrocyte related axonal remodeling with no indication of immunologic sensitization in adult rat brain (Li et al., 2006). After a transient global ischemia in mice, microarray assays indicated that ischemia up-regulated 586 genes (Ohtaki et al., 2008). Transplanted human BMSCs down-regulated >10% of the ischemia-induced genes, most of which were involved in inflammatory and immune responses. It was concluded that the functional recovery after BMSC transplantation were “largely explained by their modulation of inflammatory and immune responses, apparently by alternative activation of microglia and/or macrophages” (Ohtaki et al., 2008). In the present investigation, we show that BMSC transplantation attenuates macroglia activation, which is consistent with the anti-inflammatory action of BMSCs. We then focused on the effect of hypoxic preconditioning on the inflammatory gene expression in BMSCs in order to understand whether the preconditioning procedure could influence the hypoimmunogenic property of BMSCs. The broad reduction in inflammatory gene mRNAs in these cells implies that hypoxic preconditioning can reduce the immunoreactivity of BMSCs. It is assumed that the less inflammatory activity after BMSC transplantation provides an microenvironment favorable for the survival of endogenous and exogenous cells, which can directly or indirectly promote cell survival, tissue repair and functional recovery.
BMSCs can provide a resource of several trophic and growth factors that play important roles in cell survival, angiogenesis, and stimulate differentiation of BMSCs (Chen et al., 2002; Hess and Borlongan, 2008; Kurozumi et al., 2005). Exposure to sub-lethal hypoxia also activates intracellular signaling pathways involved in regenerative processes. These changes may contribute to the adaptive responses observed after hypoxia. For example, following HP, expression of hypoxia-inducible factor-1α (HIF-1α) is up-regulated, conferring cytoprotective and angiogenic effects. It was shown that administration of HIF-1α combined with skeletal myoblast transplantation enhanced cell engraftment, survival, and angiogenesis in the ischemic heart of rats (Azarnoush et al., 2005). Hypoxia can prolong the half-life of several mRNAs, such as HIF-1α, VEGF and EPO (Sharp et al., 2001). An earlier study showed that ex vivo HP up-regulates the synthesis of VEGF mRNA and stimulates endothelial differentiation of bone marrow stem cells, which together contribute to improved angiogenesis in the ischemic hindlimb after transplantation (Li et al., 2002). Transplanted BMSCs can stimulate angiogenesis after myocardial ischemia by secreting multiple angiogenic factors and differentiating into endothelial cells (Hamano et al., 2000). Recent animal studies have linked increased angiogenesis to improved performance in neurological and behavioral tests (Caplan and Dennis, 2006; Hess and Borlongan, 2008). In ischemic stroke patients, the number of new vessels surrounding injured tissue correlates with longer survival (Krupinski et al., 1994). Studies have shown the therapeutic potential of angiogenesis in the restoration of local blood flow and functional recovery in ischemic diseases (Hamano et al., 2000). It is thus reasonable to propose that enhancing the ability of BMSCs to promote angiogenesis will increase the therapeutic effect of BMSC transplantation and benefit functional recovery after stroke.
Intravenous administration of BMSCs over-expressing GDNF or BDNF protects against injury and results in greater functional recovery in cerebral ischemia models (Kurozumi et al., 2004). Recent studies have demonstrated the paracrine effect of BMSCs as a potential mechanism for angiogenesis and functional recovery after transplantation (Zacharek et al., 2010). Our previous work demonstrated that microvascular proliferation and remodeling occur after cerebral ischemia in the cortex, with increased blood flow and stimulated collateral growth (Whitaker et al., 2007). We recently showed that brain region targeted peripheral (whisker) stimulation following barrel cortex ischemic stroke increased angiogenic factors and endogenous angiogenesis in the penumbra region (Whitaker et al., 2007). These investigations suggest multiple and often synergic benefits of supplementary approaches in stem cell therapy that can lead to a more effective combination therapy for ischemic stroke.
The mechanism of BMSC therapy for ischemic stroke has been under debate. It is well known that the homing of systemically administered BMSCs to the ischemic cortex is extremely low (e.g. ~0.01% of total injected cells) (Chen et al., 2001). Because of the low homing and survival rates of BMSCs and of the observation that injection of BMSC-conditioned culture media showed similar effects as BMSC themselves, the functional benefits after BMSC transplantation has been attributed to increased trophic support but not cell replacement (Caplan and Dennis, 2006; Chen et al., 2002; Hess and Borlongan, 2008). In agreement with previous reports, we did not observe many eGFP-transfected or BrdU-pre-labeled N-BMSCs in the ischemic core and penumbra. However, as we have shown in previous and present investigations, hypoxia-treated BMSCs survive better and more of them home to ischemic region and exhibit a superior property of promoting angiogenesis and neurogenesis (Hu et al., 2011; Hu et al., 2008). Although cell fusion or leakage of BrdU from BMSCs cannot be completely excluded, the increased number of eGFP and NeuN double positive cells after H-BMSC transplantation supports differentiation from transplanted cells. These data provide a possibility that BMSC therapy can be improved not only by enhancing trophic support but also by optimizing cell differentiation and replacement. We believe that recognizing this possibility is of great clinical significance for the development of a better stroke therapy using BMSCs.
In our experiments, we observed that HP markedly inhibited a broad spectrum of pro-inflammatory chemokines/cytokines in BMSCs, such as CC3, CC5, CC17, CCL4, CXCR3, and CXCL10. These factors are potent chemoattractants for monocytes, macrophages and T cells (Angiolillo et al., 1995; Dufour et al., 2002). For example, CCL4, also known as macrophage inflammatory protein-1β (MIP-1β), is a chemoattractant for natural killer cells, monocytes and a variety of other immune cells (Bystry et al., 2001). The decrease of these factors in BMSCs surely helped to prevent attraction of inflammatory cells. Microglia, cells of the monocyte/macrophage lineage in the brain, have been implicated in the pathogenesis of a number of neurodegenerative conditions such as stroke, Alzheimer’s disease, HIV dementia, and multiple sclerosis (Danton and Dietrich, 2003; Gonzalez-Pal et al., 1999). As part of the innate immune defense mechanism, microglia can defend the central nervous system against damage, but increasing evidence suggest that excessive or sustained microglia activation can significantly contribute to acute and chronic neuropathologies and apoptotic cell death. Dysregulation of microglial cytokine production could thereby promote harmful actions of the defense mechanisms, result in direct neurotoxicity, as well as disturb neural cell functions (Hanisch, 2002; Ohmi et al., 2003). Bone marrow transplantation led to suppression of activated microglia and to a delay of neuronal death (Ohmi et al., 2003). This is highly consistent with our observation and, moreover, we demonstrate that HP pretreatment further improves the anti-inflammatory action of BMSCs, which should contribute to the enhanced neuroprotection after H-BMSC transplantation.
Previous reports showed that intravenous administration of BMSCs cultured under normoxic condition improved neurological function (Chen et al., 2001). In our study, we didn’t observe a significant improvement of motor function in the N-BMSC transplantation group. This difference may be attributable to several differences in these investigations. Previous work injected many more BMSC cells (e.g. 3×106 cells × 2 times) (Chen et al., 2001), while we only injected 1×106 cells. The fewer cells used in our investigation was based on the assessment that cells could survive much better with the HP strategy so fewer cells are needed for transplantation (Theus et al., 2008). The significant functional improvement in the H-BMSC group supports the assumption that HP can help to develop efficient and effective stem cell therapy with fewer cells. This can be critical in clinical practice when the availability or expending of cells becomes a limiting factor. It also needs to be noticed that only rotarod motor function was tested in our study, but a battery of neurological activities was used in the previous report (Chen et al., 2001).
In conclusion, hypoxic preconditioning of transplanted cells provides a new and effective strategy for cell-based therapy in the treatment of ischemic diseases such as stroke and heart attack (Francis, 2010; Hu et al., 2011; Hu et al., 2008; Ogle et al., 2009; Theus et al., 2008). The mechanism of the therapeutic benefits must be multifaceted, at least involve enhanced expression and release of trophic/growth factors that provide autocrine and paracrine effects of protection and stimulation of regenerative responses. We propose that HP-induced other potential beneficial effects such as promoting migration and homing of transplanted cells should be further studied for the purpose of improving the efficacy of cell-based therapy.
Supplementary Material
Highlights.
Hypoxia preconditioning (HP) upregulates pro-survival and pro-regenerative genes in BMSCs.
HP has a profound effect of down-regulating inflammatory genes in BMSCs.
BMSC transplantation into the ischemic brain suppresses inflammatory activity.
HP promotes neuronal differentiation and vascular endothelial cell differentiation of BMSCs.
Transplantation of HP-treated BMSCs results in better functional recovery after stroke.
Acknowledgments
This work was supported by NIH grants NS058710 (LW), NS062097 (LW), NS075338 (LW), NS057255 (SPY), NS073378 (SPY) and the American Heart Association Established Investigator Award (LW). It was also supported by the NIH grant NS055077 to the ENNCF (Emory Neurology-NINDS Core Facility).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal R, et al. Genetic modification of ex-vivo expanded stem cells for clinical application. Front Biosci. 2010;15:854–71. doi: 10.2741/3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiolillo AL, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–62. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarnoush K, et al. Enhancement of the functional benefits of skeletal myoblast transplantation by means of coadministration of hypoxia-inducible factor 1alpha. J Thorac Cardiovasc Surg. 2005;130:173–9. doi: 10.1016/j.jtcvs.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Bystry RS, et al. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen X, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–9. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Coaxing bone marrow stromal mesenchymal stem cells towards neuronal differentiation: progress and uncertainties. Cell Mol Life Sci. 2006;63:1649–57. doi: 10.1007/s00018-006-6019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–36. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Dufour JH, et al. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death & Diseases. 2010;1:e22. doi: 10.1038/cddis.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pal S, et al. [Frontal lobe dysfunction in patients with epilepsy and chronic psychosis] Rev Neurol. 1999;28:219–23. [PubMed] [Google Scholar]
- Hamano K, et al. Angiogenesis induced by the implantation of self-bone marrow cells: a new material for therapeutic angiogenesis. Cell Transplant. 2000;9:439–43. doi: 10.1177/096368970000900315. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008;41(Suppl 1):94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa K, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–61. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- Hu X, et al. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011;301:C362–72. doi: 10.1152/ajpcell.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- Keogh CL, et al. The effect of recombinant human erythropoietin on neurovasculature repair after focal ischemic stroke in neonatal rats. J Pharmacol Exp Ther. 2007;322:521–8. doi: 10.1124/jpet.107.121392. [DOI] [PubMed] [Google Scholar]
- Krupinski J, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–8. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–97. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lennon DP, et al. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345–55. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- Li TS, et al. Improved angiogenic potency by implantation of ex vivo hypoxia prestimulated bone marrow cells in rats. Am J Physiol Heart Circ Physiol. 2002;283:H468–73. doi: 10.1152/ajpheart.00261.2002. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456:120–3. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313–25. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Liu XB, et al. Therapeutic strategy of erythropoietin in neurological disorders. CNS Neurol Disord Drug Targets. 2008;7:227–34. doi: 10.2174/187152708784936617. [DOI] [PubMed] [Google Scholar]
- Malgieri A, et al. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248–69. [PMC free article] [PubMed] [Google Scholar]
- Momin EN, et al. Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther. 5:326–44. doi: 10.2174/157488810793351631. [DOI] [PubMed] [Google Scholar]
- Nandoe Tewarie RD, et al. Bone marrow stromal cells for repair of the spinal cord: towards clinical application. Cell Transplant. 2006;15:563–77. doi: 10.3727/000000006783981602. [DOI] [PubMed] [Google Scholar]
- Ogle ME, et al. Primed for lethal battle: a step forward to enhance the efficacy and efficiency of stem cell transplantation therapy. J Thorac Cardiovasc Surg. 2009;138:527. doi: 10.1016/j.jtcvs.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi K, et al. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci U S A. 2003;100:1902–7. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–43. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacary E, et al. Synergistic effects of CoCl(2) and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J Cell Sci. 2006;119:2667–78. doi: 10.1242/jcs.03004. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11:1255–65. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- Rismanchi N, et al. Cell death and long-term maintenance of neuron-like state after differentiation of rat bone marrow stromal cells: a comparison of protocols. Brain Res. 2003;991:46–55. doi: 10.1016/j.brainres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Sharp FR, et al. Hypoxia-inducible factor in brain. Adv Exp Med Biol. 2001;502:273–91. doi: 10.1007/978-1-4757-3401-0_18. [DOI] [PubMed] [Google Scholar]
- Shyu WC, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–54. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- Singer NG, Caplan AI. Mesenchymal Stem Cells: Mechanisms of Inflammation. Annu Rev Pathol. 2011;6:457–78. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- Snider BJ, et al. Cycloheximide reduces infarct volume when administered up to 6 h after mild focal ischemia in rats. Brain Res. 2001;917:147–57. doi: 10.1016/s0006-8993(01)02822-0. [DOI] [PubMed] [Google Scholar]
- Theus MH, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–70. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Tse WT, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- Wei L, et al. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005;19:183–93. doi: 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Whitaker VR, et al. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:57–68. doi: 10.1038/sj.jcbfm.9600318. [DOI] [PubMed] [Google Scholar]
- Wislet-Gendebien S, et al. Astrocytic and neuronal fate of mesenchymal stem cells expressing nestin. Brain Res Bull. 2005;68:95–102. doi: 10.1016/j.brainresbull.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Woodbury D, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Zacharek A, et al. Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke. 2010;41:524–30. doi: 10.1161/STROKEAHA.109.568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.