Abstract
Study Objective:
Recently, the use of multicomponent insomnia treatment has increased. This study compares the effect of single component and multicomponent behavioral treatments for insomnia in older adults after intervention and at 3 months and 1 yr posttreatment.
Design:
A randomized, controlled study.
Setting:
Veterans Affairs medical center.
Participants:
179 older adults (mean age, 68.9 yr ± 8.0; 115 women [64.2%]) with chronic primary insomnia.
Interventions:
Participants were randomly assigned to 6 wk of stimulus control therapy (SCT), sleep restriction therapy (SRT), the 2 therapies combined into a multicomponent intervention (MCI), or a wait-list control group.
Measurements and Results:
Primary outcomes were subjective (daily sleep diary) and objective (actigraphy) measures of sleep-onset latency (SOL), wake after sleep onset (WASO), total sleep time (TST), time in bed (TIB), and sleep efficiency (SE). Secondary outcomes were clinical measures including response and remission rates. There were no differences between the single and multicomponent interventions on primary sleep outcomes measured by diary and actigraphy. All treatments produced significant improvement in diary-reported sleep in comparison with the control group. Effect sizes for sleep diary outcomes were medium to large. Treatment gains were maintained at follow-up for diary and actigraph measured SOL, WASO, and SE. The MCI group had the largest proportion of treatment remitters.
Conclusions:
For older adults with chronic primary insomnia, the findings provide initial evidence that SCT, SRT, and MCI are equally efficacious and produce sustainable treatment gains on diary, actigraphy, and clinical outcomes. From a clinical perspective, MCI may be a preferred treatment due to its higher remission rate.
Clinical Trial Information:
Behavioral Intervention for Insomnia in Older Adults. NCT01154023. URL: http://clinicaltrials.gov/ct2/show/NCT01154023?term=Behavioral+Intervention+for+Insomnia+in+Older+Adults&rank=1.
Citation:
Epstein DR; Sidani S; Bootzin RR; Belyea MJ. Dismantling multicomponent behavioral treatment for insomnia in older adults: a randomized controlled trial. SLEEP 2012;35(6):797-805.
Keywords: Insomnia, behavior therapy, aged
INTRODUCTION
Insomnia is a prevalent and chronic problem among older adults that is associated with depressive symptoms, increased hypnotic use, falls, increased health care utilization, functional impairment, and decreased quality of life.1–4 Insomnia's prevalence and its effect on multiple aspects of older persons' lives underscore the need for effective treatment. The efficacy of single and multicomponent treatments for insomnia in older adults is supported by results of meta-analyses and a systematic review.5–8 Despite the success of behavioral methods in reducing insomnia and the recommendation for use as a first-line therapy,9 to our knowledge there are no published comparisons of the 2 commonly used single-component interventions, stimulus control therapy (SCT) and sleep restriction therapy (SRT), in older adults with primary or comorbid insomnia. In older persons, SCT was compared with imagery therapy,10,11 relaxation therapy,12,13 and a control condition.14 SRT was tested against relaxation therapy and sleep hygiene.15,16 Studies using multicomponent treatment with older persons have included SCT and SRT17–23 but have not involved a comparison of the single interventions.
The use of multicomponent insomnia intervention has increased over the past decade in research24 and clinical treatment recommendations.25,26 This multifaceted approach targets the circadian, homeostatic, and conditioned factors that lead to the development and maintenance of a chronic insomnia condition. Multicomponent insomnia interventions have demonstrated larger effect sizes than single-component treatments,6 yet it remains unclear whether the efficacious single-treatment components commonly included in treatment packages are as effective as the multicomponent interventions. The dismantling of a commonly used multicomponent behavioral intervention, consisting of SCT and SRT, may determine if both components are essential for change in sleep outcomes and which component, if any, can be eliminated without loss of efficacy in older persons. The identification of the most effective component(s) would provide future direction for modifications of behavior therapy that could achieve and maintain the same level of effectiveness while enhancing efficiency and feasibility within clinical practice. The aim of the study was to compare the effect of single-component and multicomponent behavioral treatments for chronic primary insomnia in older adults on subjective and objective sleep outcomes after intervention and at 3 mo and 1 yr posttreatment.
METHODS
Participants
Community-dwelling older adults with chronic primary insomnia were recruited through media advertisements in newspapers, radio, and television. Participants were considered for inclusion if they were 55 yr or older; had sleep onset or maintenance insomnia of 45 min or longer per night for at least 3 nights per wk as ascertained through 14 days of sleep diaries; had an insomnia duration of at least 6 mo; and complained of impaired daytime functioning as a consequence of insomnia. Exclusion criteria included psychopathology evidenced by the Brief Symptom Inventory27 (BSI) Global Severity Index T score > 60; cognitive impairment as ascertained by the Mini-Mental State Examination28 score < 27; current psychotherapy or medical treatment for major depression or other psychopathology; current and regular use of over-the-counter medication or prescription medication for sleep (verified through urinalysis), or any medication affecting sleep; a major physical or mental illness directly related to the onset and course of insomnia; substance abuse ascertained per interview; suspicion of sleep apnea as determined by an Epworth Sleepiness Scale29 score of 11 or higher, a respiratory disturbance index > 15 as established through in-home overnight use of the EdenTec Model 3711 Digital Recorder (Nellcor Puritan Bennett, Pleasanton, CA), and interview with a bed partner, if available; suspicion of restless leg syndrome, periodic limb movement disorder, or circadian rhythm sleep disorders as determined through the participant and bed partner interviews. Human subject protection and research methods were approved by the medical center Institutional Review Board. Written informed consent was obtained from each participant. There was no compensation for participation.
Design
The study was a randomized trial using a wait-list control condition (WLC) to compare the efficacy of a multicomponent behavioral intervention and its single components. A 4 (group) × 4 (measurement occasions) factorial design with repeated measures on the second factor was used. Using a random numbers table, participants were assigned to 1 of 4 conditions: MCI (SCT, SRT); SCT; SRT; or WLC. The study included a 2-wk baseline assessment, 6 wk of treatment, and a 2-wk posttreatment, 3-mo and 1-yr follow-up assessment. Treatment was conducted in waves so that the participants randomly assigned to the 4 conditions received treatment and assessments over an identical time period, i.e., the same start and end dates. Participants in the treatment conditions received the intervention immediately. Participants in the WLC group received delayed treatment; they were told they would receive behavioral intervention 10 wk later when the immediate treatment conditions were completed. The WLC group's delayed treatment was conducted apart from this study's protocol and the data are not reported here.
Measures
The treatment and WLC conditions completed measures at baseline and post-treatment but only treated participants received the 3-mo and 1-yr follow-up measures. Sleep outcomes were assessed with daily diaries and wrist actigraphy simultaneously. Diary data, but not actigraphy data, were collected during the intervention period for the treatment conditions.
Sleep outcomes
Daily sleep diaries and wrist actigraphy were used to measure changes in sleep. The diaries provided data for sleep-onset latency (SOL), wake after sleep onset (WASO), total sleep time (TST), time in bed (TIB), sleep efficiency (SE), and a sleep quality (SQ) self-rating question. Participants called responses each morning to a voice- mail service to avoid retrospective estimates of sleep during the 14-day baseline, post-treatment, and follow-up, and 6-wk treatment measurement periods. Daily diaries have been efficiently used in most of the treatment studies on insomnia in older adults.
The Actiwatch (Mini Mitter, Sunriver, OR, Model AW64), a lightweight activity monitor about the size of a sports watch, was worn on the nondominant wrist during nighttime sleep throughout the 14-day baseline, posttreatment, and follow-up measurement phases. The Actiwatch uses a piezoelectric accelerometer to detect physical movement. The data are stored in its memory at user-determined intervals for as long as needed. The movement data are translated to numeric digital data for analysis. The data were analyzed with Sleepwatch software (Version 3.4, Mini Mitter). “Lights out” and “final awakening” were recorded using the device's event marker. Wrist actigraphy is a reliable, objective measure of sleep in persons with insomnia.30 Wrist actigraphy is useful for measuring treatment outcomes31 and was sensitive to the effects of behavioral treatment for insomnia in older adults.32
Clinical outcomes
The participants' perception of insomnia was measured with the brief, self-report Insomnia Severity Index33 (ISI). The 7 ISI items measure perceived insomnia and its severity and effect on daily functioning using a 5-point Likert-type response format (0 to 4). The ISI scores can be placed in 4 severity categories: absence of insomnia (0-7), subthreshold insomnia (8-14), moderate insomnia (15-21), and severe insomnia (22-28) to facilitate clinical interpretation. The ISI has established psychometric properties,33 is sensitive to change as an outcome of behavioral treatment for insomnia in older adults,34 and is used as a clinical significance indicator.35 Significant others rated the insomnia severity of the participants using a parallel version of the ISI, the Significant Other Questionnaire, to validate treatment outcomes.35 Depression and anxiety were measured by the Geriatric Depression Scale36 and the State-Trait Anxiety Inventory, respectively.37 Treatment session attendance was recorded at each group and telephone session. Treatment compliance was self-rated at posttreatment using a Likert-type 5-point rating from “not at all” to “very much” (0 to 4). In addition, questions to ascertain compliance with specific intervention recommendations were included in the treatment diaries. The questions were pertinent to the type of treatment received. For example, the SCT and MCI groups were asked about getting out of bed when awake longer than 15 min, whereas this question was not used for the SRT condition.
Procedures and Treatments
Potential participants were initially screened by telephone to determine if they met the basic study criteria. The participants then received a sleep and health status interview to further ascertain eligibility criteria, completed the baseline assessment, and took home a wrist actigraph, 2 wk of sleep diaries, and a Significant Other Questionnaire, if applicable. The participants whose baseline sleep diary data met the inclusion criteria were randomly assigned to the 4 treatment conditions.
The single-component treatment conditions were SCT and SRT.38 The amount of TIB scheduled for sleep restriction was never set below 5 hr. The MCI consisted of the two single-component interventions. In the MCI condition, SCT was modified to accommodate SRT, i.e., participants were to go to bed only when sleepy but not before the scheduled bedtime. All treatment conditions included sleep education and sleep hygiene instructions. Although we did not include a structured cognitive therapy component, we incorporated a cognitive approach in a manner similar to that described by Sánchez-Ortuño and Edinger,39 i.e., within the context of a sleep education module, and as seen in studies labeled as “cognitive-behavioral treatment of insomnia”.40,41 The module was tailored to older adults and focused on sleep processes and function, sleep architecture, sleep regulation, changes in sleep with aging, individual sleep needs, sleep expectations, the development and maintenance of insomnia, and supportive information about sleep. Sleep hygiene consisted of standard recommendations.42
The 3 treatment groups received 6 consecutive and concurrent weeks of intervention. Four wk of treatment were implemented in a small group format with 4 to 6 participants. The final 2 wk of treatment were conducted by telephone. Each group and telephone session followed a standard format using a treatment manual based on several sources.15,42–45 The interventions were delivered in the first session, which lasted approximately 2 hr. The following 3 group sessions were approximately 1 hr in length and focused on reviewing diaries, discussing progress to date, troubleshooting, devising strategies to enhance treatment adherence, and adjusting TIB schedules for conditions with SRT. In each group session, bar graphs of the previous week's sleep diary variables were used to shape awareness of change and address adherence. The telephone sessions in wk 5 and 6 followed the same treatment structure as the group sessions and lasted approximately 15 min.
Treatment was implemented by a masters' level psychiatric-mental health clinical nurse specialist, with some substitution for vacations and illness by a PhD level nurse (DRE) with the same clinical background. The master's level nurse was an experienced mental health therapist. The nurse was trained in the insomnia treatments by the first author. The training involved didactic presentation of basic sleep science and behavior therapy for insomnia, observation of the first author delivering the MCI, and supervision in the delivery of a pilot MCI group. Therapy tapes during the study were used for weekly supervision to ensure treatment fidelity.
Data Analysis
The sample size was determined using a power analysis performed for WASO. Effect sizes were drawn from meta-analyses.6,46 Based on the study design, using 4 levels for treatment condition, 2 levels for the pretreatment to posttreatment time factor, an alpha of 0.05, and assuming a large effect size for time and an intermediate effect size for group, power was determined at 0.80 for group, 0.99 for pretreatment to post-treatment change, and 0.80 for interaction using 40 participants per group. Therefore, a total sample size of 160 participants was needed.
Descriptive statistics were used to characterize the participants in terms of demographic and clinical variables assessed during baseline. The chi-square test and one-way analysis of variance were used to examine differences in baseline characteristics among the groups. The sleep outcomes were computed from daily sleep diary and actigraph data and averaged over the 14-day period at each time point. The total scores on clinical outcomes were calculated using available instrument instructions. Analysis of covariance (ANCOVA), using linear mixed models, was calculated to examine differences among the treatment groups and WLC group in posttest sleep and clinical outcomes with pretreatment scores used as covariates in the analysis. Significant main effects were followed up with preplanned pair-wise Tukey-Kramer tests. Repeated-measures ANCOVA was used to compare the 3 treatment groups over time. To determine any treatment differences over time, we tested the interaction between treatment and time. Significant interaction effects were followed by preplanned contrasts to determine where the differences occurred. To look at patterns of change over time, contrasts between pretreatment and post-treatment, post-treatment and 3 mo, post- treatment and 1 yr, and between 3 mo and 1 yr were constructed. Analyses were performed using all available data, including participants who subsequently dropped out. Statistical analyses were done using SAS PROC Mixed, Version 9.1.3 (SAS Institute Inc, Cary, NC). The formula for Cohen's-d was used to calculate effect sizes for each treatment group compared with the WLC group at posttreatment.47
A response and remission analysis was conducted. Because there are no standards available to determine response and remission rates, we used guidance from the insomnia literature. We chose the ISI as the outcome for the analysis because it has a consensually recognized normative cutoff score,48 is a commonly used indicator of clinical significance,35 and was used in response and remission analysis in recent insomnia treatment studies.49,50 Furthermore, there is a recommended minimally important difference for the ISI, i.e., a 6-point reduction.51 A response to treatment was defined as an ISI change score of ≥ 6 points from baseline to posttreatment. Remission from insomnia was a posttreatment ISI score < 8 (absence of insomnia). The chi-square test was used to compare proportions for treatment response and remission among the four groups.
RESULTS
Sample
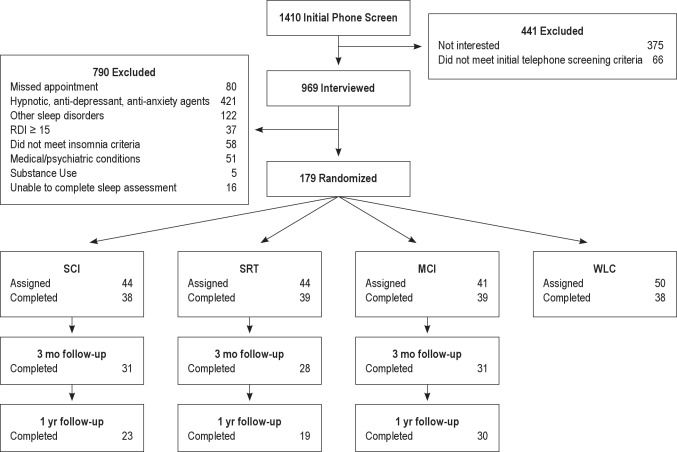
Figure 1 shows the flow of participants through the study. The major reason for exclusion (43%) was the use of hypnotic, antidepressant, and antianxiety agents.
Figure 1.
Participant flow and treatment assignment. WLC, wait-list control condition; MCI, multicomponent intervention; RDI, respiratory disturbance index; SCT, stimulus control therapy; SRT, sleep restriction therapy.
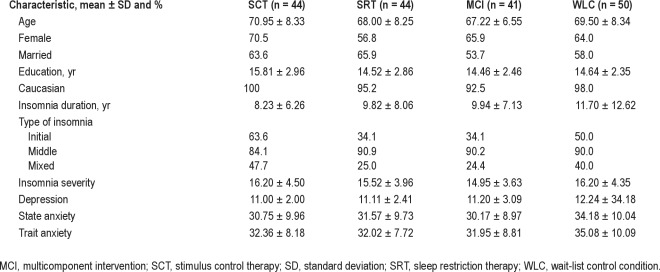
Table 1 presents the demographic and clinical characteristics of the sample. The 179 participants were primarily Caucasian, female, married, and well educated. The participants had long-standing insomnia that was predominantly a sleep maintenance problem. For the demographic and clinical characteristics, there were no differences among groups at baseline. For daily sleep diary and actigraph-measured sleep, the 4 groups were equivalent except on daily sleep diary measured SOL (F3,175 = 5.82, P = 0.001). The SCT group had the highest mean value (Ps ≤ 0.01).
Table 1.
Baseline demographic and clinical characteristics of participants
The results of comparisons among participants who dropped out and those who completed treatment on baseline demographic and clinical variables showed that dropouts tended to be older males (F1,177 = 6.60, P = 0.011; F1,177 = 4.91, P = 0.028) with more severe insomnia (F1,177 = 4.64, P = 0.033).The study attrition rate differed by group (F3,175 = 8.45, P < 0.0001). The MCI condition had a significantly lower dropout rate than the SCT, SRT, and WLC groups (Ps < 0.05). The SCT condition had a lower attrition rate than the control group (P = 0.004). The study's overall attrition rate was 38.54%. Participants dropped out of the treatment mainly because they disliked the intervention or started a sleep medication.
Sleep Outcomes
Posttreatment
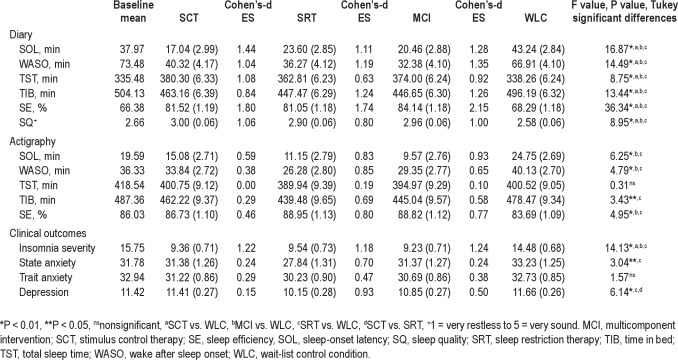
Table 2 presents the adjusted means, Cohen's-d effect sizes, and comparisons among the 3 treatment and the WLC groups for sleep diary and actigraphy variables. There were significant between-group differences at posttreatment for all diary outcomes. The treatment groups differed significantly from the WLC condition in the expected directions (Ps ≤ 0.031). There were no significant differences among the 3 treatment groups. There were significant between-group differences for actigraph measured outcomes except TST. The SRT and MCI groups had significantly lower SOL (Ps ≤ 0.003) and WASO (Ps ≤ 0.031), and higher SE (Ps ≤ 0.007) than the WLC group. The SRT group had significantly less TIB than the WLC group (P = 0.023). There were no significant differences among the 3 intervention groups for actigraphy variables. All treatments showed a large effect size for self-reported sleep outcomes, with the exception of the moderate effect size for SRT on TST. Effect sizes were small to large in magnitude for all treatment groups on actigraph measures except TST in the SCT group, which showed no effect.
Table 2.
Adjusted results for posttreatment comparisons (adjusted mean, standard error)
3-mo and 1-yr follow-up
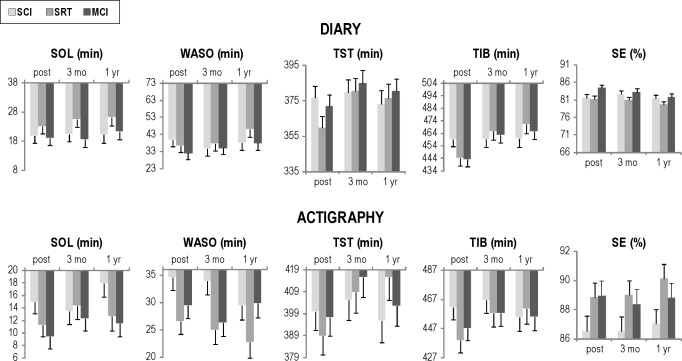
Figure 2 depicts changes from posttreatment to 3 mo and 1 yr in the treatment groups. For diary measured outcomes, TST for the SRT condition significantly increased over time (F2,156 = 5.84, P = 0.004) from posttreatment to 3 mo (P = 0.04). TIB changed significantly over time (F2,156 = 12.26, P < 0.0001) and by condition (F4,156 = 2.92, P = 0.0230). Post hoc tests revealed the MCI and SRT groups increased TIB (Ps ≤ 0.03) and in comparison to the SCT condition (Ps ≤ 0.022) over the follow-up phases. There were no significant changes during the follow-up phases for diary-measured SOL, WASO, SE, and SQ. Actigraph measured TIB changed significantly over the follow-up phase (F2,137 = 3.17, P = 0.05). There was an increase in TIB for the SRT group from posttreatment to 1 yr compared with the SCT group (P = 0.0288). Actigraph-measured SOL, WASO, TST, and SE did not change significantly over the follow-up period.
Figure 2.
SCT, SRT, and MCI diary and actigraphy outcomes at posttreatment, 3 mo, and 1 yr. MCI, multicomponent intervention; SCT, stimulus control therapy; SE, sleep efficiency; SOL, sleep-onset latency; SRT, sleep restriction therapy; TIB, time in bed; TST, total sleep time; WASO, wake after sleep onset.
Clinical Outcomes
Table 2 provides the adjusted means, Cohen's-d effect sizes, and comparisons among groups at posttreatment for insomnia severity reported by participants, anxiety, and depression. There was a significant between-group difference at posttreatment for insomnia severity. The treatment participants rated their insomnia as less severe than the WLC group (Ps < 0.0001). There were no differences among the 3 treatment groups and no change during the follow-up period. At baseline, the significant others (n = 99) who completed the ISI were primarily spouses (88.88%) and 86.86% shared the bedroom. Significant others' rating of insomnia severity were comparable for all groups at baseline but differed at posttreatment (F3,70 = 8.10, P = 0.0001). The significant others indicated the participants' insomnia was less severe in the SCT, SRT, and MCI groups than the WLC group (Ps ≤ 0.02). There were no changes in significant others' rating of insomnia severity over the follow-up phases.
There were no significant changes in trait anxiety at posttreatment or over the follow-up period. There were significant between-group differences for state anxiety at posttreatment (F 3,141 = 3.04; P = 0.0310). The SRT group had lower state anxiety than the WLC group (P = 0.018). There was a significant change over time in state anxiety (F2,153 = 188.02, P < 0.0001). Post hoc tests revealed that the SCT, MCI, and SRT groups decreased anxiety from posttreatment to 1 yr (Ps = 0.0001). At posttreatment, there were significant between-group differences for depression (F3,142 = 6.14, P = 0.0006). The SRT group had less depression than the SCT (P = 0.007) and WLC groups (P = 0.0007). There was a significant change in depression over the follow-up period (F2,153 = 3.92, P < 0.0218). There was a difference between the MCI and SRT groups from posttreatment to 1 yr (P = 0.011) and 3 mo to 1 yr (P = 0.026). Depression increased slightly in the MCI group whereas it decreased in the SRT condition.
Treatment Attendance and Compliance
Participants attended an average of 5.86 (± 0.35), 5.60 (± 0.55), and 5.83 (± 0.45) sessions in the SCT, SRT, and MCI conditions, respectively. There was a significant difference in the number of session attended among groups (F2,104 = 3.48, P = 0.034). The SCT and MCI participants attended more sessions than those in the SRT condition (Ps ≤ 0.034). Participants' and significant others' rating of compliance to treatment recommendations revealed no significant posttreatment differences among the conditions. All participants and significant others rated compliance equally high (≥ 3.00 on 0 to 4 scale). The overall mean compliance rates calculated from the daily sleep diaries for each treatment condition were 89.3% (± 9.5) for SCT, 87.3% (± 10.2) for SRT, and 90.0% (± 8.5) for MCI. There were no significant differences among groups.
Treatment Response and Remission
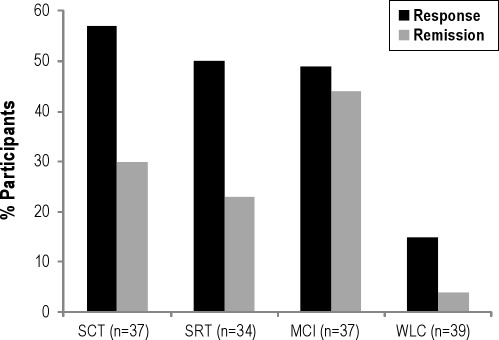
Treatment response was achieved by 42.18% of all participants and 51.80% of treated participants. The SCT group had the largest proportion of responders (56.76%) followed by the SRT (50.00%), MCI (48.65%), and WLC (15.38%) conditions (χ2 = 16.19, P = 0.001). Remission was attained by 24.02% of all participants and 32.06% of treated participants. The MCI condition had the largest proportion of participants who remitted (43.90%), followed by the SCT (29.55%), SRT (22.73%), and WLC (4.00%) groups (χ2 = 20.64, P = 0.0001). Figure 3 illustrates the response and remission rates across the 4 conditions.
Figure 3.
Response and remission rates among the four conditions. MCI, multicomponent intervention; SCT, stimulus control therapy; SRT, sleep restriction therapy; WLC, wait-list control condition.
DISCUSSION
To our knowledge, this is the first report of a study in older adults comparing a behavioral treatment package for insomnia with its component interventions. The results of the current study provide initial evidence that SCT, SRT, and MCI are equivalent in improving diary- and actigraph-measured sleep in older adults with chronic primary insomnia. The behavioral treatments, in single and multicomponent form, were superior to the control condition. This finding supports immediate and long-term benefits found in other older adults studies using similar treatments.10–13,21 In the current study, post-treatment comparisons between the intervention conditions and the control group produced large effect sizes for diary-measured sleep. Medium to large effect sizes for self-reported sleep outcomes (SOL, WASO, SE, and SQ) were found in a meta-analysis of randomized controlled trials of cognitive-behavioral treatment packages, behavioral treatments, and relaxation-based interventions for primary insomnia in middle-aged and older adults.5 The effect sizes obtained for self-reported SOL, TST, and SQ were comparable to or larger than those found in a meta-analysis of the efficacy of benzodiazepines and zolpidem for chronic insomnia.52
The immediate sleep outcomes for all treatment conditions were essentially maintained through 1 yr after treatment completion. An increase in TST and TIB were found for the SRT and MCI groups over the follow-up period and in comparison with the SCT condition. These changes most likely reflect an easing of SRT recommendations over time by the participants. We set the initial sleep prescription at the average TST, rather than an alternative method that adds 30 min to accommodate normative amounts of nighttime wakefulness.53 This manipulation of the homeostatic sleep mechanism may have created daytime sleepiness. Therefore, once treatment was completed participants continued to increase their TIB and TST. Other studies in older adults using sleep restriction as a single treatment and in MCI have noted increases in TST at follow-up.15,21,54
Equivalent clinical outcomes were also found in comparisons among the SCT, SRT, and MCI groups. The reduction in perceived insomnia severity and its effect was comparable among the treatment conditions. Clinically significant improvement was reflected in the change from moderately severe insomnia at baseline to the subclinical insomnia range at posttreatment and in significant others' validation of the participants' self-report and maintenance of treatment effect. Treatment studies in older adults have included insomnia severity assessment for almost 2 decades with consistent demonstration of improvement,11,13,21,23,34 including significant others' reports.21,34
Although there was a statistically significant difference among treatment groups for depression at posttreatment, overall, the change in mood was minimal and not clinically significant. At baseline the participants' depression ratings were at or slightly above the established cutoff score of 11 on the Geriatric Depression Scale.36 The participants' anxiety levels at baseline were below the average normative score seen in older working adults.55 Insomnia treatment studies of older adults with primary insomnia have found modest and no differences in anxiety and depression at posttreatment.11,12,18,21 Persons with significant psychopathology are typically excluded from primary insomnia studies.35 This study did not use a structured psychiatric interview; therefore, symptoms of psychopathology were not formally ruled out. A structured psychiatric assessment would have placed more confidence in the report of mood.
One explanation for the lack of statistically significant sleep outcome differences among treatment conditions is that stimulus control and sleep restriction each address the circadian timing and sleep homeostasis factors underlying the maintenance of chronic insomnia. Conditioned aspects of insomnia may have been addressed through SRT and SCT, which build the sleep drive and consolidate sleep so the behaviors that contribute to conditioning did not emerge. Because all treatments were efficacious, single-component treatment may be sufficient for older adults with primary insomnia; however, the remission analysis provides an alternative interpretation. In the current study, the MCI group had a higher remission rate than the single-component treatments and twice the rate of the SRT condition. Because remission is the goal of therapy, intervention should include strategies with the greatest likelihood of achieving remission. The lower dropout rate in the MCI group is an additional clinically meaningful factor that contributes to support for MCI versus the single-component interventions.
The study has several limitations. This was a highly selected sample of primarily Caucasian and well-educated older adults, which limits the generalizability of the findings. Furthermore, the differences between those who dropped out and those who remained in the study suggest the findings may not generalize to older men with severe insomnia. The screening assessment of psychopathology and other sleep disorders was limited; therefore, participants' presenting insomnia symptoms may have been associated with mental health and occult sleep disorders. The design would have been strengthened by a control group with nonspecific treatment factors to determine if the positive findings were due to the treatment or elements such as therapist contact and treatment attendance. This study used 1 therapist to deliver the intervention. The study would have been better balanced by several therapists with different educational background and representing multiple disciplines. Behavioral treatment for insomnia is based in psychological theory and has been delivered primarily by psychologists or psychology graduate students. The current study and others17,56 provide support for supervised masters-level psychiatric-mental health nurses to deliver behavioral intervention for primary and comorbid insomnia. Nurses without graduate degrees or mental health background have been trained to effectively treat patients with a psychologist's supervision.57 The use of nurses as therapists could address the need for skilled practitioners and improve access to insomnia treatment in routine clinical practice settings.38
The current findings are applicable to older adults with primary insomnia. Treatment studies indicate that positive outcomes are achievable for insomnia in the context of medical and psychiatric conditions.17,49,56,58 In clinical practice, the use of 1 treatment over another may be driven by patient characteristics such as contraindications for the use of SRT, frail and mobility-impaired patients who cannot get out of bed easily during the night to follow SCT, or when a behavioral treatment may increase anxiety. Additional studies are needed to test the effect of a dismantled MCI approach on sleep and remission outcomes in persons with medical and psychiatric co-morbidities.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Bootzin has received research support from Takeda Pharmaceuticals. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Funded by NIH grant NR04951. This research was supported with resources and the use of facilities at the Phoenix Veterans Affairs Health Care System. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
REFERENCES
- 1.Avidan AY, Fries BE, James ML, Szafara KL, Wright GT, Chervin RD. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc. 2005;53:955–62. doi: 10.1111/j.1532-5415.2005.53304.x. [DOI] [PubMed] [Google Scholar]
- 2.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 4.Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–97. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 5.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–80. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery P, Dennis J. A systematic review of non-pharmacological therapies for sleep problems in later life. Sleep Med Rev. 2004;8:47–62. doi: 10.1016/S1087-0792(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 8.Pallesen S, Nordhus IH, Kvale H. Nonpharmacological interventions for insomnia in older adults: a meta-analysis of treatment efficacy. Psychotherapy. 1998;35:472–82. [Google Scholar]
- 9.NIH. State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 10.Morin CM, Azrin NH. Stimulus control and imagery training in treating sleep-maintenance insomnia. J Consult Clin Psychol. 1987;55:260–62. doi: 10.1037//0022-006x.55.2.260. [DOI] [PubMed] [Google Scholar]
- 11.Morin CM, Azrin NH. Behavioral and cognitive treatments of geriatric insomnia. J Consult Clin Psychol. 1988;56:748–53. doi: 10.1037//0022-006x.56.5.748. [DOI] [PubMed] [Google Scholar]
- 12.Engle-Friedman M, Bootzin RR, Hazlewood L, Tsao C. An evaluation of behavioral treatments for insomnia in the older adult. J Clin Psychol. 1992;48:77–90. doi: 10.1002/1097-4679(199201)48:1<77::aid-jclp2270480112>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Pallesen S, Nordhus IH, Kvale G, et al. Behavioral treatment of insomnia in older adults: an open clinical trial comparing two interventions. Behav Res Ther. 2003;41:31–48. doi: 10.1016/s0005-7967(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 14.Puder R, Lacks P, Bertelson AD, Storandt M. Short-term stimulus control treatment of insomnia in older adults. Behav Ther. 1983;14:424–29. [Google Scholar]
- 15.Friedman L, Bliwise DL, Yesavage JA, Salom SR. A preliminary study comparing sleep restriction and relaxation treatments for insomnia in older adults. J Gerontol. 1991;46:1–8. doi: 10.1093/geronj/46.1.p1. [DOI] [PubMed] [Google Scholar]
- 16.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13:17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011:E1–E9. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichstein KL, Wilson NM, Johnson CT. Psychological treatment of secondary insomnia. Psychol Aging. 2000;15:232–40. doi: 10.1037//0882-7974.15.2.232. [DOI] [PubMed] [Google Scholar]
- 19.McCrae CS, McGovern R, Lukefahr R, Stripling AM. Research evaluating brief behavioral sleep treatments for rural elderly (RESTORE): a preliminary examination of effectiveness. Am J Geriatr Psychiatry. 2007;15:979–82. doi: 10.1097/JGP.0b013e31813547e6. [DOI] [PubMed] [Google Scholar]
- 20.McCurry SM, Logsdon RG, Vitiello MV, Teri L. Successful behavioral treatment for reported sleep problems in elderly caregivers of dementia patients: a controlled study. J Gerontol. 1998;53B:P122–29. doi: 10.1093/geronb/53b.2.p122. [DOI] [PubMed] [Google Scholar]
- 21.Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clin Psychol. 1993;61:137–46. doi: 10.1037//0022-006x.61.1.137. [DOI] [PubMed] [Google Scholar]
- 22.Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychol Aging. 2002;17:288–98. [PubMed] [Google Scholar]
- 23.Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, Davis A. A placebo-controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults. J Consult Clin Psychol. 2005;73:1164–74. doi: 10.1037/0022-006X.73.6.1164. [DOI] [PubMed] [Google Scholar]
- 24.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 25.Edinger JD, Carney C. Overcoming insomnia: a cognitive-behavioral therapy approach, therapist guide. New York: Oxford University Press; 2008. [Google Scholar]
- 26.Morin CM, Espie CA. Insomnia: a clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum; 2003. [Google Scholar]
- 27.Derogatis L. Melisaratos, N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Johns M. Daytime sleepiness, snoring, and obstructive sleep apnea: The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 30.Littner M, Kushida C, Anderson W, et al. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 31.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–39. [PubMed] [Google Scholar]
- 32.Brooks JO, Friedman L, Bliwise DL, Yesavage JA. Use of the wrist actigraph to study insomnia in older adults. Sleep. 1993;16:151–55. doi: 10.1093/sleep/16.2.151. [DOI] [PubMed] [Google Scholar]
- 33.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 34.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. JAMA. 1999;281:991–99. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 35.Morin CM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med Rev. 2003;7:263–279. doi: 10.1053/smrv.2002.0274. [DOI] [PubMed] [Google Scholar]
- 36.Yesavage JA, Brink T, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD, Sydeman SJ. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcome assessment. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994. pp. 292–321. [Google Scholar]
- 38.Bootzin RR, Epstein DR. Understanding and treating insomnia. Ann Rev Clin Psychol. 2011;7:435–58. doi: 10.1146/annurev.clinpsy.3.022806.091516. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Ortuno MM, Edinger JD. A penny for your thoughts: patterns of sleep-related beliefs, insomnia symptoms and treatment outcome. Behav Res Ther. 2010;48:125–33. doi: 10.1016/j.brat.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26:177–82. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 41.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: A randomized controlled trial. JAMA. 2001;285:1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 42.Lacks P. Behavioral treatment for persistent insomnia. New York: Pergamon Press; 1987. [Google Scholar]
- 43.Bootzin RR, Epstein D, Engle-Friedman M, Salvio M-A. Sleep disturbances. In: Carstensen LL, Edelstein BA, Dornbrand L, editors. The practical handbook of clinical gerontology. Thousand Oaks, California: Sage Publications; 1996. pp. 389–420. [Google Scholar]
- 44.Glovinsky PB, Spielman AJ. Sleep restriction therapy. In: Hauri P, editor. Case studies in insomnia. New York: Plenum; 1991. pp. 49–63. [Google Scholar]
- 45.Morin CM. Insomnia: psychological assessment and management. New York: Guilford Press; 1993. [Google Scholar]
- 46.Murtagh D, Greenwood KM. Identifying effective psychological treatments for insomnia: A meta-analysis. J Consult Clin Psychol. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 47.Cohen J. Statistical power analysis for the behavioral sciences. Mahwah, New Jersey: Lawrence Erlbaum; 1988. [Google Scholar]
- 48.Buysse DJ, Ancoli-Israeli S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 49.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morin CM, Vallières A, Guay B, Ivers H, Savard J, Merette C, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25:2487–94. doi: 10.1185/03007990903167415. [DOI] [PubMed] [Google Scholar]
- 52.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, III, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278:2170–77. [PubMed] [Google Scholar]
- 53.Wohlgemuth WK, Edinger JD. Sleep restriction therapy. In: Lichstein KL, Morin CM, editors. Treatment of late-life insomnia. New York: Sage Publications; 2000. pp. 147–66. [Google Scholar]
- 54.Morin CM, Bastien C, Guay B, Radouco-Thomas M, Leblanc J, Vallieres A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161:332–42. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- 55.Spielberger C, Gorsuch RC, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, California: Consulting Psychologists Press; 1983. [Google Scholar]
- 56.Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum. 2007;34:E51–59. doi: 10.1188/07.ONF.E51-E59. [DOI] [PubMed] [Google Scholar]
- 57.Espie CA, MacMahon KMA, Kelly H-L, Broomfield NM, Douglas NJ, Engleman HM, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30:574–84. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 58.Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004;99:1121–32. doi: 10.1111/j.1360-0443.2004.00835.x. [DOI] [PubMed] [Google Scholar]