Abstract
Study Objective:
Sleep responses to chronic sleep restriction (CSR) might be very different from those observed after short-term total sleep deprivation. For example, after sleep restriction continues for several consecutive days, animals no longer express compensatory increases in daily sleep time and sleep intensity. However, it is unknown if these allostatic, or adaptive, sleep responses to CSR are paralleled by behavioral and neurochemical measures of sleepiness.
Design:
This study was designed to investigate CSR-induced changes in (1) sleep time and intensity as a measure of electrophysiological sleepiness, (2) sleep latency as a measure of behavioral sleepiness, and (3) brain adenosine A1 (A1R) and A2a receptor (A2aR) mRNA levels as a putative neurochemical correlate of sleepiness.
Subjects:
Male Sprague-Dawley rats
Interventions:
A 5-day sleep restriction (SR) protocol consisting of 18-h sleep deprivation and 6-h sleep opportunity each day.
Measurement and Results:
Unlike the first SR day, rats did not sleep longer or deeper on days 2 through 5, even though they exhibited significant elevations of behavioral sleepiness throughout all 5 SR days. For all SR days and recovery day 1, A1R mRNA in the basal forebrain was maintained at elevated levels, whereas A2aR mRNA in the frontal cortex was maintained at reduced levels.
Conclusion:
CSR leads to a decoupling of sleepiness from sleep time and sleep intensity, suggesting that there are at least two different sleep regulatory systems: one mediating sleepiness (homeostatic) and the other mediating sleep time/intensity (allostatic). The time course of changes observed in adenosine receptor mRNA levels suggests that the basal forebrain and cortical adenosine system might mediate sleepiness rather than sleep time or intensity.
Citation:
Kim Y; Bolortuya Y; Chen L; Basheer R; McCarley RW; Strecker RE. Decoupling of sleepiness from sleep time and intensity during chronic sleep restriction: evidence for a role of the adenosine system. SLEEP 2012;35(6):861–869.
Keywords: Chronic sleep restriction, rat, allostasis, sleep latency, adenosine, receptor
INTRODUCTION
Many people in modern society voluntarily reduce the amount of time they sleep for vocational or lifestyle reasons. However, experimental studies in humans reveal that sleeping 2 to 3 h less than their normal sleep time, even for only a few consecutive days, leads to significant impairment in cardiovascular, immune, endocrine, and cognitive functions.1 Consistent with these reports are epidemiological studies which suggest that habitual short sleep duration is associated with obesity,2 heart disease,3 and mortality.4
While most studies have focused on the health consequences of chronic sleep restriction (CSR), only a few studies have investigated the sleep responses to CSR. In two well-designed human CSR studies on neurobehavioral cognitive functions, subjects consistently rated their subjective sleepiness as “mild” as CSR continued.5,6 In contrast, these subjects clearly showed severe sleepiness when objectively assessed using the multiple sleep latency test. In addition, they exhibited cumulative impairments in daily cognitive performance. These results indicate that chronically sleep restricted humans suffer from worsening performance without recognizing it is due to insufficient sleep. Although these two studies together gave insights into how CSR alters subjective and objective sleepiness, as well as cognitive performance, it remains unknown how CSR alters the brain's sleep-wake regulatory systems.
A recent study by the first author and colleagues7 investigated the homeostatic sleep response in a rat model of CSR. In this study, following daily 20-h sleep deprivation, animals did not exhibit enhanced NREM delta power (which is widely used as a measure of sleep intensity) or increased total sleep time (TST) during the daily 4-h sleep opportunity (SO) after the first 2 days of sleep restriction (SR), despite the daily accumulation of sleep pressure. These data demonstrated that, unlike the homeostatic response to acute sleep loss, CSR induces an adaptive, or allostatic sleep response. Allostasis is achieving stability through change.8
The inhibitory neuromodulator adenosine has been proposed as an endogenous sleep factor that mediates sleepiness.9,10 During periods of prolonged wakefulness, extracellular adenosine levels rise in the basal forebrain (BF), leading to inhibition of wake-active neurons, which have widespread projections to the cerebral cortex and other brain areas.10
The present study was designed to test the hypothesis that the adenosine system mediates the CSR-induced changes in sleepiness. First, we measured sleep amount and sleep latency to determine if the rats are sleepier when compensatory increases in sleep time/intensity are absent during 5 days of CSR. Second, changes in brain adenosine receptor mRNA levels during CSR were examined to determine whether the time course of adenosine receptor mRNA changes follows the time course of the homeostatic or the allostatic sleep response to CSR. Here we show the decoupling of sleepiness from sleep time/intensity in rats under the CSR condition and report new evidence that changes in the brain adenosine receptor system may mediate sleepiness, but not sleep time/intensity.
METHODS
Subjects
Three-month-old male Sprague-Dawley rats were housed individually and maintained on a 12:12h light-dark cycle (light on at 10:00 AM) with free access to food and water. Protocols were approved by the Institutional Animal Care and Use Committee at the VA Boston Healthcare System.
Experimental Design 1: EEG/EMG Recording
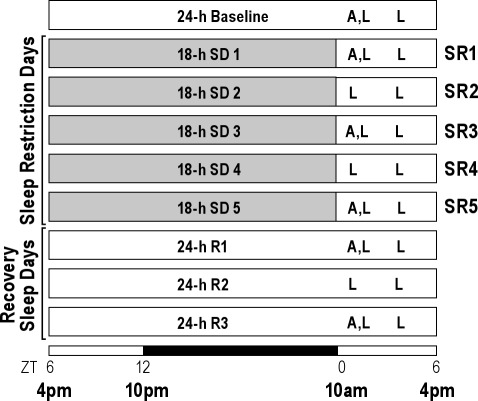
Following 2 weeks of recovery from EEG/EMG surgery, animals (N = 9) were habituated to the sleep deprivation wheels for 2 h per day for 2 consecutive days. On experiment day 1, sleep was recorded in their home cages for a 24-h baseline (BL) period beginning 6 h after light onset (Zeitgeber time 6, ZT6 = 4 PM), as depicted in Figure 1. For the next 5 consecutive sleep restriction days (SR1-SR5), animals were sleep deprived for 18 h (ZT6-24), followed by a restricted 6-h free sleep opportunity (ZT0-6). The protocol was designed with the 6-h blocks of SO at the beginning of the rats' rest period (light onset) to model typical human CSR. Following the last day of SR, animals were allowed unrestricted recovery sleep for 3 days (R1-R3). EEG/EMG recordings were continued throughout the entire protocol. The sleep latency of each rat was measured twice during the 6-h SO periods: ZT1 and ZT5, which are 1 h after the end of sleep deprivation and 5 h after the start of SO on SR days.
Figure 1.
Schematic diagram of experimental design. A 24-h baseline sleep-wake recording was collected beginning 6-h after light onset (zeitgeber time 6 [ZT6] = 4 PM). Over the next 5 sleep restriction days (SR1-SR5), animals were sleep-deprived (SD) for 18 h each day followed by a 6-h sleep opportunity (ZT0-6). Thereafter, animals had a 3-day unrestricted period allowing sleep recovery (R1-R3). The 12-h:12-h light-dark cycle is indicated at the bottom (open bar = light phase; black bar = dark phase). “A” represents brain tissue collection time points (ZT0) for mRNA measurements, and “L” indicates sleep latency test at ZT1 (11 AM) and ZT5 (3 PM).
Experimental Design 2: mRNA Measurements
A separate group of animals without EEG/EMG surgery went through the same experimental procedure designed for the EEG/EMG recording experiment. Brains were collected at the light onset (i.e., immediately following 18-h sleep deprivation on SR days) on baseline, sleep restriction day 1, 3, 5, and recovery sleep day 1, and 3. Changes in mRNA levels for adenosine A1 (A1R) and A2a receptor (A2aR) were analyzed using a reverse transcription – polymerase chain reaction (RT-PCR) technique in 5 different brain areas: the BF, frontal cortex, anterior cingulate cortex, hippocampus, and thalamus. These brain areas are chosen because previous studies reported that sleep deprivation induces changes in adenosine and/or its receptors level in the BF, frontal cortex, anterior cingulate cortex, and hippocampus.11–13 The thalamus was chosen as a control area.
EEG/EMG Surgical Procedures
Rats were anesthetized with isoflurane (2.5%), positioned in a stereotaxic apparatus, and surgically implanted with EEG and EMG electrodes. For monitoring EEG signals, 2 stainless steel screw electrodes were implanted, one above the frontal cortex (2 mm anterior to bregma, 2 mm lateral to the central suture), and a second above the cerebellum area (2 mm posterior to lambda on the extended line of the central suture). Differential recording with this EEG electrode configuration maximizes the amount of EEG delta activity acquired. EMG activity was monitored using nylon-insulated stainless steel wires placed bilaterally in the nuchal muscle in the dorsal neck region.
Sleep Deprivation
Animals were sleep deprived by placing each animal in a periodically rotating wheel (14 inches in diameter × 4.3 inches in width, Lafayette Instrument, product #80860) programmed on a repeated cycle of 4-s on (3 m/min) and 12-s off during the daily 18-h periods of sleep deprivation (Animal Wheel Monitor software, Lafayette Instrument). The wheel is situated vertically between a solid aluminum plate on one side and a transparent sheet of acrylic on the other, which supports the food bin and water bottle. Animals had access to food and water during the whole sleep deprivation periods. The acrylic sheet has a vertical slit to enable the tether to be connected to a swivel commutator (SL6C, Plastics One, Inc.) so that the EEG/EMG activity of animals could be continually recorded during the sleep deprivation period. After the daily 18-h sleep deprivation period, animals were quickly returned to their sleep recording home cage for the 6-h SO. During the 18-h sleep deprivation, the rats slept less than 1.3 h even on the last SR day, which corresponds to only 7% of the entire 18-h period, indicating the sleep deprivation procedure produced ≥ 93% wakefulness during the CSR protocol. This sleep deprivation procedure is far more effective than the disc-over-water method, producing only about 85% wakefulness,14 or equivalent to the disc-over-water along with constant monitoring by an experimenter (approximately 93% wakefulness).15
Sleep Latency
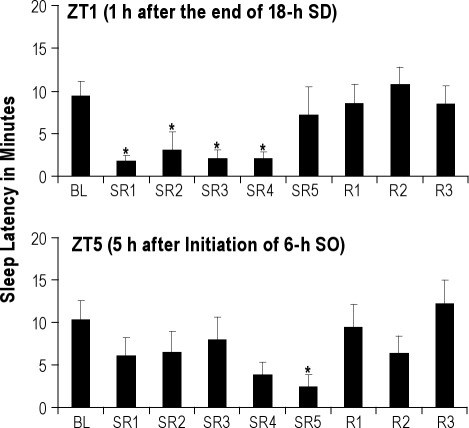
Sleepiness is defined operationally the propensity to fall asleep.16 To assess sleepiness, a sleep latency test was administered at 2 time points during the daily 6-h SO period: first at 1 h after the end of 18-h sleep deprivation (ZT1) to detect the sleep pressure built during the sleep deprivation period, and second at 5 h after the start of 6-h SO (ZT5) to detect any sleep pressure remained after 5 h of sleep opportunity (see Figure 1). This study used only 2 sleep latency trials to minimize the amount of additional sleep loss produced by the sleep latency tests themselves. However, sleep latency data were highly variable, resulting in large error bars and inconsistent data at a single time point (see Figure 5, top panel). Nonetheless, we reported sleep latency data per trial to show an important trend over sleep opportunity time (for detail, see Discussion).
Figure 5.
Sleep latency during baseline (BL), sleep restriction (SR1-SR5) and recovery sleep (R1-R3) days. The top panel indicates that rats were behaviorally sleepy during the first 4 days of sleep restriction (SR1-SR4) when tested at ZT1, which is 1 h after the end of the 18-h sleep deprivation (SD) period. The sleep latency data (mean ± SEM) on SR5 is considered an outlier because 3 of the 9 rats did not follow the group trend at this time point. The bottom panel suggests that the 5 h of sleep opportunity (SO) does not fully discharge the sleep pressure accumulated over 5 days of SR. The asterisk (*) indicates statistical significance (P < 0.05, N = 9) compared to the baseline.
Briefly, animals were awakened for 5 min by a mild noise (e.g., tapping cages), and then given an uninterrupted sleep opportunity. The sleep latency was calculated as the time between the moment when the brief forced wakefulness was stopped and the time when the rat fell asleep, exhibiting NREM (and REM) sleep episodes in at least 5 of 6 consecutive 10-s epochs. A maximum sleep latency value of 20 min was used.
Sleep Data Collection
Using Grass 15A94 Quad Neuroamplifiers (Astro-Med Inc., West Warwick, RI), EEG signals were amplified 10,000 times with high and low pass filters set at 0.3 and 100 Hz, respectively; and EMG signals were amplified 5,000 times with high- and low-pass filters set at 30 and 300 Hz. Both signals were then digitized at 256 Hz and waveforms were collected using Gamma Research Data Acquisition – Analysis System v4.6 (Astro-Med Inc., West Warwick, RI).
Sleep Data Analysis
The EEG and EMG signals were visually scored in 10-sec epochs as wake (low voltage, high frequency EEG; high amplitude EMG), NREM sleep (high voltage, mixed frequency EEG; low amplitude EMG), or REM sleep (mixed frequency EEG with a predominance of theta activity (6-10 Hz); very low amplitude EMG). For quantitative analysis of the EEG signal, each epoch were subjected to a fast Fourier transformation using the Research Sleep Stager program (v 3.2, Grass Technologies, West Warwick, RI). For all epochs of wake, NREM, and REM sleep, the EEG power in the delta (0.5-4 Hz) frequency range was calculated. Epochs containing EEG artifact were eliminated from power spectral analysis (13.0% ± 1.8% of artifacts per day in average). NREM delta power was not corrected over 9 experimental days because the total power was not changed significantly between BL and R3 (paired 2-tailed t-test, N = 6, P = 0.83).
Depending on the particular analysis, wake, NREM sleep and REM sleep time, as well as delta power, were determined in 6, 18, or 24-h time blocks. For statistical comparisons of sleep-wake parameters across BL, SR1-SR5, and R1-R3 conditions, a repeated-measures analysis of variance (ANOVA) test was used. Post hoc comparisons, when indicated, were made using the Fisher LSD test. For comparing 3 days of recovery sleep from the baseline sleep in 2-h intervals, paired 2-tailed t-test was used. Comparisons were considered significant if P < 0.05.
RT-PCR
RT-PCR procedures have been previously described by Basheer et al.17 Briefly, brain tissue samples were punched (2 mm in diameter) from brain slices for the 5 different brain areas. The RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA) and reverse transcribed using Oligo(dT)20 and SuperScript III (Invitrogen). Real time PCR was performed using Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA) on rat A1R and A2aR (Cat# Rn00567668_m1 and Rn00583935_m1, respectively), as well as beta-actin (Cat# 4352340E) to serve as an internal control to normalize RNA concentration variation among samples. Relative quantification was done using the comparative Ct method (ΔΔCt method).18 The fold-difference in the levels of mRNA expression was calculated as described previously.19 The Wilcoxon signed-rank test was used to compare ΔΔCt values from experimental groups to the baseline values. A P value less than 0.05 was considered significant.
RESULTS
Following a baseline sleep day, rats underwent 18-h sleep deprivation each day followed by 6-h SO given during the first 6 h of the light period (10 AM to 4 PM, or zeitgeber time [ZT] 0-6). Sleep deprivation was done by placing rats in periodically rotating wheels which were programmed on a repeated cycle of 4-s on and 12-s off. This SR protocol was repeated for 5 consecutive days, followed by 3 unrestricted recovery sleep days (R1-R3). Sleep time and NREM EEG delta power were analyzed in 2-h, 6-h (ZT0-6), 18-h (ZT6-24) and 24-h time blocks.
Total Sleep Time Did Not Increase During the 6-h SO on SR Days 2 through 5
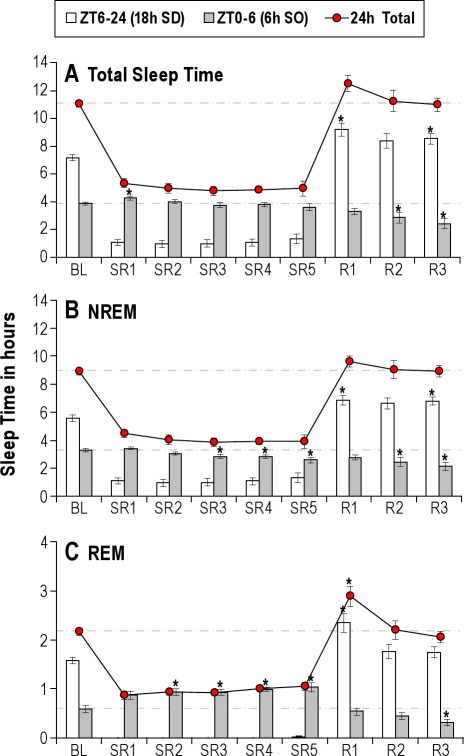
On the BL day, rats slept 11.1 h on average over the 24-h period, derived from 7.2 h in the ZT6-24 time block (18 h) and 3.9 h in the ZT0-6 time block (6 h) (Figure 2A, BL). During the 18-h sleep deprivation period (ZT6-24) on the 5 SR days, the rats slept only about 1.1 h on SR1 and 1.3 h on SR5; this amount of sleep corresponds to 5.9% to 7.4% of the entire 18-h period, indicating the sleep deprivation procedure produced 92.6% to 94.1% wakefulness during the sleep deprivation periods of the SR protocol. The TST achieved during the18-h sleep deprivation periods was not significantly different among 5 SR days. During the 6-h SO (ZT0-6) on SR1, the TST was significantly increased (P = 0.009) by +23.4 min, compared to the corresponding time period of the BL day. However, this significant increase in TST during the 6-h SO on SR1 was not observed on days SR2 through SR5, despite the continued accumulation of sleep debt across the 5 SR days. Following the 5 days of SR, rats had 3 days of unrestricted sleep opportunity. During these 3 recovery days, a trend towards an increase in TST (P = 0.054) was observed only on the first recovery day (R1), but not on days R2 and R3 (Figure 2A). In total, rats lost 30.4 h of their normal sleep time by the end of 5 days of SR and gained only 1.5 h during 3 recovery sleep days (R1-R3), resulting in net loss of 28.9 h of sleep.
Figure 2.
Sleep time during baseline (BL), sleep restriction (SR1-SR5) and recovery sleep (R1-R3) days. The amount (mean h ± SEM) of total (A), NREM (B) and REM (C) sleep time was determined across 18-h (zeitgeber time [ZT] 6-24, open bar), 6-h (ZT0-6, gray bar) and 24-h (ZT0-24, filled circle) time blocks. Total sleep time during 6-h sleep opportunity (SO) was increased on SR1, compared to the corresponding BL, but not from SR2 to SR5. NREM sleep time was even decreased on SR3 to SR5. Even though REM sleep time was significantly elevated across all SR days, the magnitude of increases was relatively small compared to the net loss. During the 3 recovery sleep days following 5 days of SR, rats showed no significant increase in sleep time over the 24-h time block except the REM sleep time on the first day (R1). All comparisons are made to the corresponding baseline and the asterisk (*) indicates statistical significance (P < 0.05, N = 8).
NREM Sleep Time Decreased During the 6-h SO on SR Days 2 through 5
The pattern of changes in NREM sleep time (Figure 2B) was similar to that of TST changes (Figure 2A). However, there were 3 major differences: (1) A significant increase in NREM sleep time was absent on the first day of SO (ZT0-6). (2) More surprisingly, relative to the BL day, NREM sleep time during the 6-h SO decreased significantly on SR3 (P = 0.024), SR4 (P = 0.004) and SR5 (P = 0.002) days. (3) On the first recovery day (R1), following the 5 days of SR, the increase in NREM sleep time over the 24-h period was almost absent, unlike the TST (Figure 2A).
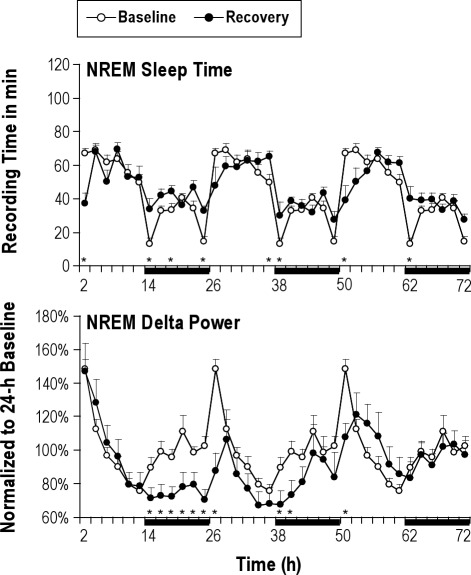
The time course of NREM sleep time in 2-h intervals during the first 72 h immediately following the end of the last 18-h sleep deprivation on SR day 5 is shown in Figure 3 (top panel). It should be noted that the hours 0-6 are the same as the 6-h SO on the last day of sleep restriction (SR5). On the first recovery day, there was a significant decrease in NREM sleep time in the first 2 h of recovery (P = 0.0037 for hours 0-2), followed by a significant increase during the dark period compared to corresponding BL levels (P < 0.05 for hours 12-14, 16-18, and 22-24). The increases in NREM sleep time were observed mainly in or near the dark period on the recovery day 2 (P < 0.05 for hours 34-36, 36-38, and 48-50) and 3 (P < 0.05 for hours 60-62).
Figure 3.
Baseline sleep versus sleep on the 3 unrestricted recovery sleep days. Overall, NREM sleep time and NREM delta power showed significant differences from the baseline (BL) level mainly on the first 24 h of recovery sleep opportunity and returned to the BL level by the third recovery day. NREM delta power was continuously lower than the corresponding BL level over the first 48 h. NREM sleep time (top panel, mean min ± SEM) and NREM delta power (bottom panel, mean % of 24-h baseline ± SEM) are depicted for the 24-h BL (open circles) and the 3 recovery days (filled circles) in 2-h intervals. The recovery data were taken immediately after the last 18-h sleep deprivation period on sleep restriction day 5 and therefore begin at light onset (ZT0). Note that the 24-h BL distribution is re-plotted 3 times in order to allow visual comparisons with all 3 recovery days. The 12-h dark periods are indicated with black bars at the bottom. The paired-t test was used to compare recovery sleep vs. baseline sleep in 2-h intervals. The asterisk (*) indicates statistical significance (P < 0.05, N = 8) compared to the baseline.
REM Sleep Time Increased Mildly During the 6-h SO on SR Days 2 through 5
Changes in REM sleep time over 9 experimental days showed a different pattern from that of TST and NREM sleep time (Figure 2C). On the BL day, rats spent an average of 2.2 h in REM sleep over the 24-h period, derived from 1.6 h during the ZT6-24 time block and 0.6 h during the ZT0-6 time block. During the 18-h sleep deprivation (ZT6-24) on 5 SR days, REM sleep was completely absent. During the daily 6-h SO (ZT0-6), REM sleep time was significantly increased throughout SR days (all P < 0.008), except SR1 (P = 0.066). However, the magnitude of this gain (16.8 min on SR1 to 27.0 min on SR5 relative to baseline) was far less than the amount of REM sleep lost each day of the CSR protocol (∼1.6 h per day). On recovery sleep days, significant increase (P = 0.007) in REM sleep time over 24-h period was observed only on the first day (R1); thereafter, REM sleep time returned to the BL levels on days R2 and R3. Rats lost 6.0 h of their normal REM sleep time by the end of 5 days of SR and gained only 0.7 h during 3 recovery sleep days (R1-R3), resulting in net loss of 5.4 h of REM sleep time.
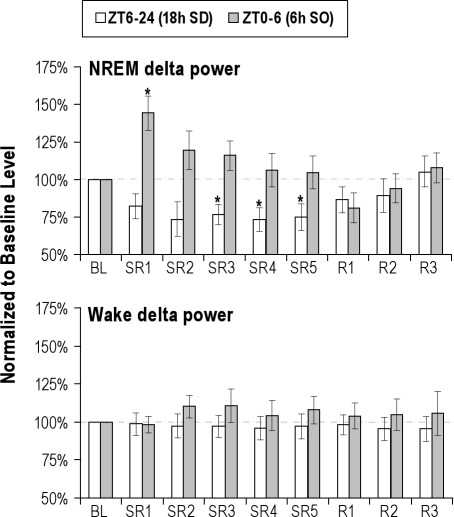
NREM Delta Power Did Not Increase During the 6-h SO on SR Days 2 through 5
The sleep time data revealed that rats did not exhibit compensatory increases in sleep time from SR2 through SR5. NREM EEG delta power was examined to determine whether animals compensate for their loss of sleep time by an increase in their sleep intensity (Figure 4). Following the rats' first exposure to sleep deprivation (18-h sleep deprivation on SR1), the NREM delta power during the subsequent 6-h SO was significantly increased by 44.2% (P = 0.006), compared to the corresponding BL level (Figure 4, top panel). However, in the subsequent SR days (SR2-SR5), the magnitude of NREM delta power rebound was not significant, and, indeed, gradually declined to the BL level from SR2 to SR5. For the brief periods of NREM sleep (∼1.2 h/day) that rats obtained during the daily 18-h sleep deprivation periods, NREM delta power did not increase, but actually decreased significantly on sleep deprivation day 3 to 5 (all P < 0.026).
Figure 4.
NREM and Wake EEG delta power. Delta power (mean % of baseline ± SEM) was determined across 18-h (ZT6-24, open bar) and 6-h (ZT0-6, gray bar) time blocks on baseline (BL), sleep restriction (SR1-SR5) and recovery sleep (R1-R3) days. NREM delta power (top panel) was increased significantly after the first 18-h of sleep deprivation (SR1), but not on SR2 through SR5. For ∼ 1 h of sleep obtained during the 18-h sleep deprivation (SD) periods, NREM delta power did not increase, but actually decreased significantly on SR3 to SR5 (all P < 0.05). The wake delta power (bottom panel) was not significantly increased over 9 experimental days, suggesting the absence of dissipating delta power into the rats' wake state. The asterisk (*) indicates statistical significance (P < 0.05, N = 8) compared to the corresponding baseline.
Next, to assess possible intrusion of delta power into the rats' arousal state, wake delta power was assessed. As shown in the bottom panel of Figure 4, no significant changes of wake EEG delta power were found either during the 18-h sleep deprivation or during the 6-h SO periods over 9 experimental days.
The time course of NREM delta power in 2-h intervals during the first 72 h immediately following the end of the last 18-h sleep deprivation on SR day 5 is shown in Figure 3 (bottom panel). On the first 12 h of the first recovery day, there was no significant changes in NREM delta power compared to corresponding BL levels, followed by long-lasting decreases, mostly in the dark period of the recovery day 1 (all P < 0.05 for hours 12 to 26) and 2 (all P < 0.05 for hours 36 to 40 and 48-50). The NREM delta power returned to the BL level in the dark period of the recovery day 3.
Sleepiness Remained Increased Over the 5 Days of SR
To determine whether rats exhibit behavioral sleepiness despite of the absence of compensatory increases in sleep time/intensity, the sleep latency was measured twice a day: at ZT1 and ZT5. The sleep latency value at ZT1 indicates the sleep propensity induced during the previous sleep deprivation, while the one at ZT5 indicates sleep propensity remaining after 5 h of free SO. Sleep latencies 1 h after the end of 18-h sleep deprivation (ZT1) were significantly reduced to 3.0 min or less from SR1 through SR4 (all P < 0.043), compared to 9.4 min on the BL day (Figure 5, top panel). However, 3 of the 9 rats stayed awake until the cutoff time (20 min) on SR5, and the decrease in latency was not statistically significant (P = 0.526).
For the sleep latency at ZT5 (after the rats had experienced 5 h of SO on the SR days), the rats still showed a trend towards decreased sleep latency on SR4 (P = 0.090), and this trend only became a significant decrease on SR5 (P = 0.036), as shown in the bottom panel of Figure 5.
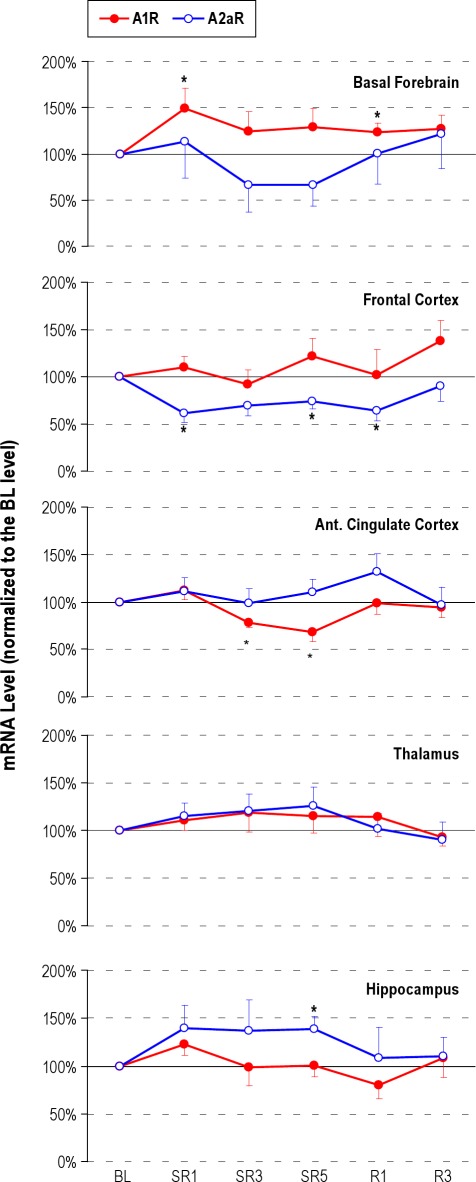
Adenosine Receptor MRNA Levels Exhibited Site Specific Changes
A1R and A2aR mRNA levels were measured using a RT-PCR technique. Brain tissue was collected at the light onset (ZT0), which was immediately after the end of the 18-h sleep deprivation period on SR days. As shown in the top panel of Figure 6, A1R mRNA levels in the BF were significantly increased on SR1 (+49%, P = 0.024) and remained elevated throughout the 5 SR days (+25% on SR3 and +29% on SR5), and the 3 recovery sleep days (+24% on R1 [P = 0.038] and +27% on R3). In contrast, A1R mRNA levels were significantly decreased in the anterior cingulate cortex on SR3 (−22%, P = 0.017) and SR5 (−32%, P = 0.025). A2aR mRNA levels were significantly decreased in the frontal cortex from SR1 (−38%, P = 0.017) through SR5 (−26%, P = 0.022) and R1 (−36%, P = 0.047). The other brain areas did not show any significant changes except the A2a mRNA in the hippocampus on SR5 (+38%, P = 0.018).
Figure 6.
Adenosine A1 (A1R) and A2a receptor (A2aR) mRNA levels on baseline (BL), sleep restriction (SR1, SR3 and SR5) and recovery sleep days (R1 and R3). The brain tissue was collected immediately after 18-h sleep deprivation on SR days. The increase in the basal forebrain A1R, and decrease in the frontal cortex A2aR mRNA levels (mean ± SEM) were consistent throughout the days of sleep restriction; this time course of adenosine receptor mRNA changes resembles that of the sleep latency changes observed throughout the 5 days of sleep restriction, suggesting that the basal forebrain and cortical adenosine receptor changes may mediate sleepiness rather than sleep time or intensity. The asterisk (*) indicates statistical significance (P < 0.05, N = 7∼11) compared to the baseline.
DISCUSSION
This is the first study demonstrating that, after the first day of the 5-day SR protocol, animals do not sleep longer or deeper, even though they show elevated sleepiness throughout the 5 SR days. Therefore, we propose that there are at least two different sleep regulatory systems: one mediating sleepiness and the other mediating sleep time and intensity. The results of adenosine receptor mRNA measurements suggest that alterations in brain adenosinergic tone may be a neurochemical mechanism underlying the continuous increase in sleepiness observed during CSR (a homeostatic response), rather than the adaptive change of sleep time/intensity (an allostatic response).
CSR Induces an Allostatic Sleep Response in Sleep Time and Intensity
When rats were sleep deprived for 18 h on the first SR day, they showed a compensatory increase in sleep time and sleep intensity during the subsequent 6-h SO (Figures 2 and 4). This is a well-characterized sleep response to short-term sleep deprivation. However, NREM sleep time was not increased after 18-h sleep deprivation on SR day 1. This finding is consistent with previous reports where a compensatory increase in NREM sleep time following sleep deprivation is absent when recovery sleep is given at a normal rest period.20–22
The typical homeostatic sleep response was no longer observed from the second SR day (Figures 2 and 4). These data demonstrated that CSR induces allostatic sleep responses, which have been also observed in previous studies using a different protocol: 24-h total sleep deprivation for 4 days14 or 12-h sleep deprivation for 5 days.22 Interestingly, the allostatic sleep responses were not observed in a recent study that used Wistar-Kyoto rats,15 a strain that is hypersensitive to stress23 and does “not adapt” to a chronic stress.24 Furthermore, two recent human CSR studies with 4-h SO per day for 5 days showed that slow wave activity (SWA) on the first SO following 20-h sleep deprivation was only slightly increased25 or did not increase.26 This lack of a robust SWA response following acute sleep deprivation in these two studies makes it difficult to interpret the additional findings that SWA on SO5 was similar to that of SO1.
Our results rule out the possibility that animals preserve intact homeostatic sleep drive under the CSR condition. One possible explanation on the absence of compensatory increases in NREM delta power is that animals dissipate sleep pressure in a form of wake delta power during forced wakefulness.15,27 Another possibility is that animals sleep very briefly but frequently between the wheel movements. Since these microsleeps typically last only 2∼3 s, the whole 10-s epoch is often scored as wake rather than sleep. However, both cases should result in increased delta power in the epochs designated as wakefulness. We found no significant changes of wake EEG delta power either during the 18-h sleep deprivation or during the 6-h SO periods over 9 experimental days (Figure 4, bottom panel). Therefore, this finding is contrary to both possibilities; we thus conclude that the absence of NREM delta power rebound on SR days 2 through 5 is not because of microsleeps or delta power intrusion into periods of wakefulness. This observation is consistent with our previous report that animals do not increase total delta power (i.e., sum of wake, NREM, and REM delta power divided by total number of epochs) during forced wakefulness over 5 SR days (see Kim et al.,7 SI Figure 2). Rechtschaffen and colleagues also reported that the total delta power during 4 days of total sleep deprivation was only 65.5% of the BL level.14 The recent CSR study by Leemburg et al.15 also failed to observe any increases in wake delta power in the rat frontal cortex during 5 days of sleep restriction, although significant increases were observed in the parietal and occipital cortex.
CSR Induces a Homeostatic Response in Sleepiness
We measured sleep latency to objectively measure the animals' behavioral sleepiness in addition to the EEG signature of sleepiness. In contrast to the absence of an increase in NREM delta power from the second SR day, sleepiness was elevated over all 5 SR days (Figure 5, top panel). Furthermore, even when sleep latencies were measured near the end of the daily 6-h SO (ZT 5), the rats still exhibited elevated sleepiness on the last 2 days of SR (Figure 5, bottom panel). This implies that CSR-induced sleepiness seems to be “cumulative” in nature and proportional to the number of days of SR. These findings are consistent with the results of human CSR study which used a multiple sleep latency test.6 However, this human study failed to detect a cumulative effect because the sleep latency had already reached its minimum level on the second SR day. In contrast, the cumulative effects of CSR on daily performance have been well characterized in humans.5,6 Consequently, we postulate that the sleep latency test is so sensitive to the manipulation of sleep deprivation or sleep restriction that it results in subjects reaching the minimal level very quickly (i.e., a floor effect). To detect a gradual change in the sleep pressure accumulated over days, a better strategy might be to measure sleep latencies after allowing a few hours of sleep recovery period so that human or animal subjects could release some portion of the sleep pressure.
CSR Leads to Decoupling of Sleepiness from Sleep Time and Intensity
It is traditionally and widely accepted that sleepiness and sleep propensity can be best measured by slow wave activity (or NREM EEG delta power).28,29 NREM delta power may be also correlated with sleep intensity or sleep depth.28,30 The results of the present study suggest that there are at least two different sleep regulatory systems in the brain: one mediating sleepiness and the other mediating sleep time and intensity. Our data indicate, for the first time, that CSR leads to a decoupling of sleepiness from sleep time/intensity. In response to acute sleep loss (e.g., ≤ 24 h), these two systems exhibit a parallel response. However, with longer periods of repeated sleep loss (e.g., ≥ 3 days), the response pattern of these two systems diverges. In other words, when sleep duration is reduced chronically, we are unable to sleep longer or deeper because the brain sleep-wake regulatory system has adapted to the new condition (i.e., an allostatic sleep response). In humans, subjective sleepiness also showed an allostatic sleep response.5,6 However, this allostatic change was not without cost because cognitive performance kept declining and objective sleepiness continued to be elevated throughout CSR.5,6
The Brain Adenosinergic System May Mediate Sleepiness Rather than Sleep Time or Sleep Intensity
To assess dynamic changes in adenosine system during CSR, we measured adenosine receptor mRNA levels. We hypothesized that changes in adenosine receptors are a better indicator of adenosinergic tone for long periods of sleep disruption than measures of extracellular adenosine levels, which are better suited to assess shorter periods of sleep loss. Indeed, previous studies revealed that brain adenosine levels have already reached a plateau even during 6-h sleep deprivation.12,31
We found that A1R mRNA in the basal forebrain was maintained at elevated levels throughout the 5 days of SR and recovery day 1, while A2aR mRNA in the frontal cortex was maintained at significantly reduced levels (see Figure 6). This is consistent with the previous finding that following 3 and 6-h sleep deprivation, the levels of A1R in the BF and A2aR in the olfactory tubercle changed in opposite directions.17 It appears that, in the BF and frontal cortex, elevated extracellular levels of the ligand downregulates excitatory receptors (e.g., A2aR) and upregulates inhibitory receptors (e.g., A1R). However, the direction of change seems also brain area-specific since we also found decreased A1R mRNA in the anterior cingulate cortex and increased A2aR in the hippocampus.
How might alterations in adenosine receptors mediate sleepiness? Via the A1R, adenosine is hypothesized to inhibit wake-active BF neurons which project to the cerebral cortex and promote wakefulness.10,32 CSR-induced increase in the number of A1R in the BF will amplify the inhibitory action of elevated extracellular adenosine levels, which in turn results in less arousal during wakefulness, leading to elevated sleepiness. However, it is unknown how A2aR in the frontal cortex regulates sleep-wake states. A recent microdialysis study33 reported that A2aR are involved in releasing acetylcholine in the prefrontal cortex and brain stem of the mouse. Thus, downregulation of excitatory A2aR during CSR might reduce acetylcholine release from cholinergic neuron terminals in the frontal cortex, also resulting in reduced cortical arousal and wakefulness. Therefore, simultaneous upregulation of inhibitory A1R in the BF and a downregulation of excitatory A2aR in the frontal cortex might have the same net effect: decreasing cortical arousal and increasing behavioral sleepiness.
Unlike mice with constitutive A1R knockouts,34 recently Bjorness et al.35 using mice with a conditional CNS knock-out of the A1R found that NREM delta power (3∼4.5 Hz) did not increase during 48 h of intermittent sleep deprivation, suggesting that the A1R is necessary for the NREM delta power rebound. However, this study did not measure sleepiness, which is frequently associated with increases in SWA in a baseline sleep or recovery sleep following acute sleep deprivation condition. In addition, their study compared absolute delta power between two groups of mice, which is an inherently difficult and unusual analysis. Therefore, whether adenosine system mediates sleep time/intensity or sleepiness is still inconclusive. To this end, CSR studies may be needed using mice with selective and complete conditional knock out of adenosine receptors in the BF or other specific brain regions.
This study includes a few limitations. Our findings of changes in adenosine receptor mRNA levels do not fit precisely with the pattern of changes in sleep latency. Specifically, on recovery day 1, BF A1R mRNA levels remained elevated and frontal cortex A2aR mRNA levels remained decreased, while sleep latency returned to the baseline level. Although the reason for this discrepancy is unknown, it is possible that the adenosine itself or receptor protein may correlate better with the sleepiness on recovery day 1. Future studies are needed to investigate factors causing the discrepancy by assessing each component of overall adenosine tone. In addition, awakening rats for the sleep latency tests could actually interfere with their sleep opportunity, reducing the amount of sleep obtained. To minimize this possibility, we used only two sleep latency trials consisting of 5-min waking per trial during 6-h sleep opportunities, which is less than 3% of their total sleep opportunity. Finally, the present study, and most studies to date, has measured the EEG effects of sleep deprivation using signals derived from the frontal cortex, where the largest amplitude of SWA is observed. However, findings from human and rodent CSR studies15,25 illustrate the importance of measuring cortical EEG changes from more widespread cortical regions. Recent progress in measuring high-density EEG in behaving rodents36 is likely to resolve the issue of topographic changes in cortical SWA.
To conclude, our data indicate that the time pattern of changes in mRNA levels of the BF A1R and the frontal cortex A2aR resembles that of sleepiness during CSR (cf. Figures 5 and 6). These results imply that the brain adenosine system is more likely to mediate sleepiness rather than sleep time or intensity. Even though future studies are needed to confirm that changes in adenosine system directly mediate the elevated sleepiness observed in CSR, this is the first study to reveal a possible neurochemical mechanism underlying the homeostatic and allostatic changes in sleep observed during CSR.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Ritchie E. Brown and Michael A. Christie for helpful discussions. This research was supported by: Department of Veterans Affairs Medical Research Service Award (RES), T32 HL07901 (YK), R37 MH039683 (RWM – RES), P50 HL060292 (RES – RWM), HL095491 (RWM – RES).
REFERENCES
- 1.Dinges DF, Rogers NL, Baynard MD. Chronic sleep deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier Saunders; 2005. pp. 67–76. [Google Scholar]
- 2.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 3.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 4.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 6.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697–702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 9.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 10.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–96. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Elmenhorst D, Meyer PT, Winz OH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–5. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–17. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 13.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci. 2011;31:6956–62. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- 15.Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010;107:15939–44. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenna JT, Cordeira JW, Christie MA, et al. J Sleep Res. 2008. Assessing sleepiness in the rat: a multiple sleep latencies test compared to polysomnographic measures of sleepiness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basheer R, Halldner L, Alanko L, McCarley RW, Fredholm BB, Porkka-Heiskanen T. Opposite changes in adenosine A1 and A2A receptor mRNA in the rat following sleep deprivation. Neuroreport. 2001;12:1577–80. doi: 10.1097/00001756-200106130-00013. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Thakkar MM, Winston S, Bolortuya Y, Basheer R, McCarley RW. REM sleep changes in rats induced by siRNA-mediated orexin knockdown. Eur J Neurosci. 2006;24:2039–48. doi: 10.1111/j.1460-9568.2006.05058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobler I, Borbely AA. The effect of 3-h and 6-h sleep deprivation on sleep and EEG spectra of the rat. Behav Brain Res. 1990;36:73–8. doi: 10.1016/0166-4328(90)90161-7. [DOI] [PubMed] [Google Scholar]
- 21.Endo T, Schwierin B, Borbely AA, Tobler I. Selective and total sleep deprivation: effect on the sleep EEG in the rat. Psychiatry Res. 1997;66:97–110. doi: 10.1016/s0165-1781(96)03029-6. [DOI] [PubMed] [Google Scholar]
- 22.Lancel M, Kerkhof GA. Effects of repeated sleep deprivation in the dark- or light-period on sleep in rats. Physiol Behav. 1989;45:289–97. doi: 10.1016/0031-9384(89)90130-3. [DOI] [PubMed] [Google Scholar]
- 23.Pearson KA, Stephen A, Beck SG, Valentino RJ. Identifying genes in monoamine nuclei that may determine stress vulnerability and depressive behavior in Wistar-Kyoto rats. Neuropsychopharmacology. 2006;31:2449–61. doi: 10.1038/sj.npp.1301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zafar HM, Pare WP, Tejani-Butt SM. Effect of acute or repeated stress on behavior and brain norepinephrine system in Wistar-Kyoto (WKY) rats. Brain Res Bull. 1997;44:289–95. doi: 10.1016/s0361-9230(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 25.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4:e5874. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009;32:217–22. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones SG, Vyazovskiy VV, Cirelli C, Tononi G, Benca RM. Homeostatic regulation of sleep in the white-crowned sparrow (Zonotrichia leucophrys gambelii) BMC Neurosci. 2008;9:47. doi: 10.1186/1471-2202-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 29.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4 ed. Philadelphia: Elsevier Saunders; 2005. pp. 405–17. [Google Scholar]
- 30.Tobler I. Phylogeny of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4 ed. Philadelphia: Elsevier Saunders; 2005. pp. 77–90. [Google Scholar]
- 31.McKenna JT, Tartar JL, Ward CP, et al. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–73. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porkka-Heiskanen T, Alanko L, Kalinchuk A, Stenberg D. Adenosine and sleep. Sleep Med Rev. 2002;6:321–32. doi: 10.1053/smrv.2001.0201. [DOI] [PubMed] [Google Scholar]
- 33.Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci. 2009;29:871–81. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res. 2003;12:283–90. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 35.Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29:1267–76. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi JH, Koch KP, Poppendieck W, Lee M, Shin HS. High resolution electroencephalography in freely moving mice. J Neurophysiol. 2010;104:1825–34. doi: 10.1152/jn.00188.2010. [DOI] [PubMed] [Google Scholar]