Abstract
Study Objectives:
GABA is increasingly recognized as an important neurotransmitter for the initiation and maintenance of sleep. We sought to measure cortical GABA content through proton magnetic resonance spectroscopy (MRS) in persons with and without primary insomnia, and relate brain GABA levels to polysomnographic sleep measures.
Design:
Two-group comparison study.
Setting:
Outpatient study at a university research clinic.
Participants:
Non-medicated persons with primary insomnia (N = 16) and no sleep complaints (N = 17).
Interventions:
Participants kept sleep diaries and a regular time-in-bed schedule for 9 days, culminating in 2 consecutive nights of ambulatory polysomnography and a single proton MRS session. The main outcome measure was occipital GABA/creatine ratios; secondary measures included sleep measurements and relationship between polysomnographically measured time awake after sleep onset and occipital GABA content.
Measurements and Results:
The primary insomnia group was distinguished from persons with no sleep complaints on self-reported and polysomnographically measured sleep. The two groups did not differ in age, sex, body mass index, habitual bed- and wake-times, napping, use of caffeine, or use of cigarettes. Mean occipital GABA level was 12% higher in persons with insomnia than in persons without sleep complaints (P < 0.05). In both groups, GABA levels correlated negatively with polysomnographically measured time awake after sleep onset (P < 0.05).
Conclusions:
Increased GABA levels in persons with insomnia may reflect an allostatic response to chronic hyperarousal. The preserved, negative relationship between GABA and time awake after sleep onset supports this notion, indicating that the possible allostatic response is adaptive.
Citation:
Morgan PT; Pace-Schott EF; Mason GF; Forselius E; Fasula M; Valentine GW; Sanacora G. Cortical GABA levels in primary insomnia. SLEEP 2012;35(6):807-814.
Keywords: Insomnia, GABA, hyperarousal, magnetic resonance spectroscopy, polysomnography
INTRODUCTION
Insomnia is a widespread health problem, affecting between 10% and 30% of the population, depending on how it is defined.1,3–4 Insomnia is most often chronic, with over 80% of persons with insomnia reporting more than one year of symptoms, and over 40% reporting over 10 years of symptoms.5 The consequences of insomnia are varied and considerable. Insomnia is associated with twice or greater the odds of having another medical problem like heart disease, high blood pressure, neurologic conditions, or breathing problems,6 and is very strongly associated with mental health problems including depression and anxiety.7 Health-related quality of life is poor in persons with insomnia who rate such quality as worse than persons with congestive heart failure or depression,8 and accident rates are higher9 and productivity lower10 in persons with insomnia. Despite the personal and societal consequences of insomnia, the pathophysiology of insomnia is not yet well understood.
Available evidence for the pathophysiology of insomnia points to a disorder of hyperarousal,11,12 with evidence for increased brain metabolism, increased sympathetic activity, increased high-frequency EEG activation, and hormonal changes consistent with hyperarousal.13–19 This view of insomnia is consistent with the role of arousal promoting neurotransmitters in the regulation of sleep and wakefulness. Histaminergic, noradrenergic, serotonergic, and cholinergic neurotransmission are all highly active during wakefulness, and this activity drops precipitously with the normal transition to sleep.20,21 Furthermore, pharmacologic agents that inhibit such arousal promoting neurotransmission are often used to promote sleep. Not directly addressed in the hyperarousal model of insomnia, however, is the role of the inhibitory neurotransmitter GABA.
GABA is strongly implicated in the regulation of sleep,22,23 with GABA activity emanating from the ventrolateral preoptic nucleus of the hypothalamus dramatically inhibiting wakefulness-associated neurotransmitter activity.21 In addition, extrasynaptic inhibition by GABA may account for a substantial proportion of GABAergic inhibition in the CNS,24 with glial sources likely prominent.25 GABA promoting pharmacologic agents are widely recognized as sleep inducing, and several GABA agents are FDA approved for the treatment of insomnia.26 Hence, examining a potential role of GABA in insomnia may be essential to understanding the pathophysiology of insomnia, and measuring GABA content in persons with primary insomnia is a first step in that direction.
Proton magnetic resonance spectroscopy (1H-MRS) offers a noninvasive method of measuring total tissue GABA content in the brain. Several prior studies employing similar 1H-MRS methods have previously demonstrated abnormal occipital cortex GABA content in a variety of neuropsychiatric disorders including depression,27–31 panic disorder,32 and schizophrenia,33 suggesting that occipital GABA measurement can be useful in studying other disorders, including insomnia. Recent evidence further suggests that the total occipital tissue GABA content determined by 1H-MRS is strongly correlated with regional brain activity as measured by MEG and fMRI in association with both visual function and motor activity.34–36 Moreover, the abnormal GABA content in the occipital cortex has now been shown to be associated with functional changes in visual processing in both MDD and schizophrenia.33,37 Hence, abnormal occipital GABA content appears to reflect functional differences with potential pathophysiological relevance for a variety of neuropsychiatric disorders.
A 1H-MRS study averaging GABA content from several brain regions (i.e., not specifically occipital cortex) found GABA levels reduced by approximately 30% in primary insomnia subjects.38 In the present study, we sought to specifically examine the relationship between occipital cortex GABA content and primary insomnia. We hypothesized that occipital cortex GABA content in primary insomnia would differ from healthy persons, and that the direction of that difference would reflect the pathophysiological significance of GABA in insomnia.
METHODS
Participants
One hundred individuals who responded to flyers and internet postings were screened for participation in this study between 2007-2010. Thirty-three individuals between 25 and 55 years old met inclusion criteria and agreed to participate in this protocol. All participants provided written, informed consent as approved by the Yale University Institutional Review Board. No participant had a history of major medical or neurological illness and none exhibited signs or symptoms of current medical or neurological illness as determined by physical examination and screening laboratory testing. Potential participants were evaluated by unstructured psychiatric and medical interviews and were excluded if they reported a lifetime history of substance dependence, reported substance abuse in the past 6 months, had a positive urine test for drugs at screening (THC, opiates, cocaine, amphetamine, methamphetamine, benzodiazepines, barbiturates, and PCP), or drank more than the caffeine-equivalent of 3 cups of coffee per day (or used caffeine after 19:00 more than once every 2 weeks). Use of neuroactive prescription medication (including but not limited to sedative/hypnotics, antidepressants, anxiolytics, antipsychotics, opiate pain medication, muscle relaxants, and stimulants) in the past 3 months was also exclusionary. Potential participants were excluded for a previous diagnosis of or historical evidence for sleep disordered breathing, restless leg syndrome, periodic limb movement disorder, sleep paralysis, nocturia, enuresis, narcolepsy, REM behavior disorder, unresolved chronic pain, or gastroesophageal reflux disease. In addition to being excluded for a personal history of psychiatric disorders, potential participants were also excluded if a first-degree relative was suspected of having depression.
The control group participants (N = 17) were included for an Insomnia Severity Index (ISI) score < 4. The insomnia group participants (N = 16) met DSM-IV criteria for primary insomnia at time of screening with onset of symptoms ≥ 1 year prior to participation and were included for an ISI score ≥ 15. All participants reported a typical bedtime of between 20:00 and 01:00. All participants agreed to refrain from all psychoactive substances (including alcohol) for 2 weeks prior to undergoing MRS including the day of the MRS. Exceptions to this were that continued caffeine use equivalent to ≤ 3 cups of coffee/day was permitted during the 2 weeks prior to the MRS (so long as no caffeine was used after 19:00), and daily nicotine use could be continued. Baseline assessments included demographic information, BMI, and Pittsburgh Sleep Quality Index.39
Lead-in Period
For one week prior to and during the 2 nights of polysomnography, all participants were instructed to maintain a regular schedule for time in bed (within a 1-h window of their typical bedtime) and to assure that a total ≥ 8 h in bed was possible. Participants maintained a daily log of sleep, waking activities, and psychoactive substance use during this time using the Evening-Morning Sleep Questionnaire.40 This questionnaire assesses sleep and wake times, perceived sleep latency and time awake after sleep onset, perceived sleep quality (on a 0- to 100-mm visual analog scale), and compliance with psychoactive substance use restrictions. No participant reported using any psychoactive substance other than caffeine or nicotine during these 9 days of the study.
Polysomnography (PSG)
Each of the 33 participants was set up for ambulatory PSG on 2 consecutive nights, immediately following the one-week lead in period. Set-up occurred in the laboratory, following which participants went home to sleep at their regular bedtime. PSG recording of C3, C4, ROC, LOC, chin EMG, and EKG was performed using the TEMEC ambulatory sleep monitor, an 8-channel universal recording device. The device is about 2 × 3 × 5 inches and is placed inside a pouch that is worn on a belt, from which the electrode wires emanate. Participants were shown how to dress and undress with the equipment attached. The PSG was turned on in the laboratory, and participants were required to press a button to mark the time when they lay down to sleep and at the time of final awakening.
Of the 66 nights on which PSG data was collected, data from 56 nights (85%) were scored and included in the analysis. Reasons for data not being included in the analysis were related to data quality (e.g., recording stopped prematurely due to battery failure or participant intervention, 6 nights; electrodes fell off or were removed, 1 night) or external issues (e.g., participants reported that their sleep was disturbed by an external factor like a phone call or housemate, 3 nights). All participants had ≥ 1 night of scored PSG data. There were 6 missing data nights in the healthy group and 4 missing data nights in the insomnia group.
All sleep records were visually scored for sleep stage in 30-sec epochs according to Rechtsaffen and Kales41 by the same registered PSG technologist, who was blinded to the study design and group membership. PSG sleep data including time in bed, total sleep time, time in each sleep stage, time awake, and sleep onset latency from the first and second nights were compared within each group. There were no statistically significant differences between the first and second night in either the healthy group or the insomnia group (all uncorrected P > 0.4). For all further analysis, the average of the 2 nights of PSG data was used.
MRS
On the second night of PSG recording and prior to being set up for PSG, participants arrived at the Yale Magnetic Resonance Research Center for a 1H-MRS study that was scheduled to occur approximately 2-3 h before the participant's usual bedtime (mean MR midpoint time was 20:10 ± 1:10 [SEM] in the control group and 20:00 ± 1:20 in the insomnia group).
1H MRS studies were performed with a 4T magnet (Oxford Magnetic Technology, Oxford, England) and a Bruker spectrometer (Bruker Instruments, Billerica, MA). The head of the subject was comfortably secured to a platform with the region of interest apposed to an 8-cm distributed capacitance surface coil tuned to 170 MHz. The patients were asked to remain awake for the duration of the scanning session and a member of the research team remained in the magnet room at all times to frequently remind the subjects of this request. A T1- weighted multi-slice MRI was obtained for anatomical localization. From the image, a 3 × 1.5 × 3 cm region of interest was selected centered on the midline of the occipital cortex, 1.5 cm deep from the dura. Automated first- and second-order shimming was applied in the volume of interest.42
Detection of the 3.0 ppm GABA C4 resonance was performed for 20 min using J-editing.43 Briefly, pairs of subspectra were obtained, one with a frequency selective inversion pulse applied to the GABA C3 resonance, and one without the inversion pulse. Subspectra were acquired in interleaved fashion, toggling between individual inverted and uninverted acquisitions, in 2-min blocks run for 20 min to yield 10 pairs of subspectra. The subspectra were subtracted to obtain difference spectra that contained a combined measure of GABA and the GABA-containing dipeptide homocarnosine. Localization was achieved with selective excitation, 3-dimensional, image-selected, in vivo spectroscopy (ISIS), outer volume suppression, and a surface spoiler coil. The spectral acquisition parameters were as follows: repetition time, 2.5 sec; echo time, 68 ms; sweep width, 15,000 Hz; and acquisition time, 510 ms. The free induction decay was zero-filled to 32 K, processed with −2-Hz Lorentzian/6 Hz Gaussian broadening, and Fourier-transformed. The complete procedure took 1-1.5 hours.
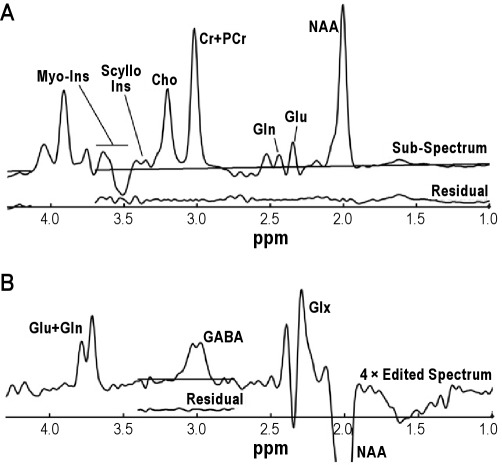
The subspectrum obtained without the editing pulse was fitted simultaneously with the J-edited difference spectrum of GABA, using a basis set of metabolite spectra measured with the J-editing acquisition sequence (Figure 1). The metabolites fitted were aspartate, GABA, glutamate, glutamine, NAA, NAAG, creatine, phosphocreatine, myoinositol, choline, phosphorylcholine, glycerophosphorylcholine, and scylloinositol. Because the spectral resolution in vivo is limited, some metabolites could not be distinguished from structurally similar chemicals. Therefore, the results for NAA and NAAG were summed and reported as NAA; creatine and phosphocreatine were combined and recorded as creatine; and the 3 choline-containing compounds were combined and recorded as choline. As part of the implementation of the editing method, coedited macromolecular contamination was evaluated in the GABA spectrum, using 2 methods: metabolite nulling44–46 and frequency switching symmetrically about the coupled macromolecular resonance47; both cases showed no macromolecular contamination of the resonance. Therefore, the basis set for fitting did not include a macromolecular signal. Aspartate was barely visible at TE = 68 ms and so was poorly determined that it was not considered in the statistical analyses. Uncertainties of individual measurements were determined using a Monte Carlo analysis, in which the least-squares spectral fits were treated with random Gaussian noise whose standard deviation was equal to that of the raw data and refitted, using 20 repetitions to estimate the standard deviations of the uncertainty for each metabolite measure. For each metabolite, a threshold for rejection was set at twice the average noise-based standard deviation of the respective metabolite. GABA levels whose uncertainties were > 11%, glutamine levels whose uncertainties were > 20%, and glutamate levels whose uncertainties were > 16% were not included in subsequent analysis.
Figure 1.
Typical spectra obtained from a primary insomnia subject with baselines superimposed and fitting residuals immediately beneath each spectrum. (A) Subspectrum obtained with the frequency-selective editing pulse applied at 1.31 ppm. The subspectra were used to determine levels of myo-inositol (Myo-Ins), total choline (Cho), total creatine (Cr+PCr), glutamine (Gln), glutamate (Glu), and N-acetylaspartate (NAA). Because of variable impact of the CHESS water suppression on the region beyond Myo-Ins, the metabolite resonances from 3.65 to 4.2 ppm were not fitted for the subspectra. (B) Edited spectrum of the difference of spectra obtained with the editing pulse applied at 1.31 ppm (shown in A) and at 1.89 ppm (not shown). The edited spectrum is displayed at four times the vertical scale so that the small GABA resonance is more easily seen. Because of power-dependent variations in the bandwidth of the editing pulse applied at 1.89 ppm, the Glu, Gln, and NAA resonances in the difference spectrum varied from subject to subject and were not used for quantification, so only the region around 3 ppm was fitted. Because of similar power-dependent variations of the water suppression pulses, the resonances around 4 ppm were also not fitted.
Statistical Method
The GABA/Cr ratio was the primary outcome measure of this study. GABA/Cr distributions from each experimental group were found to have no evidence of non-normality by the Lilliefors test and were compared with the 2-sample, 2-tailed t-test. The t-test was also used for between-group comparisons of baseline data and secondary outcomes. PSG measured time spent awake after sleep onset (WASO) and PSQI were selected a priori for comparison to GABA/Cr. Linear correlation was used to assess these relationships. The Fisher transformation of the Pearson correlation coefficients was used to assess for differences between coefficients and confidence intervals for those differences. Post hoc correlation analyses were similarly performed for self-reported time awake after sleep onset and polysomnographically measured total sleep time in relation to GABA/Cr.
RESULTS
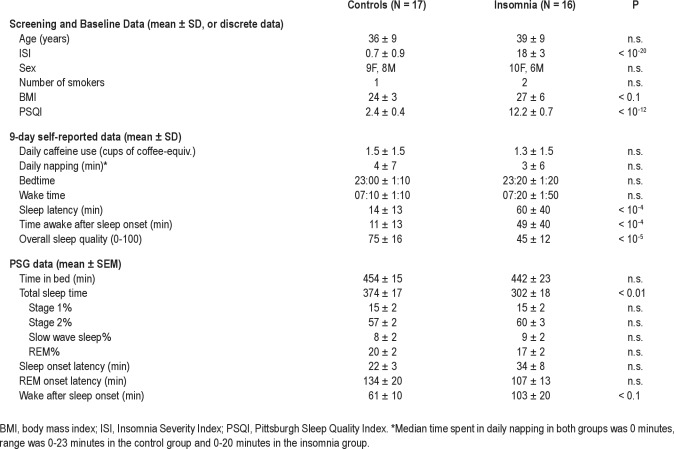
Screening and baseline data, 9-day lead-in data, and PSG data are shown in Table 1. The control and insomnia group participants were well matched in age and sex and had similar numbers of nicotine users. Body mass index was nonsignificantly higher in the insomnia group (P < 0.1). The Insomnia Severity Index score and PSQI score in the insomnia group were significantly higher than in the control group (P < 10−20 and P < 10−12, respectively).
Table 1.
During the 9-day lead-in period, both groups reported similar amounts of caffeine use and time spent napping, and similar bedtimes and wake times. Self-reported sleep latency was significantly higher in the insomnia group (P < 10−4), as was self-reported time awake after sleep onset (P < 10−4). Self-reported overall sleep quality was significantly greater in the control group (P < 10−5).
PSG measured total sleep time was 72 min longer in the control group than in the insomnia group (P < 0.01). Time awake after sleep onset was nonsignificantly longer (∼40 min) in the insomnia group. There were no differences between the 2 groups in time spent in bed, percent of time spent in each sleep stage, latency to sleep onset, or latency to REM sleep.
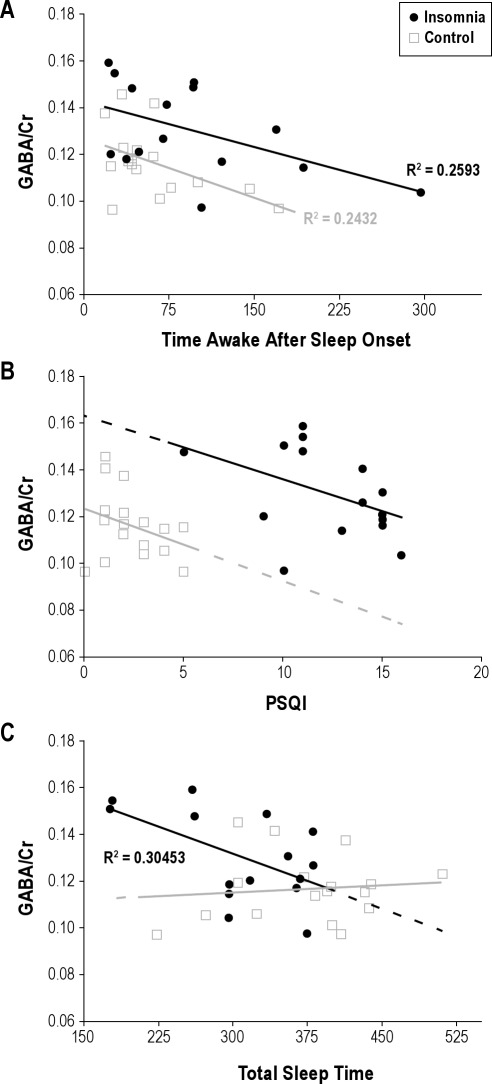
The mean occipital GABA/Cr ratio was significantly higher in the insomnia group than in the control group (0.130 ± 0.005 vs. 0.116 ± 0.004; P < 0.05). GABA/Cr ratios were compared to PSG-measured WASO and baseline PSQI score in each group. GABA/Cr ratio and time awake after sleep onset were correlated negatively in both the insomnia and control groups (P < 0.05; Figure 2A). GABA/Cr ratio and PSQI were correlated negatively but nonsignificantly in both groups with distinct intercepts (Figure 2B). Fisher transformed correlation coefficients were not different between groups for GABA/Cr versus WASO (Rdifference = 0.021, 90% CI = ± 0.561), nor for GABA/Cr versus PSQI (Rdifference = 0.13, 90% CI = ± 0.561).
Figure 2.
(A) GABA/Cr is negatively correlated to time awake after sleep onset in persons with primary insomnia (linear correlation, P < 0.05) and in controls (P < 0.05). There is no difference in R between groups (Rdiff = 0.021 ± 0.561 [90% confidence interval]). Mean GABA/Cr is higher in persons with insomnia (P < 0.05). (B) Relationship between GABA/Cr and PSQI in the insomnia and control groups. A trend toward a significant correlation was observed in the insomnia group (R = 0.19, P < 0.1). The projected y-intercepts are non-overlapping at confidence intervals of ∼90% (0.163 ± 0.03 [90% CI] in the insomnia group and 0.123 ± 0.01 [90% CI] in the control group). (C) Shorter total sleep time is correlated with higher GABA/Cr in persons with primary insomnia (P < 0.05) but not in controls.
In post hoc analyses, 9-day, mean, self-reported wake time after sleep onset was negatively correlated with GABA/Cr in the insomnia group (R = −0.60, P < 0.05) but not in the control group (R ≈ 0), where mean self-reported time awake after sleep onset was low and varied little (11 min ± 14 min [SD]). Polysomnographically recorded TST was negatively correlated with GABA/Cr in the insomnia group (R = −0.55, P < 0.05) but not in the control group (R = 0.01; Figure 2C). Polysomnographically recorded TST and WASO were negatively correlated in the control group (R = −0.67, P < 0.01) but were not correlated in the insomnia group (R = −0.06). Polysomnographically recorded sleep latency showed a trend correlation with GABA/Cr in the insomnia group (R = 0.44, P < 0.1) but not in the control group (R = −0.06). No significant differences were found between groups in measures of the other metabolites examined in the study: glutamate, glutamine, NAA, creatine, myoinositol, choline, or scylloinositol (P values ranged between 0.2 and 0.99 for all comparisons).
DISCUSSION
We report that occipital GABA is higher in persons with primary insomnia than in a well-matched control group. This finding contrasts with that reported in Winkelman et al., who reported lower whole brain GABA in persons with primary insomnia.38 However, similar to Winkelman et al., we found that GABA levels correlated inversely with time awake after sleep onset in persons with primary insomnia. In addition we report for the first time that this relationship is also present in a control group without primary insomnia.
The observed relationship between time awake after sleep onset and GABA levels is consistent with our understanding of the role of GABA in sleep and insomnia. GABA-containing neurons in the ventrolateral preoptic nucleus of the hypothalamus promote sleep through suppression of arousal systems.48 Pharmacologic promotion of synaptic GABA activity with benzodiazepines and benzodiazepine-like drugs tends to promote sleep and reduce symptoms of insomnia in humans.22 Similarly, tonic effects of gaboxadol on extrasynaptic GABA-A receptors in the thalamus may promote the thalamocortical rhythms of slow wave sleep.49 Hence, it is reasonable to hypothesize that symptoms of insomnia be associated with decreased GABA-related neurotransmission, and finding a negative relationship between GABA levels and time awake after sleep onset may reflect the apparent role of GABA in promoting sleep. We found such a relationship in persons with primary insomnia (both on PSG-measured and self-reported time awake after sleep onset) and in persons without sleep complaints. In addition, we observed this relationship with ambulatory PSG data, and it was previously observed in persons with primary insomnia in a sleep laboratory setting.38 That this same relationship occurred in persons with or without sleep complaints and in an ambulatory or sleep laboratory setting suggest that this relationship is robust.
However, the finding of increased occipital GABA in persons with primary insomnia may seem at first counterintuitive given this basic understanding of the role of GABA in sleep. Indeed, Winkelman et al.38 have previously reported that brain GABA levels were lower in persons with insomnia compared to controls. However, we suggest that increased GABA in persons with insomnia is more consistent with our understanding of the etiology of insomnia.
Current thinking describes insomnia as a disease of chronic hyperarousal.12 Increased metabolism, increased sympathetic activity, increased high-frequency EEG activation, and hormonal changes are some of the observed markers of hyperarousal in persons with insomnia.12 In addition, 18FDG PET studies have shown greater whole-brain glucose metabolism in persons with insomnia than controls during sleep, as well as lesser decline in glucose metabolism from waking to early NREM sleep in areas of the brain that promote arousal, specifically structures of the ascending reticular activating system.18 Lesser decline was also seen in the thalamus, insula, amygdala, hippocampus, anterior cingulate, and medial prefrontal cortex. Additionally, Nofzinger and colleagues have shown that, in insomniacs, wake time after sleep onset (WASO) in NREM correlates with glucose metabolism in the pons and anterior thalamocortical circuits, again indicative of a hyperaroused state.50 Hyperarousal in insomnia compared to controls is also associated with increased EEG spectral power at higher frequencies such as beta51 and gamma19 and decreases in delta.52 Moreover, these EEG spectral changes are clearly associated with subjective measures of stress,53,54 as well as with cardiovascular indices of sympathetic arousal.12,54,55
In addition to descriptive evidence of endogenously driven hyperarousal in insomnia, experimental interventions have illustrated that chronic, exogenously driven hyperarousal leads to the seemingly paradoxical state of insomnia wherein daytime fatigue is accompanied by decreased ability to sleep.56 Similarly, an animal model of insomnia has found both cellular and more grossly neurophysiological evidence of concurrent arousal and sleep system activation.57 In this understanding of insomnia, the sleep system has not acquiesced to arousal, but is competing against hyperarousal, possibly via mechanisms such as increased GABAergic inhibition of orexinergic58 and aminergic arousal systems.21,59 In such a case, GABA levels would be chronically increased as an allostatic response to increased activity in arousal systems or to intrinsic ineffectiveness in GABA signaling pathways. Extracellular GABA may be similarly increased across widespread areas of the brain in response to chronic hyperarousal to augment tonic neuronal inhibition via extransynaptic GABA receptors.
In addition to initiating and maintaining sleep, GABAergic tone in the hypothalamic paraventricular nucleus (PVN) is essential to moderating the HPA stress cascade,60 as well as outflow to brainstem and spinal cord (preganglionic) sympathetic effectors61 following stimulation of the PVN by its limbic and cortical afferents in response to stress-evoking stimuli. The increased sympathetic tone and cortisol levels in insomniacs12 suggest that the PVN is another crucial site at which maintenance of homeostasis might require an increased GABAergic response. In either case, this hypothesis posits that the sleep and/or arousal systems are relatively resistant to the effects of GABA, and hence the increase in GABA levels observed in the present study would be neuroadaptive. Importantly, this interpretation is nonetheless consistent with the observed relationship between GABA levels and time awake after sleep onset, and our understanding of the role of GABA in promoting sleep and moderating the stress response. In particular, within each experimental group GABA levels were correlated with less time awake after sleep onset. Hence the direction of effect of GABA signaling is not changed, but rather we suggest that a relative “GABA resistance” could cause an adaptive increase in GABA levels in persons with insomnia.
Supporting this notion, we found that PSG measured total sleep time correlated negatively with GABA/Cr in the insomnia group. This correlation suggests that insomnia severity, as measured by total sleep time, is associated with greater GABA/Cr, and is consistent with adaptive increases in GABA levels in persons with insomnia. The difference in direction in the relationship between the degree of insomnia as measured by TST and WASO and GABA/Cr is striking. Importantly, the correlation between TST and GABA/Cr was found only in persons with insomnia. Whereas the robust relationship between WASO and GABA/Cr may reflect how short-term variability in GABA/Cr generally affects sleep, the relationship between TST and GABA/Cr in persons with insomnia may reflect longer-term changes associated with chronic hyperarousability and insomnia. This interpretation may be surprising, as TST can show a high degree of short-term variability; but it is perhaps relevant that time in bed was not restricted in this study, so although WASO would not be improved by staying in bed longer, TST could be increased. Another possible interpretation is that TST and WASO could have increased together in the insomnia group, such that the negative correlation between WASO and GABA/Cr resulted in finding a similar negative correlation between TST and WASO. This was potentially possible as persons who had substantial WASO may have stayed in bed longer and ultimately achieved disproportionate increases in TST. We did not find such a correlation between WASO and TST in the insomnia group, however, suggesting that the latter explanation is unlikely.
The apparent difference in results between the present study and the prior work requires careful scrutiny. Several possible differences between this study and the study of Winkelman et al.38 may have contributed to the observed differences in GABA levels between persons with insomnia and healthy controls. Study differences include those related to the acquisition of MRS data, timing of study interventions, and study participants.
Differences in MRS acquisition between the studies may be important. This study used homonuclear editing using a specifically designed localized J-editing pulse sequence regionally localized the occipital cortex, while the study of Winkelman et al.38 employed 1H-CSI combined with J-resolved-MRS to determine GABA metabolite levels averaged from rectangular volumes that covered the basal ganglia, thalamus, and temporal, parietal, and occipital cortices. Thus, while both studies provided measures of GABA/Cr ratios, the methodological differences between the methods used in the two studies make it difficult to draw exact comparisons. In addition, MRS acquisition occurred at an unspecified time within approximately 2 weeks of polysomnography in the study of Winkelman et al., but occurred on the evening approximately 2-3 hours prior to bedtime of the second of two consecutive nights of polysomnography in the present study. It is possible that the evening MRS yielded different results than in the previous study, and as noted above, the sleep recordings themselves may have influenced GABA measurement.
The apparent outcome differences between the present work and that of Winkelman et al. may also be explained by differences in the populations studied. In the present study, the Insomnia Severity Index was used to distinguish the insomnia group (ISI ≥ 15) and the healthy control group (ISI < 4). Use of the ISI for grouping resulted in highly statistically significant differences in Pittsburgh Sleep Quality Index between groups, as well as several other measures of sleep quality. Nevertheless, there was a large range in WASO in the control group, with several participants exhibiting WASO over 60 minutes. Hence some control participants in the current study, despite not having significant sleep complaints as measured by the ISI, and reporting normal PSQI scores, exhibited some sleep impairment when measured polysomnographically. This finding leaves open the possibility that the PSG measurement, here performed on the night prior to and the night following MRS measurement, influenced participants' sleep and GABA/Cr levels. Notably, the difference between self-reported, 9-day average WASO and PSG measured WASO was similar in both groups, with PSG measured WASO about 50 minutes more. Another non-exclusive possibility is that the control group in Winkelman et al. may represent a group of more consistently good sleepers (compared to the control group studied here), who may therefore have unusually low WASO and high GABA/Cr levels.
The lack of PSG measurement of sleep in healthy controls in Winkelman et al. precludes this comparison between studies. PSG measures of sleep were notably different between the insomnia groups, however. For example, mean total sleep time in the insomnia group in the current study was 302 ± 72 minutes (SD), whereas in Winkelman et al. it was 354 ± 60 minutes at screening and 384 ± 70 minutes at the inpatient study point. Those sleep time durations were closer to that measured in the control group in the current study (374 ± 70 min). Although conditions differed between the two studies (i.e., sleep laboratory versus ambulatory PSG), these rather large differences suggest a possible difference in the studied populations between studies. In particular, it is possible that participants in the present study had more severe insomnia than their counterparts in Winkelman et al.38
The present results confirm a relationship between brain GABA levels and objectively measured sleep. Measurement of GABA levels was limited to the occipital cortex, however, so the observed relationship may not occur in other brain regions, and may be particular to the study groups studied, as the ISI and unstructured clinical interview were used to screen participants instead of a structured clinical interview. The results also introduce the possibility that the hyperarousal syndrome of primary insomnia may result in adaptive increases in GABA, or that inefficient GABA signaling with compensatory increases in cortical GABA may contribute to primary insomnia. Longitudinal studies beyond the scope of the present work would be necessary to test such possibilities. Further research testing these findings may contribute substantially to our understanding of the pathophysiology of primary insomnia, and promote more effective long-term treatments.
DISCLOSURE STATEMENT
This work was supported by grants from Sepracor. Dr. Sanacora has received consulting fees form Abbott, AstraZeneca, Bristol-Myers Squibb, Evotec, Eli Lilly & Co., Hoffman La-Roche and Johnson & Johnson, Novartis, and Novum Pharmaceuticals. He has also received additional grant support from AstraZeneca, Bristol-Myers Squibb, Hoffman La-Roche, Merck & Co., and Sepracor (Sunovion) Inc. He is also a co- inventor on filed patent application by Yale University (PCTWO06108055A1). The other authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 741.
REFERENCES
- 1.Ishigooka J, Suzuki M, Isawa S, Muraoka H, Murasaki M, Okawa M. Epidemiological study on sleep habits and insomnia of new outpatients visiting general hospitals in Japan. Psychiatry Clin Neurosci. 1999;53:515–22. doi: 10.1046/j.1440-1819.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22(Suppl 2):S347–53. [PubMed] [Google Scholar]
- 3.Ohayon MM, Caulet M, Lemoine P. Comorbidity of mental and insomnia disorders in the general population. Compr Psychiatry. 1998;39:185–97. doi: 10.1016/s0010-440x(98)90059-1. [DOI] [PubMed] [Google Scholar]
- 4.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–64. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 8.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–35. [PubMed] [Google Scholar]
- 9.Leger D, Guilleminault C, Bader G, Levy E, Paillard M. Medical and socio-professional impact of insomnia. Sleep. 2002;25:625–9. [PubMed] [Google Scholar]
- 10.Kuppermann M, Lubeck DP, Mazonson PD, et al. Sleep problems and their correlates in a working population. J Gen Intern Med. 1995;10:25–32. doi: 10.1007/BF02599573. [DOI] [PubMed] [Google Scholar]
- 11.Roth T, Roehrs T, Pies R. Insomnia: pathophysiology and implications for treatment. Sleep Med Rev. 2007;11:71–9. doi: 10.1016/j.smrv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Lushington K, Dawson D, Lack L. Core body temperature is elevated during constant wakefulness in elderly poor sleepers. Sleep. 2000;23:504–10. [PubMed] [Google Scholar]
- 16.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 17.Lichstein KL, Wilson NM, Noe SL, Aguillard RN, Bellur SN. Daytime sleepiness in insomnia: behavioral, biological and subjective indices. Sleep. 1994;17:693–702. doi: 10.1093/sleep/17.8.693. [DOI] [PubMed] [Google Scholar]
- 18.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 19.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 20.John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–34. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 22.Gottesman CV, Intraub H. Surface construal and the mental representation of scenes. J Exp Psychol Hum Percept Perform. 2002;28:589–99. doi: 10.1037//0096-1523.28.3.589. [DOI] [PubMed] [Google Scholar]
- 23.Siegel JM. The neurotransmitters of sleep. J Clin Psychiatry. 2004;65(Suppl 16):4–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009;29:1779–94. doi: 10.1111/j.1460-9568.2009.06716.x. [DOI] [PubMed] [Google Scholar]
- 25.Koch U, Magnusson AK. Unconventional GABA release: mechanisms and function. Curr Opin Neurobiol. 2009;19:305–10. doi: 10.1016/j.conb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Krystal AD. The treatment of primary insomnia. CNS Spectr. 2009;14:6–10. doi: 10.1017/s1092852900003953. [DOI] [PubMed] [Google Scholar]
- 27.Bhagwagar Z, Wylezinska M, Jezzard P, et al. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–12. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 28.Bhagwagar Z, Wylezinska M, Jezzard P, et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11:255–60. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 29.Price RB, Shungu DC, Mao X, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanacora G, Gueorguieva R, Epperson CN, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–13. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 31.Sanacora G, Mason GF, Rothman DL, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 32.Goddard AW, Mason GF, Almai A, et al. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Arch Gen Psychiatry. 2001;58:556–61. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- 33.Yoon JH, Maddock RJ, Rokem A, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–81. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–6. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–61. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muthukumaraswamy SD, Evans CJ, Edden RA, Wise RG, Singh KD. Individual variability in the shape and amplitude of the BOLD-HRF correlates with endogenous GABAergic inhibition. Hum Brain Mapp. 2012;33:455–65. doi: 10.1002/hbm.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golomb JD, McDavitt JR, Ruf BM, et al. Enhanced visual motion perception in major depressive disorder. J Neurosci. 2009;29:9072–7. doi: 10.1523/JNEUROSCI.1003-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS) Sleep. 2008;31:1499–506. doi: 10.1093/sleep/31.11.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–49. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service, University of California; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 42.Shen J, Rycyna RE, Rothman DL. Improvements on an in vivo automatic shimming method [FASTERMAP] Magn Reson Med. 1997;38:834–9. doi: 10.1002/mrm.1910380521. [DOI] [PubMed] [Google Scholar]
- 43.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–6. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothman D, Behar K, Petroff OA. Improved quantitation of shor TE 1H NMR human brain spectra by removal of short T1 macromolecule resonances. Soc Magn Reson, 2nd Meeting; 1994. p. 47. [Google Scholar]
- 45.Behar K, Rothman DL, Spencer DD, Petroff OAC. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 46.Shen J, Yang J, Choi IY, Li SS, Chen Z. A new strategy for in vivo spectral editing. Application to GABA editing using selective homonuclear polarization transfer spectroscopy. J Magn Reson. 2004;170:290–8. doi: 10.1016/j.jmr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45:517–20. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 49.Wafford KA, Ebert B. Gaboxadol--a new awakening in sleep. Curr Opin Pharmacol. 2006;6:30–6. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Nofzinger EA, Nissen C, Germain A, et al. Regional cerebral metabolic correlates of WASO during NREM sleep in insomnia. J Clin Sleep Med. 2006;2:316–22. [PubMed] [Google Scholar]
- 51.Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5:363–74. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- 52.Perlis ML, Kehr EL, Smith MT, Andrews PJ, Orff H, Giles DE. Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleeper controls. J. Sleep Res. 2001;10:93–104. doi: 10.1046/j.1365-2869.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- 53.Hall M, Buysse DJ, Nowell PD, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 54.Hall M, Thayer JF, Germain A, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007;5:178–93. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- 55.De Zambotti M, Covassin N, De Min Tona G, Sarlo M, Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. J Sleep Res. 2011;20:318–25. doi: 10.1111/j.1365-2869.2010.00871.x. [DOI] [PubMed] [Google Scholar]
- 56.Bonnet MH, Arand DL. Caffeine use as a model of acute and chronic insomnia. Sleep. 1992;15:526–36. [PubMed] [Google Scholar]
- 57.Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28:10167–84. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alam MN, Kumar S, Bashir T, et al. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–82. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGinty D, Szymusiak R. Hypothalamic regulation of sleep and arousal. Front Biosci. 2003;8:s1074–83. doi: 10.2741/1159. [DOI] [PubMed] [Google Scholar]
- 60.Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 2002;71:457–68. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 61.Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. J Physiol. 2009;587:4645–60. doi: 10.1113/jphysiol.2009.173435. [DOI] [PMC free article] [PubMed] [Google Scholar]