Abstract
Study Objectives:
To investigate possible differences in the effect of repeated sleep restriction (RSR) during adolescence and adulthood on sleep homeostasis and spatial learning and memory ability.
Design:
The authors examined electroencephalograms of rats as they were subjected to 4-h daily sleep deprivation that continued for 7 consecutive days and assessed the spatial learning and memory by Morris water maze test (WMT).
Participants:
Adolescent and adult rats.
Measurements and Results:
Adolescent rats exhibited a similar amount of rapid eye movement (REM) and nonrapid eye movement (NREM) sleep with higher slow wave activity (SWA, 0.5-4 Hz) and fewer episodes and conversions with prolonged durations, indicating they have better sleep quality than adult rats. After RSR, adult rats showed strong rebound of REM sleep by 31% on sleep deprivation day 1; this value was 37% on sleep deprivation day 7 in adolescents compared with 20-h baseline level. On sleep deprivation day 7, SWA in adult and adolescent rats increased by 47% and 33%, and such elevation lasted for 5 h and 7 h, respectively. Furthermore, the authors investigated the effects of 4-h daily sleep deprivation immediately after the water maze training sessions on spatial cognitive performance. Adolescent rats sleep-restricted for 7 days traveled a longer distance to find the hidden platform during the acquisition training and had fewer numbers of platform crossings in the probe trial than those in the control group, something that did not occur in the sleep-deprived adult rats.
Conclusions:
Repeated sleep restriction (RSR) altered sleep profiles and mildly impaired spatial learning and memory capability in adolescent rats.
Citation:
Yang SR; Sun H; Huang ZL; Yao MH; Qu WM. Repeated sleep restriction in adolescent rats altered sleep patterns and impaired spatial learning/memory ability. SLEEP 2012;35(6):849-859.
Keywords: Adolescent rat, repeated sleep restriction (RSR), slow wave activity (SWA), Morris water maze test (WMT)
INTRODUCTION
Sleep can provide optimal neurobiologic conditions for consolidation of memories for long-term storage.1,2 Adequate sleep can ensure the extensive reconstruction of the regulation regions of learning and memory such as the hippocampus and prefrontal cortex.2–5 Several studies indicated that only sleep, which occurred at an early specific time window after learning, effectively facilitated memory consolidation. Hagewoud et al. observed that 6-h sleep deprivation immediately after training in the light period impaired contextual fear condition. The results of Van Der Werf et al. showed that immediate sleep is necessary for the enhancement of a motor skill through prior observation.6–10
Adolescence is a major transitional period from childhood to adulthood. Adolescents ages 12-18 yr generally sleep on average considerably less than the recommended 9 h during weekdays.11 The consequences of chronic, insufficient sleep are daytime sleepiness; mood and behavioral problems; increased vulnerability to catastrophic accidents, drugs, and alcohol; and development of major disorders of the sleep-wake cycle.12–14 The proposed hypothesis of the current study was to assess the effects in young rats of short daily sleep deprivation, which continued for 7 days during the critical phase of memory consolidation immediately following the water maze training sessions, on sleep pattern and hippocampus-dependent learning and memory performance.
Sleep deprivation is a useful approach in assessing the consequences of insufficient sleep on neuronal functions. Acute sleep deprivation usually lasts a few hours to maximally 12-48 h, whereas chronic sleep deprivation often continues for days to weeks.15 Studies have found that acute sleep deprivation impairs retrieval of stored memories.1,16 The available information about the effects of prolonged sleep restriction on learning was mainly assessed in humans.17–19
The effects of acute sleep loss on sleep pattern have been widely studied. Acute short-term sleep deprivation (3 h or 6 h) in the early part of the light period primarily resulted in a compensatory increase in nonrapid eye movement (NREM) sleep, but not rapid eye movement (REM) sleep,20 whereas long-term (24 h) sleep deprivation mainly led to a rebound of REM and NREM sleep and an initial increase of slow wave activity (SWA) after sleep deprivation followed by a prolonged negative rebound.21 So far, few studies have examined the effects of repeated sleep restriction (RSR) on the sleep-wake system and learning ability.22–26 Ward et al. found that 24-h sleep fragmentation by a treadmill to awaken the animals every 2 min produced spatial learning and memory deficits and reduced hippocampal CA1 pyramidal cell excitability.27,28 Animal references mainly focus on sleep deprivation in adult rats for a long period each day, which is quite different from the real sleep situation in humans. Moreover, the effects of sleep restriction on sleep architecture and learning in adolescent rats have not been reported. In the current study, both the adult and adolescent rats were deprived of sleep daily for 4 h during their rest phase and given a 20-h sleep opportunity that continued for 7 days. We will try to address the following questions. How does repeated partial sleep deprivation affect sleep quality and quantity in adolescent rats compared with adult rats? Does the 4 h of daily repeated sleep deprivation during the light phase immediately following learning damage the acquisition and memory capability during spatial tasks? Can adult rats endure more sleep deprivation than adolescents without affecting spatial performance?
METHODS
Animals
All experiments were carried out on male Sprague-Dawley rats obtained from the Laboratory Center of Chinese Academy of Sciences (Shanghai, China). The animals were housed 3-4 per cage under an ambient temperature of 22 ± 0.5°C with a relative humidity of 60 ± 2% and an automatically controlled 12-h light/12-h dark cycle (light on at 07:00, illumination intensity ≈ 100 lux). The animals had free access to food and water. Experimental protocols were approved by the Shanghai Medical Experimental Animal Administrative Committee. Every effort was made to minimize the number of animals used and any pain and discomfort.
The developmental epoch most similar to adolescence in rats appears to range from postnatal days (PNDs) 28 to 53.29 In the sleep recording test, 32 adolescent rats at PND 22-27 (weight 55-75 g) and 13 adult rats at PND 65-72 (weight 250-290 g) were implanted with electroencephalogram (EEG) and electromyogram (EMG) electrodes. After 1 wk of recovery from the procedure, repeated sleep deprivation began both during adolescence, at PND 29-34 (weight 82-132 g), and during adulthood, at PND 72-79 (weight 290-346 g).
In the water maze test (WMT) at the start of the experiment, 29 adolescent rats at PND 31-44 (weight 98-180 g) and 31 adult rats at PND 60-85 (weight 246-360 g) were used.
Surgical Procedures and EEG and EMG Recording
Rats were anesthetized (chloral hydrate 3.6 mg/kg, intraperitoneally) and surgically implanted with EEG and EMG electrodes for sleep-wake recording. EEG electrodes were placed on the skull surface (1.0 mm anterior to bregma, 1.0 mm right of the central suture and 1.0 mm anterior to lambda on the extended line of the central suture). EMG activity was monitored by using stainless steel Teflon-coated wires placed bilaterally in the nuchal muscle in the dorsal neck region. All electrodes were attached to a microconnector and fixed to the skull with dental cement. After a recovery period of 7 days, each rat was connected to the slip ring (designed so that behavioral movement would not be restricted) and habituated for 3 days with a cable in a soundproof and transparent recording chamber before polygraphic recording (Figure 1A).
Figure 1.
Experiment schedule in adult and adolescent rats. (A) Design for the sleep architecture analysis after repeated sleep deprivation (SD) in which the rats were kept awake by gentle interference for 4 h during the light phase from 08:00 to 12:00. The rats were implanted with EEG and EMG electrodes and then recovered for 7 days. Polygraphic recording started after 3 days of habituation to the recording cable. (B) Design for the effects of sleep deprivation on spatial learning and memory. The water maze test was conducted from 05:00 to 8:00 immediately before sleep deprivation. The rats left undisturbed of sleep were used as a control group.
Sleep Data Acquisition and Analysis
Cortical EEG and EMG signals were amplified and filtered (EEG 0.5–30 Hz; EMG 20–200 Hz) and digitized at a sampling rate of 128 Hz and recorded by using SleepSign software (Kissei Comtec, Nagano, Japan). Polygraphic recordings were automatically scored offline by 10-sec epochs as wake, REM, and NREM sleep by SleepSign 3.0 according to standard criteria.30–33 As a final step, defined sleep–wake stages were examined visually and corrected, if necessary.
For quantitative analysis of the EEG signal, especially for the determination of SWA, defined as the EEG power in the delta frequency range (0.5-4 Hz) during NREM sleep, each 10-sec scored epoch of NREM sleep per hour was subjected to fast Fourier transformation and the results were saved as spectral power. The spectral power of each epoch of NREM sleep was divided into wavebands with a frequency range of 0-24.5 Hz at the bandwidth of 0.5 Hz and then averaged over all epochs of NREM sleep per hour for each rat. To determine the time course of SWA of 1 h, total delta (0.5-4 Hz) energy (μV2) (a) and total energy (μV2) of 0-24.5 Hz (b) of the mean power density across all epochs of NREM sleep per hour was summed, respectively. Because the absolute power is quite variable, the SWA for each rat was expressed as a percentage of a in b [i.e., SWA = 100 %*a/b].26,34–36 The SWA is normalized by the total NREM power for all bands of 0-24.5 Hz for each rat to determine if there were changes in the proportions of delta power in the different experimental groups to remove the potential differences in signal strength between animals. Epochs containing artifact were eliminated from power spectral analysis.
Sleep Deprivation Procedure
Sleep deprivation was achieved by gentle handling that included tapping or shaking the cage, introducing novel objects into the cage, or removing the rat from the cage when behavioral signs of sleep were observed.37,38 Rats were sleep deprived on a daily basis for 4 h during the light phase from 08:00 to 12:00 for 7 consecutive days. Undisturbed rats served as a control group.
Procedure of Morris WMT
The effects of repeated sleep deprivation immediately after training on acquisition and memory capability were evaluated in a hippocampus-dependent spatial task using a submerged platform, which remained in the same location for all trials.25 There were a total of 4 trials for each animal on each of the first 3 days of sleep deprivation and the day after a consecutive 7 days of sleep deprivation. The trials started at late dark or active phase at 05:00 and ended at early light or main sleep phase at 08:00. Rats were trained in a dimly lit room and in a water maze filled with water at 22°C. The starting position randomly varied among the 4 quadrants per trial and thus precluded the effective use of a response strategy. Each rat was placed into water with its head facing the wall of the tank. The rat was then allowed to swim until it had located the hidden platform or for a maximum of 60 sec. The rat was allowed to stay on the platform for 10 sec if it found the platform; otherwise, the rat was gently hand-guided to the platform and allowed to remain on the platform for 20 sec before it was returned to its home cage. After swimming, the rat was dried with a towel as thoroughly as possible. The time required to locate the hidden goal platform was defined as escape latency. The swim path was videotaped and quantified. On the 8th day, after a consecutive 7 days of sleep restriction, a single probe trial was conducted to test for retention of memory for the location of the hidden platform. For the probe trial, the platform was removed and each rat was allowed to swim for 60 sec39 (Figure 1B).
Statistical Analysis
All results were expressed as mean ± standard error. A value of P < 0.05 was considered to be significant for all comparisons. Unpaired 2-tailed t tests were performed to determine whether differences existed between groups.
RESULTS
Total Sleep Time
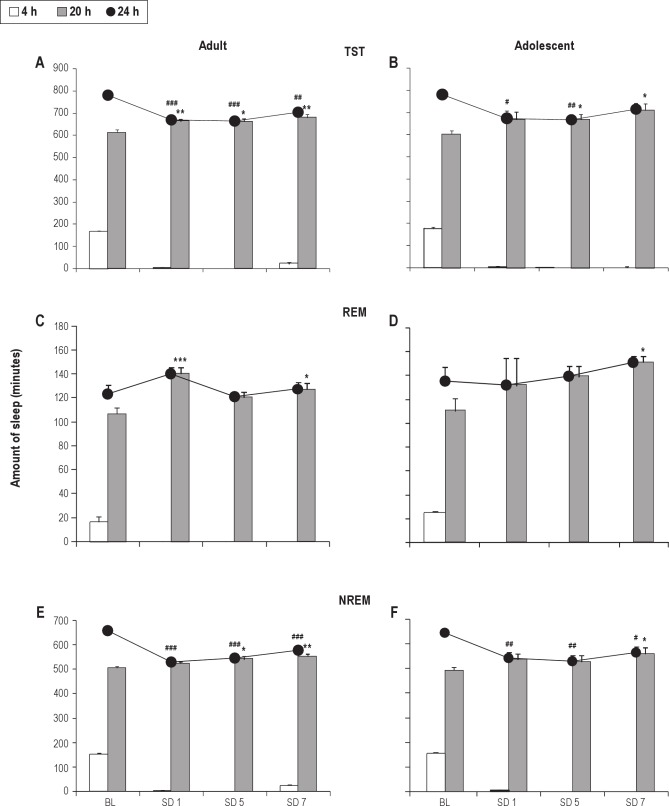
Under normal sleep conditions, adult and adolescent rats slept 13.0 ± 0.2 h and 13.0 ± 0.3 h across 24 h, including 2.8 ± 0.1 h and 3.0 ± 0.1 h during the 4-h time block (8:00-12:00) and 10.2 ± 0.2 h and 10.0 ± 0.3 h over the 20-h time block (12:00-8:00), respectively. The rats were kept awake for 4 h and given a 20-h sleep opportunity each day for 7 consecutive days. By allowing a 20-h sleep window each day, rats had the opportunity to generate a compensatory response to prior sleep loss. Within the 20 hr of allowed sleep period after the end of acute sleep deprivation on the 1st day and chronic sleep deprivation for 5 and 7 consecutive days, total sleep time (TST) of the adult rats significantly increased compared with 20-h baseline [BL] (T(13) = 3.63, P = 0.003 sleep deprivation day 1; T(11) = 3, P = 0.012 sleep deprivation day 5; T(11) = 3.77, P = 0.003 sleep deprivation day 7), which indicated the total sleep amount rebounded remarkably but had not yet reached the 24-h BL (T(13) = 7.21, P < 0.001 sleep deprivation day 1; T(11) = 6.61, P < 0.001 sleep deprivation day 5; T(11) = 3.77, P = 0.003 sleep deprivation day 7). In terms of the adolescent rats, 20-h TST slowly but significantly increased after accumulated sleep deprivation for 5 and 7 days (T(9) = 2.28, P = 0.048; T(8) = 3.15, P = 0.014, respectively). The 20-h TST was fully replenished to the corresponding 24-h BL level on sleep deprivation day 7. There is no total sleep debt after 7 days of accumulated sleep deprivation in adolescents (Figure 2A and B).
Figure 2.
Sleep time of adolescent and adult rats under baseline (BL) and repeated partial sleep deprivation (SD). (A, B) The total sleep time (TST), as well as rapid eye movement (REM) (C, D) and nonrapid eye movement (NREM) sleep states (E, F), was determined. The recordings were divided into 4-h (open bars) and 20-h (gray bars) time blocks, which corresponded to the 4 h of sleep deprivation (8:00-12:00) and 20 h of sleep opportunity (12:00-8:00) on sleep deprivation days. Sleep amounts were also summed over the 24-h recording periods. Values are mean ± standard error (n = 5-8). *P < 0.05, **P < 0.01, ***P < 0.001, versus corresponding 20-h time block of BL; #P < 0.05, ##P < 0.01, ###P < 0.001, versus 24-h BL assessed by t test.
REM Sleep Time
Under BL, adult and adolescent rats exhibited 2.1 ± 0.13 h and 2.3 ± 0.2 h of REM sleep time across 24 h, including 0.3 ± 0.08 h and 0.4 ± 0.05 h during the 4-h time block and 1.8 ± 0.09 h and 1.8 ± 0.18 h over the 20-h time block, respectively. The amount of REM sleep during the 20-h recovery period in adult rats increased significantly to the 24-h BL after acute and chronic sleep deprivation. During the first 20-h sleep opportunity on sleep deprivation day 1, the adult rats showed strong rebound of REM sleep amount, which increased from the corresponding 20-h BL levels by 1.31- fold (T(13) = 4.37, P < 0.001). Whereas on sleep deprivation day 7 after repeated sleep deprivation for 7 consecutive days, REM sleep amount increased by 1.19-fold (T(11) = 2.68, P = 0.022).
In terms of adolescent rats, the strong rebound of REM sleep was observed after 7 days of RSR. The amount of REM sleep increased 1.37-fold compared with the 20-h BL (T(8) = 2.34, P = 0.047). On each sleep restriction day, 20-h REM sleep in the adolescent rats could fully replenish to reach the 24-h BL level, which was similar to that in the adult rats. These results suggested that REM sleep in adult rats showed the strongest rebound on the 1st sleep deprivation block, whereas in adolescent rats, REM sleep rebound gradually increased after 7 days of RSR (Figure 2C and D).
NREM Sleep Time
Over the 24-h period, adult and adolescent rats produced 11.0 ± 0.13 h and 10.8 ± 0.24 h of NREM sleep, comprising 2.5 ± 0.04 h and 2.6 ± 0.08 h during the 4-h time block and 8.4 ± 0.11 h and 8.2 ± 0.19 h over the 20-h time block, respectively. During the first 20-h sleep opportunity on sleep deprivation day 1, there was no significant increase in NREM sleep amount in adult rats compared with the corresponding 20-h BL. NREM sleep time began to rebound by a statistically significant increase to 107% and 109% compared with the 20-h BL during sleep deprivation day 5 and 7, respectively (T(11) = 2.94, P = 0.013; T(11) = 4.3, P = 0.001). As for the adolescent rats, NREM sleep during the 20-h recovery period increased significantly on sleep deprivation day 7 to 114% compared with the 20-h BL (T(8) = 2.41, P = 0.042). There was no significant increase during sleep deprivation day 1 and 5 in comparison with the 20-h BL. NREM sleep rebound in both adult and adolescent rats was inadequate, as demonstrated by the dramatic reduction of NREM sleep amount during the 20-h recovery period on sleep deprivation days compared with the 24-h BL (adults: T(13) = 13.11, P < 0.001 sleep deprivation day 1; T(11) = 9.14, P < 0.001 sleep deprivation day 5; T(11) = 6.17, P < 0.001 sleep deprivation day 7; adolescents: T(9) = 4.02, P = 0.003 sleep deprivation day 1; T(9) = 4.13, P = 0.002 sleep deprivation day 5; T(8) = 2.78, P = 0.024 sleep deprivation day 7) (Figure 2E and F).
Sleep Restriction Promotes SWA in NREM Sleep
An important marker reflecting the depth of sleep is SWA, which is the EEG power between 0.5 and 4 Hz during NREM sleep. Under baseline conditions, SWA was at the lowest level immediately before and after lights off in both adolescent and adult rats, 44.2 ± 3.3% and 19.5 ± 1.6%, respectively; whereas immediately after light onset, SWA was at the highest level, 61.7 ± 3.2% and 39.5 ± 2.3%, respectively. SWA in NREM sleep power per hour within a 24-h period in the adolescent rats was significantly higher than that of adult rats (P < 0.001).
After the first day of acute or 5 to 7 days of accumulative sleep restriction, SWA in both adolescent and adult rats increased. The highest levels of SWA in both adolescent and adult rats occurred on the 7th day of sleep restriction, which increased by 33% and 47%, respectively, above BL. There was no significant difference in the degree of increase between the 2 groups. The remarkable rebound of SWA after the end of sleep deprivation in the adolescent and adult rats was kept at 5 h and 3 h, respectively, on sleep deprivation day 1; 5 h in both adolescent and adult rats on sleep deprivation day 5; 7 h and 5 h in respective adolescent and adult rats on sleep deprivation day 7 (Figure 3). On the contrary, EEG power between 6 and 10 Hz during REM sleep did not show any enhancement after repeated sleep deprivation, although the amount of REM sleep dominantly increased.
Figure 3.
Time course of slow wave activity (SWA) (%, expressed as percentage of delta power [0.5-4 Hz] in nonrapid eye movement [NREM] sleep per hour) during the 20-h recovery period after daily 4-h sleep deprivation (SD) for 7 consecutive days and 24-h baseline (BL) in adult rats (A) and adolescent rats (B). The horizontal open and filled bars on the x-axes indicate the 12-h light and dark periods, respectively. Values are mean ± standard error (n = 5-8). The horizontal filled bars under the curve indicate where there are statistical differences (P < 0.05) on sleep deprivation days in comparison with corresponding BL values. SWA (%) significantly increased in normal adolescent rats vs. the adult ones (P < 0.05). Enhancement of SWA (%) in adolescent and adult rats lasted for 7 h and 5 h, respectively, after 7 days of repeated partial sleep deprivation.
Effects of Sleep Restriction on Episode Transition and Duration
Compared with adult rats, adolescent rats had fewer episodes of both NREM sleep and wakefulness (Figure 4A: T(10) = 3.61, P = 0.005; T(10) = 3.21, P = 0.009), with the prolonged episode durations (Figure 4B: T(10) = 3.13, P = 0.011; T(10) = 3.64, P = 0.005), and had fewer conversions between NREM sleep and wakefulness (Figure 4C: T(10) = 3.34, P = 0.008; T(10) = 3.87, P = 0.003), indicating that adolescent rats have better sleep quality than adult rats.
Figure 4.
Episode numbers, mean durations, and stage transitions, respectively, in adolescent and adult rats during the 24-h baseline (BL) (A-C). Total episode numbers of sleep and wake (D, E), numbers of REM and NREM sleep episodes in different duration (F-I), and stage transitions (J, K) of adolescent and adult rats after repeated partial sleep deprivation (SD) during the 24-h (open bars) and 20-h (gray bars) time blocks, which corresponded to the 24-h BL and 20 h of sleep opportunity on sleep deprivation days. Values are mean ± standard error (n = 4-8). *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding 20-h BL; #P < 0.05, ##P < 0.01, ###P < 0.001 versus 24-h BL assessed by t test.
After sleep deprivation of 4 h per day, total REM sleep in both adult and adolescent rats could return to 24-h BL during the 20-h sleep recovery period on sleep deprivation day 1, 5, and 7. For REM sleep in adult rats, total REM episodes significantly increased compared with the corresponding 20-h BL (Figure 4D: T(13) = 4.56, P < 0.001 sleep deprivation day 1; T(11) = 5.37, P < 0.001 sleep deprivation day 7), which was mainly reflected by the remarkable increased episodes of 60-110 sec (T(13) = 2.64, P = 0.02 sleep deprivation day 1; T(11) = 4.11, P = 0.001 sleep deprivation day 7). With the repeated sleep deprivation during sleep deprivation day 7, REM episodes shorter than 30 sec also began to significantly increase (T(11) = 3.01, P = 0.012) (Figure 4F). Instead, total or each different period of REM episodes in adolescents did not change significantly after sleep restriction, except for fewer REM episodes lasting 60-110 sec on sleep deprivation day 1 compared with the 24-h BL (T(8) = 2.62, P = 0.031) (Figure 4E and G).
Adult rats had more NREM sleep episodes lasting 240-960 sec during the 20-h compensatory period after sleep restriction compared with 20-h BL (Figure 4H: T(13) = 4.02, P = 0.002 sleep deprivation day 1; T(11) = 4.89, P < 0.001 sleep deprivation day 5; T(11) = 2.98, P = 0.013 sleep deprivation day 7), which may be associated with the increased NREM sleep amount. On sleep deprivation day 1, 5, and 7, mainly because of the significant reduction of NREM episodes lasting 30-230 sec (Figure 4H: P < 0.05), total NREM sleep episodes (Figure 4D: P < 0.01) and amount were much lower than the 24-h BL. On the other hand, adolescent rats had more NREM sleep episodes lasting 480-960 sec on sleep deprivation day 7 compared with 20-h BL (Figure 4I: T(6) = 3.3, P = 0.016), which may be related to the increased NREM time. The total NREM sleep amount after the end of sleep restriction on sleep deprivation day 7 was still lower in adolescent rats than 24-h BL, mainly as a result of decreased NREM episodes of duration of 60-230 sec (Figure 4I: P < 0.01). Shorter (10-50 sec) or longer (4-8 min) episodes of NREM sleep in adolescent rats did not decrease after sleep restriction, suggesting that they were important for maintaining sleep homeostasis demonstrated as they recovered as much as possible.
Compared with the 24-h BL, adult rats showed decreased conversions in 20-h recovery period from NREM (S) to wakefulness (W) by 37%, 30%, and 32%, and from W to S by 27%, 26%, and 25% on sleep deprivation day 1, 5, and 7, respectively (Figure 4J: P < 0.01). Similarly, adolescent rats showed decreased transitions from S to W by 48% and from W to S by 33% on sleep deprivation day 7 compared with the 24-h BL (Figure 4K: P < 0.05). The episode transitions from S to R and R to W could convert back to the 24-h BL after RSR in both adult and adolescent rats. The results suggested that after repeated partial sleep deprivation, the increased rebound of NREM sleep amount compared with the 20-h BL may be related to the reduction of transitions from S to W, whereas the reduced transitions from W to S, which implied that it was difficult for the rats in the recovery period to change to NREM once awakened, may result in much less NREM time than 24-h BL level.
Effects of Sleep Restriction on Learning Performance in Morris WMT
The adolescent rats after 7 consecutive days of partial sleep deprivation spent more time finding the hidden platform underneath the water than sleep-undisturbed control rats, although this difference was not statistically significant. Total distance traveled by the sleep-deprived adolescent rats was significantly longer than that of control rats (T(13) = 2.39, P = 0.033). By contrast, sleep-deprived adult rats did not exhibit longer escape latency and swimming path length than their counterparts with normal sleep. To examine the influence of swimming ability on learning performance, mean speed in each group was calculated and no remarkable difference was found between groups, indicating that swimming speed was not a possible factor contributing to the deficit in learning ability of sleep-restricted adolescent rats in the WMT (Figure 5A-G).
Figure 5.
Effects of chronic sleep deprivation (SD) on learning and memory of adolescent and adult rats in Morris water maze test (WMT). (A) The latency of rats swimming to find the platform. (B) The distance to reach the platform. Open and filled bars show the profiles before 1st sleep deprivation and the effects after different days of sleep deprivation, respectively. On day 1, the first training day, without sleep deprivation, there were no differences in performance between different groups. Similar results showed on day 2 (sleep deprivation day 1) and day 3 (sleep deprivation day 2), while on day 8 (sleep deprivation day 7), a significant difference was observed between the groups of sleep deprivation for 4 h and control in adolescents. (C-F) Swimming tracks of adolescent (F) and adult (D) rats after sleep deprivation for 7 days versus their control (E, C) rats in WMT. The small circle represents the platform that was submerged below the surface of the water. (G) Swimming speed of rats in WMT. There was no difference between the sleep deprivation and that of the control groups. (H-J) Memory was assessed on a probe trial in the absence of the platform on the 8th day, 7 days after sleep deprivation. The sleep-restricted adolescent rats tended to generate shorter path length and spend less time in the target quadrant, and made fewer platform crossings than rats in the control group. However, there were no significant differences between the sleep-restricted adult rats and the control rats in the probe trial. Values are mean ± standard error (n = 6-9). *P < 0.05, as assessed by t test.
Regarding the retention performance, rats were given a 60-sec probe trial in the absence of the platform on the 8th day, 7 days after sleep deprivation and 4 days after the nearest prior training session. The sleep-restricted adolescent rats tended to swim a shorter path length and spent less time in the target quadrant, and made significantly fewer platform crossings relative to the control group (T(12) = 2.52, P = 0.027), indicating that RSR impaired spatial memory in adolescent rats. However, there were no significant differences between the sleep-restricted adult rats and the control rats in terms of the percentage of path length or time in the target quadrant and the number of platform crossings for reference memory (Figure 5H-J).
DISCUSSION
Teenagers are in a critical period of physical and mental development and maturation, but insufficient sleep is ubiquitous in this age group. Recognition and management of insufficient sleep is important for the health of adolescents. However, the sleep characteristics of adolescents and the effects of chronic partial sleep deprivation on cognition have not been reported. In the current study, we found there was no difference in the amount of TST, REM, and NREM sleep between adolescent and adult rats. This finding was inconsistent with previous reports in which human adolescents require more than 9 h of sleep per night, with some also requiring additional sleep during the day,14,40 or childhood is characterized by larger amounts of slow wave sleep and REM sleep.41 Although the amount of NREM sleep in adolescent rats in comparison with adult rats did not markedly increase, the pronounced SWA may play an important role in brain development. The decline of SWA with increasing age is consistent with the results in a human study in which EEG maturation over adolescence involves a decline in total power of all bands, particularly the slow wave bands,42 or the rat study in which the younger rats (age 3 mo) had higher delta power density during NREM sleep than the middle-aged (age 12 mo) and older rats (age 24 mo).34 Adolescent rats had fewer episodes of both NREM sleep and wakefulness, with prolonged episode durations, and had fewer conversions between NREM sleep and wakefulness. The above data indicate that the intensity and quality of sleep in adolescent rats were significantly better than that in adult rats.
There are 4 types of insomnia animal models that provide longer sleep deprivation: electrical stimulation (foot shock), single or multiple water platform techniques, forced locomotion, and the drug-induced method.15,43 These methods can deprive animals of sleep, cause excessive stress in rats, or result in incomplete sleep loss, and so have an unfavorable effect on experimental results. We used the method of gentle handling, which produced less stress,44 to keep rats awake for 4 h per day for 7 consecutive days. Through EEG monitoring, the rats had almost no NREM or REM sleep during the 4-h sleep deprivation period.
Our results in adult rats showed that on sleep deprivation day 5 and 7, NREM sleep increased during the 20-h recovery sleep period compared with the 20-h BL. However, the increase was inadequate as indicated by the total amount of NREM sleep, which was lower than 24-h BL on the experimental days. Similarly, the total amount of REM sleep also increased on sleep deprivation day 1, 5, and 7, but REM sleep rebounded adequately to reach the 24-h BL. The current result was in agreement with the study by Kim et al.,26 in which REM sleep in rats was found to rebound in 4 h after the 20-h sleep deprivation blocks on days 2-5. However, there are 3 main differences in this study from ours. First, according to the study by Kim et al., REM sleep could not increase to a normal level. Next, in response to the first 4-h sleep deprivation block on day 1, adult rats responded during the 20-h sleep opportunity with the highest REM sleep amount that was followed by a slight REM rebound in our experiment. In the study by Kim et al., REM rebound was found to increase with the long days of repeated sleep restriction. Then Kim et al. found that the amount of NREM sleep did not significantly rebound after sleep restriction on day 1 to 5. In fact, NREM sleep even decreased on sleep deprivation day 4 and 5 compared with the corresponding 4-h BL level.26 These differences may be due to long-term sleep deprivation (20 h) and a short sleep recovery period (4 h) in their study. In our experiment, the amount of sleep in adolescent rats was investigated. We observed the same responses in adolescent rats as in adult rats, in which REM sleep could rebound to 24 BL; however, the total amount of NREM sleep was lower than 24 BL on all sleep deprivation days.
On repeated partial sleep deprivation day 1, 5, and 7, SWA in both adult and adolescent rats was significantly elevated immediately after the 4-h sleep deprivation. The substantial enhancement of SWA was sustained 5-7 h and 3-5 h in adolescent and adult rats, respectively, and SWA returned to baseline levels following the remarkable elevation. When examined according to age, SWA in adolescent rats increased by 33%, and adults 47% above BL after 7 days of RSR. The data showed the same trend as one study in which old rats exhibited the highest delta power density from lower BL compared with young or middle-aged rats during the recovery period after 48 h of sleep deprivation.34
The human sleep-wake cycle is controlled by circadian rhythm and homeostasis. In this 2-process model of sleep regulation, SWA was used as a direct indication of sleep debt.45 The current study consisted 4 h of sleep deprivation per day and continued for 7 days, the total amount of REM sleep recovered to reach BL in all experimental days in both adult and adolescent rats. As for the response of NREM sleep to RSR, although the overall amount of NREM sleep was lower on all sleep deprivation days, this presumed debt was compensated for by increased sleep intensity reflected in higher EEG SWA. SWA in both adolescent and adult rats was elevated immediately after the 4-h sleep deprivation and then returned to BL, and on the other hand, the high SWA response was similar during all the RSR days. Furthermore, sleep episodes lasting for varying durations changed between adult and adolescent rats after RSR.
After 7 consecutive days of sleep restriction in the adolescent rats, although there was no accumulation of REM and NREM sleep loss, the spatial learning deficit was observed. Because the rats were deprived of both NREM and REM sleep during the sleep deprivation period of 4 h immediately after training, the decreased ability of spatial learning and memory in adolescent rats may be due to the lack of sleep during this critical time window to disrupt the memory consolidation, which could not be reversed by REM and NREM sleep rebound later on. However, the adult rats after 7 days of short 4-h daily sleep deprivation showed no conspicuous damage of learning and memory ability, suggesting that sleep in adolescent rats, whose better sleep quality was reflected by higher power density, longer duration, and less transition of episodes, may play a greater role in spatial memory processing.
In the current study, the sleep-restricted adolescent rats showed a tendency of longer escape latency to the hidden platform in the place navigation training and shorter distance and less time in the target quadrant in the probe trial after 7 consecutive days of RSR, however, such differences did not reach statistical significance compared with the control rats. The significant difference occurred in the distance the sleep-restricted adolescent rats swam before reaching the platform and platform crossing numbers after 7 days of repeated sleep deprivation. The performance of adolescent rats instead of adult rats, given repeated 7-day and short daily 4-h sleep deprivation during the sleep critical window of memory consolidation immediately after training sessions in the Morris WMT showed relatively mild impairment. The result agrees with the work of Walsh et al.,8 which showed only subtle effects of short term (6 h) REM sleep deprivation after training in Morris WMT in initial probe trials, not training trials. The final acquisition training and the probe trial occurred on the 8th day of the current experiment, 4 days after the nearest prior training sessions, might contribute to the slight deficit in spatial learning and memory retention ability in sleep-restricted adolescent rats.
Chronic partial sleep deprivation immediately after training in the current study in adult rats showed no significant impairment of spatial learning and memory ability in the WMT compared with control rats. However, Hagewoud et al.44 recently proposed that sleep deprivation for 12 h during the normal resting phase prior to learning impaired spatial working memory in a novel arm recognition task by reducing hippocampal glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor phosphorylation.44 Six hours of sleep deprivation immediately following the initial footshock impaired the consolidation of contextual fear memory by attenuating the hypothalamic-pituitary-adrenal (HPA) axis response and reducing 3′, 5′-cyclic AMP response-element binding protein (CREB) phosphorylation in the hippocampus and amygdala.9 Different kinds of behavior assessment, different beginning time of sleep deprivation relative to learning trials, and different duration of sleep deprivation may account for the difference in performance ability.46 In the current study, the effects of chronic sleep restriction on other physiologic and neurobiologic functions cannot be ruled out. Adult rats that were sleep-restricted for 1 wk in slowly rotating drums and allowed to have 4 h of sleep per day showed depression-like changes.47 Prolonged sleep disruption affected the basal rates of cell proliferation and neurogenesis,48 caused hyperglycemia, and decreased insulin levels in rats.49 Adolescence is a time period when the brain is continuing to develop to maturity and has unique neuropsychiatric vulnerabilities and tendencies toward risk behavior.12 Based on the previously discussed considerations, it is thought that chronic sleep restriction in both adults and adolescents has negative consequence on neuropsychiatric and/or cognitive performance. Educational programs and policies may help to improve teenagers' sleep patterns through informing adolescents, parents, and teachers about proper sleep hygiene and the risks of poor sleep habits.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Su-Rong Yang and Hui Sun contributed equally to this work. This work was performed at the Department of Pharmacology, Shanghai Medical College, Fudan University, Shanghai 200032, China. This study was supported in part by grants from The Ministry of Science and Technology of the People's Republic of China (2009ZX09303-006, 2009CB5220004), National Natural Science Foundation of China (31070957), Science and Technology Commission of Shanghai Municipality (11ZR1402000), Shanghai Leading Academic Discipline Project (B119), Shanghai Municipal Health Bureau General Program (2010234), Institutes of Brain Science of Fudan University. We are grateful to Prof. Ping Zheng of State Key Laboratory of Medical Neurobiology, Shanghai Medical College, Fudan University for providing excellent technical support.
Footnotes
A commentary on this article appears in this issue on page 745.
SUPPLEMENTAL MATERIAL
Table S1.
Monoamine and metabolite levels (nanograms per gram of protein) in brains of both adolescent and adult rats after 7 days of repeated sleep restriction
REFERENCES
- 1.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 2.Mueller AD, Pollock MS, Lieblich SE, Epp JR, Galea LA, Mistlberger RE. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1693–1703. doi: 10.1152/ajpregu.00858.2007. [DOI] [PubMed] [Google Scholar]
- 3.Boonstra TW, Stins JF, Daffertshofer A, Beek PJ. Effects of sleep deprivation on neural functioning: an integrative review. Cell Mol Life Sci. 2007;64:934–46. doi: 10.1007/s00018-007-6457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–3. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 5.Featherby T, van den Buuse M, Lubman DI, Lawrence AJ. Persistent downregulation of hippocampal CREB mRNA parallels a Y-maze deficit in adolescent rats following semi-chronic amphetamine administration. Br J Pharmacol. 2008;154:417–28. doi: 10.1038/bjp.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–43. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hennevin E, Huetz C, Edeline JM. Neural representations during sleep: from sensory processing to memory traces. Neurobiol Learn Mem. 2007;87:416–40. doi: 10.1016/j.nlm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Walsh CM, Booth V, Poe GR. Spatial and reversal learning in the Morris water maze are largely resistant to six hours of REM sleep deprivation following training. Learn Mem. 2011;18:422–34. doi: 10.1101/lm.2099011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagewoud R, Bultsma LJ, Barf RP, Koolhaas JM, Meerlo P. Sleep deprivation impairs contextual fear conditioning and attenuates subsequent behavioural, endocrine and neuronal responses. J Sleep Res. 2011;20:259–66. doi: 10.1111/j.1365-2869.2010.00895.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Der Werf YD, Van Der Helm E, Schoonheim MM, Ridderikhoff A, Van Someren EJ. Learning by observation requires an early sleep window. Proc Natl Acad Sci U S A. 2009;106:18926–30. doi: 10.1073/pnas.0901320106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loessl B, Valerius G, Kopasz M, Hornyak M, Riemann D, Voderholzer U. Are adolescents chronically sleep-deprived? An investigation of sleep habits of adolescents in the Southwest of Germany. Child Care Health Dev. 2008;34:549–56. doi: 10.1111/j.1365-2214.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- 12.Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav Neurosci. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson KP. Adolescent sleep patterns: biological, social, and psychological influences. J Am Acad Child Adolesc Psychiatry. 2004;43:374–5. [Google Scholar]
- 14.Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- 15.Longordo F, Kopp C, Luthi A. Consequences of sleep deprivation on neurotransmitter receptor expression and function. Eur J Neurosci. 2009;29:1810–19. doi: 10.1111/j.1460-9568.2009.06719.x. [DOI] [PubMed] [Google Scholar]
- 16.Vecsey CG, Baillie GS, Jaganath D, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–5. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casement MD, Broussard JL, Mullington JM, Press DZ. The contribution of sleep to improvements in working memory scanning speed: a study of prolonged sleep restriction. Biol Psychol. 2006;72:208–12. doi: 10.1016/j.biopsycho.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang F, VanDyke RD, Zhang J, Li F, Gozal D, Shen X. Effect of chronic sleep restriction on sleepiness and working memory in adolescents and young adults. J Clin Exp Neuropsychol. 2011;33:892–900. doi: 10.1080/13803395.2011.570252. [DOI] [PubMed] [Google Scholar]
- 19.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 20.Tobler I, Borbely AA. The effect of 3-h and 6-h sleep deprivation on sleep and EEG spectra of the rat. Behav Brain Res. 1990;36:73–8. doi: 10.1016/0166-4328(90)90161-7. [DOI] [PubMed] [Google Scholar]
- 21.Schwierin B, Borbely AA, Tobler I. Prolonged effects of 24-h total sleep deprivation on sleep and sleep EEG in the rat. Neurosci Lett. 1999;261:61–4. doi: 10.1016/s0304-3940(98)01006-4. [DOI] [PubMed] [Google Scholar]
- 22.Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009;32:217–22. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado RB, Suchecki D, Tufik S. Sleep homeostasis in rats assessed by a long-term intermittent paradoxical sleep deprivation protocol. Behav Brain Res. 2005;160:356–64. doi: 10.1016/j.bbr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Machado RB, Tufik S, Suchecki D. Modulation of sleep homeostasis by corticotropin releasing hormone in REM sleep-deprived rats. Int J Endocrinol. 2010;2010:1–12. doi: 10.1155/2010/326151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hairston IS, Little MT, Scanlon MD, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697–702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward CP, McCoy JG, McKenna JT, Connolly NP, McCarley RW, Strecker RE. Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res. 2009;1294:128–37. doi: 10.1016/j.brainres.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tartar JL, McKenna JT, Ward CP, McCarley RW, Strecker RE, Brown RE. Sleep fragmentation reduces hippocampal CA1 pyramidal cell excitability and response to adenosine. Neurosci Lett. 2010;469:1–5. doi: 10.1016/j.neulet.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 30.Kohtoh S, Taguchi Y, Matsumoto N, Wada M, Huang ZL, Urade Y. Algorithm for sleep scoring in experimental animals based on fast Fourier transform power spectrum analysis of the electroencephalogram. Sleep Biol Rhythms. 2008;6:163–71. [Google Scholar]
- 31.Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2008;105:19992–7. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang ZL, Qu WM, Eguchi N, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–9. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 33.Huang ZL, Mochizuki T, Qu WM, et al. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc Natl Acad Sci U S A. 2006;103:4687–92. doi: 10.1073/pnas.0600451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendelson WB, Bergmann BM. EEG delta power during sleep in young and old rats. Neurobiol Aging. 1999;20:669–73. doi: 10.1016/s0197-4580(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 35.Trachsel L, Tobler I, Borbely AA. Electroencephalogram analysis of non-rapid eye movement sleep in rats. Am J Physiol. 1988;255:R27–37. doi: 10.1152/ajpregu.1988.255.1.R27. [DOI] [PubMed] [Google Scholar]
- 36.Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010;107:15939–44. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toppila J, Alanko L, Asikainen M, Tobler I, Stenberg D, Porkka-Heiskanen T. Sleep deprivation increases somatostatin and growth hormone-releasing hormone messenger RNA in the rat hypothalamus. J Sleep Res. 1997;6:171–8. doi: 10.1046/j.1365-2869.1997.00049.x. [DOI] [PubMed] [Google Scholar]
- 38.Tobler I, Deboer T, Fischer M. Sleep and sleep regulation in normal and prion protein-deficient mice. J Neurosci. 1997;17:1869–79. doi: 10.1523/JNEUROSCI.17-05-01869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore M, Meltzer LJ. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev. 2008;9:114–21. doi: 10.1016/j.prrv.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Kopasz M, Loessl B, Hornyak M, et al. Sleep and memory in healthy children and adolescents: a critical review. Sleep Med Rev. 2010;14:167–77. doi: 10.1016/j.smrv.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: a review. Brain Cogn. 2010;72:86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep. 2010;33:621–30. doi: 10.1093/sleep/33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2010;19:280–8. doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 45.Zavada A, Strijkstra AM, Boerema AS, Daan S, Beersma DG. Evidence for differential human slow-wave activity regulation across the brain. J Sleep Res. 2009;18:3–10. doi: 10.1111/j.1365-2869.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- 46.Poe GR, Walsh CM, Bjorness TE. Both duration and timing of sleep are important to memory consolidation. Sleep. 2010;33:1277–8. doi: 10.1093/sleep/33.10.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novati A, Roman V, Cetin T, et al. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31:1579–85. doi: 10.1093/sleep/31.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev. 2009;13:187–94. doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barf RP, Meerlo P, Scheurink AJ. Chronic sleep disturbance impairs glucose homeostasis in rats. Int J Endocrinol. 2010;2010:1–6. doi: 10.1155/2010/819414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Monoamine and metabolite levels (nanograms per gram of protein) in brains of both adolescent and adult rats after 7 days of repeated sleep restriction