Abstract
Study Objectives:
We evaluated associations among subjective and objective measures of sleep and the metabolic syndrome in a multi-ethnic sample of midlife women.
Design:
Cross-sectional study.
Setting:
Participants' homes.
Participants:
Caucasian (n = 158), African American (n = 125), and Chinese women (n = 57); mean age = 51 years. Age range = 46-57 years.
Interventions:
None.
Measurements and Results:
Metabolic syndrome was measured in the clinic and sleep quality was assessed by self-report. Indices of sleep duration, continuity/fragmentation, depth, and sleep disordered breathing were assessed by in-home polysomnography (PSG). Covariates included sociodemographics, menopausal status, use of medications that affect sleep, and self-reported health complaints and health behaviors known to influence metabolic syndrome risk. Logistic regression was used to test the hypothesis that the metabolic syndrome would be associated with increased subjective sleep complaints and PSG-assessed sleep disturbances. In univariate analyses, the metabolic syndrome was associated with decreased sleep duration and efficiency and increased NREM beta power and apnea-hypopnea index (AHI). After covariate adjustment, sleep efficiency (odds ratio [OR] = 2.06, 95% confidence interval [CI]: 1.08-3.93), NREM beta power (OR = 2.09, 95% CI: 1.09-3.98), and AHI (OR = 1.86, 95% CI: 1.40-2.48) remained significantly associated with the metabolic syndrome (odds ratio values are expressed in standard deviation units). These relationships did not differ by race.
Conclusions:
Objective indices of sleep continuity, depth, and sleep disordered breathing are significant correlates of the metabolic syndrome in midlife women, independent of race, menopausal status and other factors that might otherwise account for these relationships.
Citation:
Hall MH; Okun ML; Sowers M; Matthews KA; Kravitz HM; Hardin K; Buysse DJ; Bromberger JT; Owens JF; Karpov I; Sanders MH. Sleep is associated with the metabolic syndrome in a multi-ethnic cohort of midlife women: the SWAN Sleep Study. SLEEP 2012;35(6):783-790.
Keywords: Sleep, metabolic syndrome, midlife women, race, obesity
INTRODUCTION
The menopausal transition represents a period of increased risk for the metabolic syndrome, a cluster of interrelated risk factors for incident cardiovascular disease, type 2 diabetes, and mortality.1–4 Identification of the biobehavioral pathways through which the menopausal transition increases risk for the metabolic syndrome is critical to primary and secondary prevention of cardiovascular disease and type 2 diabetes, which are leading causes of morbidity and mortality in mid- and late-life women.5–9
Sleep represents a biologically plausible, modifiable, and underexplored pathway through which the menopausal transition may increase risk for the metabolic syndrome and its consequences to cardiometabolic disease. Subjective sleep complaints, including reports of insomnia, difficulty initiating and maintaining sleep, and dissatisfaction with sleep quality, increase during the menopausal transition.10,11 Similarly, increases in sleep disordered breathing have been strongly linked to the menopausal transition.12,13 While some studies have failed to show significant differences in objective measures of sleep based on menopausal status,14,15 others have reported increased sleep fragmentation in peri- or postmenopausal women.16,17 Based on a single night of laboratory PSG studies in the predominantly Caucasian Wisconsin Sleep Cohort (n = 589), Young and colleagues reported that, despite greater dissatisfaction with their sleep, peri- and postmenopausal women had more slow wave sleep than premenopausal women.18 This paradoxical finding may reflect a re-equilibration of hormones and their influence on sleep duration, continuity/fragmentation, and depth following the menopausal transition.19
Emerging evidence suggests that the dimensions of sleep that change during the menopausal transition are linked with the metabolic syndrome. For instance, two recent cross-sectional studies observed that individuals who reported sleeping less than 6 hours or more than 8 hours per night were more likely to meet metabolic syndrome criteria than individuals who reported between 7 and 8 hours of sleep per night.20,21 These analyses were based on retrospective, self-report questions about sleep duration, which may or may not reflect physiological processes that underlie sleep. More recently, Nock and colleagues used structural equation modeling to evaluate relationships among laboratory-assessed sleep and components of the metabolic syndrome in 533 adults (mean age ∼ 40 years) with mild to moderate sleep apnea.22 Their results suggest that sleep fragmentation, decreased slow wave sleep and sleep disordered breathing co-aggregate with features of the metabolic syndrome, including obesity, insulin resistance, elevated blood pressure, and dyslipidemia. Although causal inferences cannot be drawn from these cross-sectional studies, mounting evidence from longitudinal and experimental studies suggests that sleep deprivation or curtailment, sleep fragmentation, selective restriction of slow wave sleep, and sleep disordered breathing are causally linked to key components of the metabolic syndrome including increased blood pressure, glucose dysregulation, and changes in metabolism that favor weight gain.23–29 We have also shown that self-reported difficulty falling asleep and loud snoring were prospectively associated with the development of the metabolic syndrome.30 The present study evaluated cross-sectional associations among the sleep and the metabolic syndrome in a community-based cohort of Caucasian, Chinese, and African American women transitioning into the menopause. We hypothesized that subjective sleep quality complaints and PSG-assessed indices of sleep duration, continuity/fragmentation, depth, and sleep disordered breathing would be significant correlates of the metabolic syndrome, after adjusting for race, menopausal status and other factors that might otherwise account for these relationships. In light of marked racial/ethnic differences in sleep and cardiometabolic disease risk, we also evaluated whether relationships among sleep and the metabolic syndrome differed by race/ethnicity.4,31,32
METHODS
Study Participants
Participants were drawn from the SWAN Sleep Study (n = 368), a cross-sectional study of sleep in midlife women. It is an ancillary study of the Study of Women's Health Across the Nation (SWAN), which is a community-based study of the menopausal transition and its consequences to health and functioning, as previously described. The SWAN Sleep Study enrolled a cohort of 370 Caucasian, African-American and Chinese participants from 4 study sites in the United States: Chicago, IL; Detroit area, MI; Oakland, CA; and Pittsburgh, PA. Exclusions for the SWAN Sleep Study were: current menopausal hormone replacement therapy (MHT) use; current chemotherapy or radiation; current oral corticosteroid use; regular night shiftwork; regular consumption of > 4 alcoholic drinks/day; and noncompliance with Core SWAN procedures. Metabolic syndrome data were not available in 28 Sleep Study participants; these participants did not differ from participants for whom metabolic syndrome data were available with regard to age, race, menopausal status, body mass index, subjective sleep quality or PSG-assessed indices of sleep duration, continuity/fragmentation, depth or sleep disordered breathing. Informed consent was obtained in accordance with approved protocols and guidelines of the institutional review board at each participating institution. Participants received monetary compensation for their participation.
Study Protocol
Unattended PSG sleep studies were conducted in participants' homes on the first 3 nights of the SWAN Sleep Study.31 Women who were regularly cycling were studied within 1 week of the start of their menstrual cycle. Participants whose cycles were irregular or who were postmenopausal were studied at their convenience. Study staff visited participants in their homes on each night of sleep studies to apply electrodes and calibrate monitors. Participants slept in their own beds, under their usual circumstances, at their habitual sleep and wake times, as determined by self-report. Participants turned off the PSG recorder and removed study equipment upon awakening in the morning. Self-report data were collected concurrently with sleep studies.
Sleep
Measures of sleep included subjective sleep quality and PSG-assessed indices of sleep duration, continuity/fragmentation, depth, and sleep disordered breathing. The referent for the 19-item self-report Pittsburgh Sleep Quality Index (PSQI)33 was the month prior to sleep studies. The range of scores on the PSQI was 0-21, with higher scores reflecting greater subjective sleep quality complaints.
Polysomnographic sleep data were collected with Vitaport-3 (TEMEC VP3) ambulatory recorders. Signals collected on each study night included bilateral central referential EEG channels (C3 and C4, referenced to A1-A2), electro-oculogram (EOG), submentalis electromyogram (EMG), and electrocardiogram (EKG). Additional signals were collected on the first night of sleep studies for the assessment of sleep disordered breathing (SDB; nasal pressure and oral-nasal thermistors, fingertip oximeter, and abdominal and thoracic excursion, as measured by inductance plethysmography to reflect ventilatory effort). Quality assurance assessments, scoring, and processing of all sleep study records was performed at the University of Pittsburgh Neuroscience - Clinical and Translational Research Center (N-CTRC). Visual sleep stage scoring in 20-sec epochs was conducted by trained PSG technologists with established reliability, using standard scoring criteria34 and American Academy of Sleep Medicine recommendations.35
Measures of sleep duration and continuity/fragmentation were averaged across Nights 2 and 3 due to within-subject variability in these parameters.36 Total sleep time ([TST] sleep duration) was calculated as total minutes of any stage of sleep from sleep onset to morning awakening. Although extremes of sleep duration are often associated with adverse health outcomes, including mortality and the metabolic syndrome,21,37 TST was evaluated as a continuous variable given the extremely low prevalence of long sleepers in the SWAN Sleep Study sample (only 10 participants had sleep duration ≥ 480 min). Sleep efficiency(TST / time spent in bed × 100) and arousal index (ArI; defined as an abrupt increase in EEG frequency lasting between 3 and 10 sec per hour of sleep) were used to quantify sleep continuity/fragmentation. We used 2 measures derived from power spectral analysis of the EEG to characterize sleep depth: (1) increased relative NREM EEG power in the low-frequency delta band (0.05-4.0 Hz/0.05-32.0 Hz) and (2) decreased relative NREM EEG power in the high-frequency beta band (16-32 Hz/0.05-32.0 Hz), which is an index of cortical arousal during NREM sleep.38,39 Delta and beta power were inversely correlated with one another (r = −0.27, P < 0.001), and each was significantly correlated with visually scored percent slow wave sleep (r's 0.53 and −0.26, respectively, P values < 0.001). These data were derived from power spectral analysis of the EEG during NREM sleep (Night 2 or 3 only, due to the within-subject stability of quantitative EEG during NREM sleep).40 The Apnea-Hypopnea Index ([AHI] number of apneas + number of hypopneas/hours of sleep) was used to quantify sleep disordered breathing.35
Metabolic Syndrome
The metabolic syndrome was assessed during annual Core SWAN Study assessments.4 Fasting blood draws were collected during the morning hours; samples were assayed for glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides. Standardized protocols were used to measure blood pressure and waist circumference. Briefly, blood pressure was recorded using standard mercury sphygmomanometers following a 5-min rest in the seated position. Two sequential readings were taken on the right arm, with a 2-min intervening rest; these measures were averaged for each participant. Waist circumference at the umbilicus was measured with participants in nonrestrictive undergarments. Based on National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines for women,41 the metabolic syndrome was defined as the presence of ≥ 3 of the following criteria: (1) waist circumference ≥ 88 cm for Caucasian and African American participants and ≥ 80 cm for Chinese participants; (2) blood pressure ≥ 130 mm Hg systolic, ≥ 85 mm Hg diastolic, or use of antihypertensive medication; (3) fasting serum glucose ≥ 100 mg/dL (or having ever been classified as diabetic); (4) serum triglycerides ≥ 150 mg/dL or medication for hypertriglyceridemia; and (5) HDL cholesterol ≤ 50 mg/dL or use of medication for low HDL cholesterol. The present analyses used each participant's metabolic syndrome status from the annual Core SWAN Study assessment immediately preceding their sleep study (median number of months between Core SWAN and Sleep Study assessments = 3.6 months).
Covariates
Potential covariates included sociodemographics, menopausal status, and indices of health and health behaviors previously associated with the metabolic syndrome in midlife women. Age and race/ethnicity (non-Hispanic Caucasian, Chinese, or African American) were established by self-report. Menstrual bleeding patterns were used to characterize menopausal status, according to World Health Organization criteria.42 Educational attainment, rather than income, was used as an indicator of socioeconomic status (SES) due to geographic disparities in earnings and cost of living across study sites.31 Marital status at the time of sleep studies was coded as “married or living as married” or “unmarried,” including participants who were single, separated, divorced, or widowed. Self-reported symptoms of depression were measured concurrently with in-home sleep studies using the 16-item Inventory of Depressive Symptomatology (IDS).43 The IDS, minus sleep items, was calculated as a continuous variable. Health complaints were dichotomized as “fair” or “poor” versus “good” or “excellent” based on the distribution of responses to the single-item general health rating of the SF-36.44 Health behaviors were assessed by daily diary reports of smoking (any nicotine use was coded as “yes”), alcohol use (any alcohol use was coded as “yes”), and regular exercise (reports of exercising ≥ 3 times a week were coded as “regular exercise”). Medication use, recorded at the time of sleep studies, was coded according to the World Health Organization ATC classification (http://www.whocc.no/atcddd). For the present report “medications that affect sleep” were considered to be those products associated with the following ATC classification codes: N02A (opioids), N03A (antiepileptics), N05B (anxiolytics), N05C (hypnotics and sedatives), N06A (antidepressants), and R06A (antihistamines). Use of medications that affect sleep was dichotomized as “present” or “absent.”
Statistical Analyses
Descriptive statistics were used to characterize the study sample based on presence or absence of the metabolic syndrome. Differences were evaluated by χ2 tests for categorical variables or analysis of variance for continuous variables. Skewed variables were successfully transformed prior to testing study hypotheses (see Table footnotes). One-way analysis of variance was used to evaluate univariate associations between sleep and the metabolic syndrome. To reduce the number of statistical comparisons, only those sleep measures that were significant in univariate analyses were further tested in multivariate analyses. In this subset of measures, multivariate logistic regression was used to test the hypothesis that sleep is a significant correlate of the metabolic syndrome, after adjusting for race, menopausal status, educational attainment, marital status, health complaints, use of medications that affect sleep, smoking, alcohol use, and regular exercise. Age and symptoms of depression were not included as covariates as they were unrelated to metabolic syndrome status (P values > 0.20). Separate models were used for each sleep measure given modest correlations among measures (r values < 0.20). Because obesity is strongly associated with sleep and individual components of the metabolic syndrome, BMI ≥ 30 was included as an additional covariate in a second set of logistic regression models to evaluate whether obesity attenuated relationships between sleep and the metabolic syndrome. A final set of models included an interaction term for race, with α level set at P < 0.01 due to the number of comparisons; these analyses were conducted in African Americans and Caucasians only, due to the limited number of Chinese participants with the metabolic syndrome (n = 11).
RESULTS
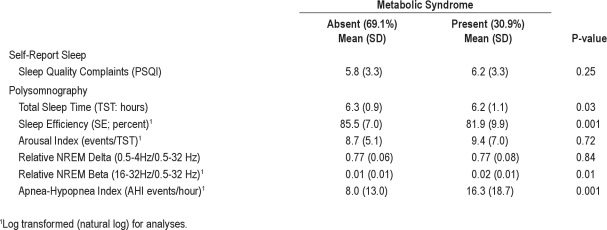
As shown in Table 1, prevalence of the metabolic syndrome in this sample of midlife women was 30.9%. Among those with the metabolic syndrome, 94% met the waist circumference criterion, 77% met the blood pressure criterion, 77% met the HDL criterion, 58% met the triglycerides criterion, and 54% met the glucose criterion. Prevalence of the metabolic syndrome differed by race, with the highest and lowest rates in African American and Chinese participants, respectively. The metabolic syndrome was more prevalent in participants who were heavier, did not exercise regularly, and who reported “fair” or “poor” health. In contrast, the metabolic syndrome was less prevalent in participants who were married or living as married and reported some alcohol use.
Table 1.
Sample characteristics according to the absence or presence of the metabolic syndrome
In univariate analyses (see Table 2), participants with the metabolic syndrome spent less time asleep (15 min, on average) and had poorer sleep efficiency and greater relative EEG beta power during NREM sleep than those without the metabolic syndrome. Moreover, the mean AHI in participants with the metabolic syndrome was double that observed in participants without the metabolic syndrome. This relationship was also evaluated using an AHI cutoff score ≥ 15 for clinically significant sleep apnea. The metabolic syndrome was present in 56.7% of participants with clinically significant sleep apnea, compared to 23.5% of participants whose AHI was < 15 (χ2 = 28.05, P < 0.001). Subjective sleep quality, arousal index, and relative EEG delta power during NREM sleep were unrelated to the metabolic syndrome.
Table 2.
Sleep characteristics according to the absence or presence of the metabolic syndrome
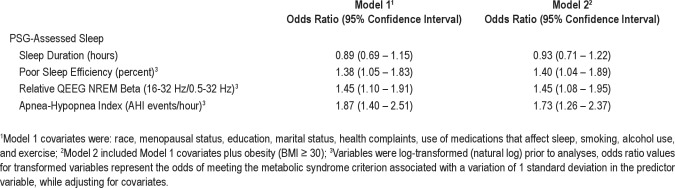
Results for multivariate logistic regression models, which adjusted for race, menopausal status, educational attainment, marital status, health complaints, medications that affect sleep, smoking, alcohol use and regular exercise, are shown in Table 3 (Model 1). Decreased sleep efficiency, increased NREM beta power, and increased AHI were each associated with increased odds for having the metabolic syndrome. To enhance interpretability of effects for these transformed variables, units of change for odds ratios and confidence intervals are standard deviation units. Thus, the AHI power estimate of 1.87 suggests that metabolic syndrome odds in midlife women nearly doubled for each one standard deviation increase in AHI. Similarly, a midlife woman was 1.38 and 1.45 times more likely to meet criteria for the metabolic syndrome for every standard deviation decrease in sleep efficiency and every standard deviation increase in relative beta power, respectively. Sleep efficiency, NREM beta power, and AHI were then entered simultaneously in a multivariate model to evaluate their independent association with the metabolic syndrome. Both sleep efficiency and AHI were independent correlates of the metabolic syndrome (sleep efficiency OR in SD units = 1.38, 95% CI: 1.02 − 1.89; AHI OR in SD units = 1.85, 95% CI: 1.36 − 2.52). In contrast, the relationship between NREM beta power and the metabolic syndrome was no longer significant (OR in SD units = 1.31, 95% CI: 0.98 − 1.76) when all 3 sleep measures were included in a single model. None of the interactions for race were statistically significant (results not shown).
Table 3.
Multivariate associations among sleep characteristics and the metabolic syndrome
A final set of models evaluated whether associations among sleep and the metabolic syndrome were independent of obesity. As shown in Model 2 of Table 3, the inclusion of obesity (BMI ≥ 30) as an additional covariate in a second set of multivariate logistic regression models did not attenuate significant associations among sleep efficiency, NREM beta power, or AHI with the metabolic syndrome. Results for sleep disordered breathing were similar when a clinical cutoff score of AHI ≥ 15 was used (OR in SD units = 2.87, 95% CI 1.43 − 5.76). When BMI was considered as a continuous variable, sleep efficiency and NREM beta power remained significantly associated with the metabolic syndrome (sleep efficiency OR in SD units = 1.47, 95% CI: 1.06 − 2.04; NREM beta power OR in SD units = 1.54, 95% CI: 1.11 − 2.12). The effects of AHI in this model approached, but did not reach, statistical significance (OR in SD units = 1.36, 95% CI: 0.97 − 1.92).
DISCUSSION
We are not aware of any published study that has evaluated relationships among subjective and objective measures of sleep with the metabolic syndrome in midlife women, who are at increased risk for developing the metabolic syndrome and incident cardiometabolic disease.1–4 As hypothesized, the metabolic syndrome was more prevalent in women whose sleep was lighter and more fragmented, as measured by increased NREM beta power and decreased sleep efficiency. Sleep disordered breathing, as measured by the continuous apnea-hypopnea index or a clinical cutoff score of AHI ≥ 15, was also robustly associated with the metabolic syndrome. Moreover, when evaluated simultaneously, both sleep efficiency and AHI emerged as significant, independent correlates of the metabolic syndrome. The richness of the SWAN database allowed us to establish that these associations were independent of other known metabolic syndrome risk factors, including race, menopausal status, education and marital status, use of medications that affect sleep, self-reported health complaints and health behaviors, and obesity. These associations were similar in African American and Caucasian participants; the limited number of Chinese participants with the metabolic syndrome precluded evaluation of this group separately.
Despite differences in sample characteristics, measures and methods, these results are consistent with Nock and colleagues' hierarchical model of the metabolic syndrome.22 Brief arousals from sleep, as measured by the arousal index, were a significant component of the metabolic syndrome in Nock et al.'s Cleveland Family Sleep Study sample. Brief arousals were less common in our sample (mean arousal index = 8.89 ± 6.69 versus 14.20 ± 8.03 in the female subsample of the Cleveland Family Sleep Study) and were unrelated to the metabolic syndrome. However, our sample of midlife women did experience marked and sustained wakefulness after sleep onset and poor sleep efficiency.31 The significant association between sleep efficiency and the metabolic syndrome in our sample is consistent with other studies that have reported an inverse association between actigraphy-assessed sleep efficiency and the metabolic syndrome or its components (e.g., increased blood pressure, drug-resistant hypertension, type 2 diabetes).45–48
Both studies found significant associations among the metabolic syndrome and indices of sleep depth. Although decreased slow wave sleep percent emerged as a significant component of the metabolic syndrome in the Cleveland Family Sleep Study sample, it was unrelated to the metabolic syndrome in our sample of midlife women.13,31 One possible explanation for these discrepant results is that midlife women generate proportionally less slow wave sleep than that observed in female participants the Cleveland Family Sleep Study sample who were, on average, ten years younger than the SWAN Sleep Study sample. In contrast to our null findings for EEG delta power, we did find a reliable and robust result for its inverse, relative EEG beta power, which is a measure of cortical arousal during NREM sleep.39 Specifically, each standard deviation increase in relative EEG beta power was associated with a nearly 50% increase in the metabolic syndrome in our sample of midlife women. This relationship was observed independent of total EEG power, the majority of which is attributed to slow wave activity.49 That relative beta power emerged as a significant correlate of the metabolic syndrome suggests that increased cortical arousal during NREM sleep is a more significant correlate of the metabolic syndrome in midlife women than are EEG slow waves.
Sleep disordered breathing, as measured by AHI, was a significant component or correlate of the metabolic syndrome in both studies. In both studies, the link between sleep disordered breathing and the metabolic syndrome was independent of obesity as defined by a BMI ≥ 30. Independence from this confounder is critical to the hypothesis that sleep disordered breathing is associated with and may contribute to the development of the metabolic syndrome, as obesity is a major risk factor for sleep apnea as well as dyslipidemia, insulin resistance, and hypertension (see reviews by Franssen et al., Kurukulasuriya et al., Pi-Sunyer, and Reaven50–53). Indeed, animal models of sleep disordered breathing have experimentally shown that intermittent hypoxia produces insulin resistance, dyslipidemia, and elevated blood pressure independently of obesity (see reviews by Dematteis et al., Drager et al., Kanagy, and O'Donnell54–57). The independent association between AHI and the metabolic syndrome was attenuated when BMI was considered as a continuous covariate in the present sample. However, this is likely related to overcontrol in the model, since BMI and AHI were highly co-linear. Similar findings regarding the association between BMI, sleep apnea, and the metabolic syndrome have been reported in the Wisconsin Sleep Cohort.58
Although cross-sectional, these results are consistent with experimental studies that have documented increased blood pressure, glucose dysregulation, dyslipidemia, upregulation of lipid biosynthesis, and changes in metabolism that favor weight gain in response to sleep deprivation, curtailment, fragmentation, intermittent hypoxia, and selective restriction of deep sleep.26–29,59,60 Potential physiological pathways linking the metabolic syndrome with sleep that is fragmented, light, and/or occurs in the context of sleep disordered breathing include HPA axis and/or sympathetic nervous system activation, inflammation, and oxidative stress, which have been implicated in the regulation of blood pressure, glucose and lipid metabolism, and selective accumulation of visceral fat.61–63 Although less often considered, disturbed sleep may also increase metabolic syndrome risk through psychological and behavioral pathways such as mood dysregulation, fatigue, and poor health behaviors including a diet high in fat and carbohydrates, physical inactivity, and smoking.28,64–67
The extent to which this relationship is causal and the pathways by which sleep disturbance contributes to the metabolic syndrome are relevant to the etiology and prevention of cardiometabolic disease and represent an important direction for systematic research. We have recently reported that self-reported difficulty falling asleep and loud snoring were prospectively associated with the development of the metabolic syndrome.30 Yet self-reports do not reliably assess specific objectively assessed dimensions of sleep shown in this and previous cross-sectional studies to be associated with the metabolic syndrome.22 Prospective studies using objective measures of sleep continuity, depth, and sleep disordered breathing are needed to identify specific dimensions of sleep associated with the metabolic syndrome. If prospective studies demonstrate a linear relationship between sleep apnea and incident metabolic syndrome, even modest decreases in symptoms of sleep apnea might positively influence cardiovascular and metabolic disease risk. Interventions that consolidate deep, restorative sleep might similarly prove to be cardioprotective.
These results cannot be generalized to men, other racial/ethnic groups or younger/older populations whose sleep and cardiovascular disease risk profiles differ significantly from midlife Caucasian, Chinese, and African American women. Moreover, although state-of-science measures were used to quantify sleep, 2 or 3 nights of PSG data may not represent habitual sleep patterns or chronicity of sleep disturbances. These issues are important given that the metabolic syndrome and cardiometabolic disease develop over years and decades. Certainly, large, prospective studies in generalizable samples across the lifespan are needed to characterize the temporal relationships among sleep and the metabolic syndrome. Finally, the cross-sectional nature of this study precludes attributions of causality.
The present study has several notable strengths. Data were drawn from a large, community-based sample of African American, Chinese, and Caucasian women at high risk for the metabolic syndrome due to changes in sex hormones and accumulation of visceral fat which characterize the menopausal transition. The size and rich nature of the SWAN dataset provided the opportunity to evaluate relationships among sleep and the metabolic syndrome independent of sociodemographic, health, and health behaviors previously associated with risk for the metabolic syndrome. We were, similarly, able to evaluate the possibility that the sleep-metabolic syndrome relationship differed by race. The use of polysomnography provided objective measures of sleep disordered breathing, sleep duration, sleep continuity/fragmentation, and quantitative indices of sleep depth. Finally, the use of in-home PSG provided more ecologically valid indices of sleep in contrast to the more artificial context of laboratory-based sleep studies.
In summary, our data extend previous studies of sleep and the metabolic syndrome by demonstrating that PSG-assessed indices of sleep are significant correlates of the metabolic syndrome in African American, Chinese, and Caucasian women transitioning through the menopause. Components of sleep associated with the metabolic syndrome included sleep disordered breathing, poor sleep continuity, and “lighter” sleep as measured by fast frequency EEG power. At a practical level, individuals and their physicians should be aware that sleep disturbances beyond sleep disordered breathing might confer increased risk for the metabolic syndrome and vice versa. Future studies are needed to evaluate causal pathways linking sleep to the metabolic syndrome, including the physiological, psychological, and behavioral mechanisms that underlie these relationships. Also needed are prospective studies and randomized clinical trials that examine whether objectively assessed sleep disturbances are associated with incident metabolic syndrome and its cardiovascular and metabolic consequences and whether interventions to improve sleep in at-risk individuals have metabolic and cardioprotective effects.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Sanders is a scientific consultant to Phillips-Respironics. Dr. Buysse serves as a consultant for Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, Sanofi-Aventis, Servier, Somnus Therapeutics, Stress Eraser, Takeda and Transcept Pharmaceuticals, Inc. He also serves as an investigator on a research study funded by Sepracor and has assisted in the production of CME materials and given paid CME lectures indirectly supported by industry sponsors. Dr. Hardin has received speaking remuneration from Phillips-Respironics and has invested funds in Reviva. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Funding for the SWAN Sleep Study is from the National Institute on Aging (Grants AG019360, AG019361, AG019362, AG019363). The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women's Health (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). Sleep data were processed with the support of RR024153 and analytic support was provided by HL104607. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Aging, National Institute of Nursing Research, Office of Research on Women's Health or the National Institutes of Health.
REFERENCES
- 1.Cameron AJ, Magliano DJ, Zimmet PZ, et al. The metabolic syndrome as a tool for predicting future diabetes: the AusDiab study. J Intern Med. 2008;264:177–86. doi: 10.1111/j.1365-2796.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 2.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–9. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Hong Y, Jin X, Mo J, et al. Metabolic syndrome, its preeminent clusters, incident coronary heart disease and all-cause mortality--results of prospective analysis for the Atherosclerosis Risk in Communities study. J Intern Med. 2007;262:113–22. doi: 10.1111/j.1365-2796.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med. 2008;168:1568–75. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001;24:1522–7. doi: 10.2337/diacare.24.9.1522. [DOI] [PubMed] [Google Scholar]
- 6.Hillier TA, Rizzo JH, Pedula KL, et al. Increased mortality associated with the metabolic syndrome in older women with diabetes. Diabetes Care. 2005;28:2258–60. doi: 10.2337/diacare.28.9.2258. [DOI] [PubMed] [Google Scholar]
- 7.Muggeo M, Verlato G, Bonora E, et al. The Verona diabetes study: a population-based survey on known diabetes mellitus prevalence and 5-year all-cause mortality. Diabetologia. 1995;38:318–25. doi: 10.1007/BF00400637. [DOI] [PubMed] [Google Scholar]
- 8.Towfighi A, Zheng L, Ovbiagele B. Sex-specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med. 2009;169:1762–6. doi: 10.1001/archinternmed.2009.318. [DOI] [PubMed] [Google Scholar]
- 9.Reibis RK, Bestehorn K, Pittrow D, Jannowitz C, Wegscheider K, Voller H. Elevated risk profile of women in secondary prevention of coronary artery disease: a 6-year survey of 117,913 patients. J Womens Health (Larchmt) 2009;18:1123–31. doi: 10.1089/jwh.2008.1082. [DOI] [PubMed] [Google Scholar]
- 10.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30:41–50. doi: 10.1016/s0378-5122(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 12.Resta O, Bonfitto P, Sabato R, De Pergola G, Barbaro MP. Prevalence of obstructive sleep apnoea in a sample of obese women: effect of menopause. Diabetes Nutr Metab. 2004;17:296–303. [PubMed] [Google Scholar]
- 13.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–5. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 14.Hachul H, Bittencourt LR, Soares JM, Jr., Tufik S, Baracat EC. Sleep in post-menopausal women: differences between early and late post-menopause. Eur J Obstet Gynecol Reprod Biol. 2009;145:81–4. doi: 10.1016/j.ejogrb.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Shaver J, Giblin E, Lentz M, Lee K. Sleep patterns and stability in perimenopausal women. Sleep. 1988;11:556–61. doi: 10.1093/sleep/11.6.556. [DOI] [PubMed] [Google Scholar]
- 16.Baker A, Simpson S, Dawson D. Sleep disruption and mood changes associated with menopause. J Psychosom Res. 1997;43:359–69. doi: 10.1016/s0022-3999(97)00126-8. [DOI] [PubMed] [Google Scholar]
- 17.Bixler EO, Papaliaga MN, Vgontzas AN, et al. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res. 2009;18:221–8. doi: 10.1111/j.1365-2869.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–72. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 19.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31:1339–49. [PMC free article] [PubMed] [Google Scholar]
- 20.Choi KM, Lee JS, Park HS, Baik SH, Choi DS, Kim SM. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes (Lond) 2008;32:1091–97. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]
- 21.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory J, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nock NL, Li L, Larkin EK, Patel SR, Redline S. Empirical evidence for «syndrome Z»: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep. 2009;32:615–22. doi: 10.1093/sleep/32.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akerstedt T, Nilsson PM. Sleep as restitution: An introduction. J Intern Med. 2003;254:6–12. doi: 10.1046/j.1365-2796.2003.01195.x. [DOI] [PubMed] [Google Scholar]
- 24.Carrington MJ, Trinder J. Blood pressure and heart rate during continuous experimental sleep fragmentation in healthy adults. Sleep. 2008;31:1701–12. doi: 10.1093/sleep/31.12.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–39. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 26.O'Donnell CP, Ayuse T, King ED, Schwartz AR, Smith PL, Robotham JL. Airway obstruction during sleep increases blood pressure without arousal. J Appl Physiol. 1996;80:773–81. doi: 10.1152/jappl.1996.80.3.773. [DOI] [PubMed] [Google Scholar]
- 27.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 29.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–49. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troxel WM, Buysse DJ, Matthews KA, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33:1633–40. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall M, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in mid-life women: The SWAN sleep study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 32.Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.Rechtschaffen A, Kales A. Washington, DC: US Government Printing Office, Department of Health Education and Welfare; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. NIH Publication 204. [Google Scholar]
- 35.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 36.Tucker AM, Dinges DF, Van Dongen HP. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16:170–80. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 37.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 38.Hall M, Thayer JF, Germain A, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007;5:178–93. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- 39.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 40.Feinberg I, Fein G, Floyd TC. Period and amplitude analysis of NREM EEG in sleep: repeatability of results in young adults. Electroencephalogr Clin Neurophysiol. 1980;48:212–21. doi: 10.1016/0013-4694(80)90306-5. [DOI] [PubMed] [Google Scholar]
- 41.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization Scientific Group. Research on the Menopause in the 1990s. Geneva: World Health Organization; 1996. [PubMed] [Google Scholar]
- 43.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 44.Ware JE, Sherbourne CD. The MOS-36-Item short form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 45.Friedman O, Bradley TD, Ruttanaumpawan P, Logan AG. Independent association of drug-resistant hypertension to reduced sleep duration and efficiency. Am J Hypertens. 2010;23:174–79. doi: 10.1038/ajh.2009.220. [DOI] [PubMed] [Google Scholar]
- 46.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–8. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trento M, Broglio F, Riganti F, et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45:225–9. doi: 10.1007/s00592-008-0047-6. [DOI] [PubMed] [Google Scholar]
- 49.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (aged 20-60 years old) Psychophysiology. 2001;38:232–42. [PubMed] [Google Scholar]
- 50.Franssen R, Monajemi H, Stroes ES, Kastelein JJ. Obesity and dyslipidemia. Med Clin North Am. 2011;95:893–902. doi: 10.1016/j.mcna.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Kurukulasuriya LR, Stas S, Lastra G, Manrique C, Sowers JR. Hypertension in obesity. Med Clin North Am. 2011;95:903–17. doi: 10.1016/j.mcna.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. 2011;95:875–92. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Dematteis M, Godin-Ribuot D, Arnaud C, et al. Cardiovascular consequences of sleep-disordered breathing: contribution of animal models to understanding the human disease. ILAR J. 2009;50:262–81. doi: 10.1093/ilar.50.3.262. [DOI] [PubMed] [Google Scholar]
- 55.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24:843–51. doi: 10.1016/j.beem.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanagy NL. Vascular effects of intermittent hypoxia. ILAR J. 2009;50:282–8. doi: 10.1093/ilar.50.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Donnell CP. Metabolic consequences of intermittent hypoxia. Adv Exp Med Biol. 2007;618:41–49. doi: 10.1007/978-0-387-75434-5_4. [DOI] [PubMed] [Google Scholar]
- 58.Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ. 2009;108:263–65. [PMC free article] [PubMed] [Google Scholar]
- 59.Mackiewicz M, Naidoo N, Zimmerman JE, Pack AI. Molecular mechanisms of sleep and wakefulness. Ann N Y Acad Sci. 2008;1129:335–49. doi: 10.1196/annals.1417.030. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Thorne LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 61.Peters A, Schweiger U, Fruhwald-Schultes B, Born J, Fehm HL. The neuroendocrine control of glucose allocation. Exp Clin Endocrinol Diabetes. 2002;110:199–211. doi: 10.1055/s-2002-33068. [DOI] [PubMed] [Google Scholar]
- 62.Kuo LE, Kitlinska JB, Tilan JU, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–11. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 63.Uusitupa MI, Karvonen MK, Pesonen U, Koulu M. Neuropeptide Y: a novel link between the neuroendocrine system and cholesterol metabolism. Ann Med. 1998;30:508–10. doi: 10.3109/07853899809002597. [DOI] [PubMed] [Google Scholar]
- 64.Atkinson G, Davenne D. Relationships between sleep, physical activity and human health. Physiol Behav. 2007;90:229–35. doi: 10.1016/j.physbeh.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buysse DJ, Thompson W, Scott J, et al. Daytime symptoms in primary insomnia: A prospective analysis using ecological momentary assessment. Sleep Med. 2007;8:198–208. doi: 10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Catrett CD, Gaultney JF. Possible insomnia predicts some risky behaviors among adolescents when controlling for depressive symptoms. J Genet Psychol. 2009;170:287–309. doi: 10.1080/00221320903218331. [DOI] [PubMed] [Google Scholar]
- 67.Grano N, Vahtera J, Virtanen M, Keltikangas-Jarvinen L, Kivimaki M. Association of hostility with sleep duration and sleep disturbances in an employee population. Int J Behav Med. 2008;15:73–80. doi: 10.1080/10705500801929510. [DOI] [PubMed] [Google Scholar]