Abstract
Study Objectives:
To test the utility of an integrated clinical pathway for obstructive sleep apnea (OSA) diagnosis and continuous positive airway pressure (CPAP) treatment using portable monitoring devices.
Design:
Randomized, open-label, parallel group, unblinded, multicenter clinical trial comparing home-based, unattended portable monitoring for diagnosis and autotitrating CPAP (autoPAP) compared with in-laboratory polysomnography (PSG) and CPAP titration.
Setting:
Seven American Academy of Sleep Medicine (AASM) accredited sleep centers.
Participants:
Consecutive new referrals, age 18 yr or older with high probability of moderate to severe OSA (apnea-hypopnea index [AHI] ≥ 15) identified by clinical algorithm and Epworth Sleepiness Scale (ESS) score ≥ 12.
Interventions:
Home-based level 3 testing followed by 1 wk of autoPAP with a fixed pressure CPAP prescription based on the 90% pressure from autotitration of PAP therapy (autoPAP) device (HOME) compared with attended, in-laboratory studies (LAB).
Measurements:
CPAP acceptance, time to treatment, adherence at 1 and 3 mo; changes in ESS, and functional outcomes.
Results:
Of 373 participants, approximately one-half in each study arm remained eligible (AHI ≥ 15) to continue in the study. At 3 mo, PAP usage (nightly time at pressure) was 1 hr greater: 4.7 ± 2.1 hr (HOME) compared with 3.7 ± 2.4 hr (LAB). Adherence (percentage of night used ≥ 4 hr) was 12.6% higher: 62.8 ± 29.2% compared with 49.4 ± 36.1% in the HOME versus LAB. Acceptance of PAP therapy, titration pressures, effective titrations, time to treatment, and ESS score change did not differ between arms.
Conclusions:
A home-based strategy for diagnosis and treatment compared with in-laboratory PSG was not inferior in terms of acceptance, adherence, time to treatment, and functional improvements.
Trial Registration:
http://www.ClinicalTrials.gov; Identifier: NCT: 00642486.
Citation:
Rosen CL; Auckley D; Benca R; Foldvary-Schaefer N; Iber C; Kapur V; Rueschman M; Zee P; Redline S. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: The HomePAP Study. SLEEP 2012;35(6):757-767.
Keywords: OSA, portable monitoring, diagnosis, randomized clinical trial, autotitration
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder with multiple comorbidities, many of which may be reduced with effective OSA treatment. In response to the growing recognition of the disorder and benefits of treatment in the community, the number of American Academy of Sleep Medicine (AASM)-accredited sleep centers in the United States has increased fourfold over the past 10 years.1 Despite this growth, it is estimated that most individuals with OSA neither receive a diagnosis nor are treated, suggesting that current resources may be inadequate to meet growing demands. Although the costs of providing quality sleep diagnostic and treatment services for OSA continue to rise, the Centers for Medicaid and Medicare Services has targeted those services for cuts in reimbursement.2 Pressures to reduce healthcare expenditures mandate more efficient approaches for diagnosing and managing chronic diseases such as OSA.
Current guidelines recommend overnight polysomnography (PSG) for the diagnosis of OSA and positive airway pressure (PAP) titration studies for treatment. Laboratory-based PSG is relatively labor intensive, expensive, and burdensome. In certain geographic areas, access to these services may be limited or delayed. High-level evidence is needed to assess the role of new technologies for OSA diagnosis and management, such as use of home-based portable monitoring and autotitration of PAP therapy (autoPAP).
This multisite, randomized controlled study tests the utility of an integrated clinical pathway for both OSA diagnosis and PAP treatment using home-based portable monitoring devices compared with gold standard laboratory-based PSG. The American Sleep Medicine Foundation sponsored the study and designed the protocol. Preliminary results of this study have been published in abstract form.3
METHODS
Participants
The targeted sample was adult patients (age 18 yr or older) referred to a sleep center with a high pretest probability (based on a clinical algorithm) of moderate to severe OSA, defined as an apnea-hypopnea index (AHI) of at least 15 events/hr by objective testing. This clinical algorithm specified that participants had an “adjusted neck circumference” of at least 43 cm.4,5 This adjusted neck circumference was calculated as the measured neck circumference (cm) plus an additional 3 cm if habitual snoring was present; 4 cm if hypertension was present; and 3 cm if apnea, gasping, or choking was present on most nights. Inclusion criteria for the study required both the high pretest probability for moderate to severe OSA based on the clinical algorithm plus an Epworth Sleepiness Scale (ESS) score ≥ 12 at the time of referral. Exclusion criteria were (1) preexisting diagnosis of OSA or treatment with continuous positive airway pressure (CPAP); (2) significant comorbid pulmonary disease (for example, chronic obstructive pulmonary disease with a forced expiratory volume (FEV)1/forced vital capacity (FVC) < 70% predicted or restrictive pulmonary disease with an FEV1 < 50% predicted), regular use of supplemental oxygen or waking oxygen level less than 92%; (3) awake hypercapnia or hypoventilation syndrome; (4) respiratory or heart failure or neuromuscular disease; (5) concerns about unsafe driving defined as report of an automobile accident due to falling asleep behind the wheel in the past year; (6) chronic narcotic use; (7) alcohol abuse defined as more than 5 alcoholic drinks per day; (8) uncontrolled psychiatric disturbance; (9) clinical features of other sleep disorders; for example, moderate to severe restless leg symptoms, chronic insomnia or other conditions resulting in less than 4 hr of sleep per night, or history of cataplexy; (10) inability to undergo home testing because of living arrangements; and (11) anticipation of upper airway or gastric bypass surgery in the next 6 months.
Participants were recruited from 7 AASM-accredited academic sleep centers across the United States in 5 cities (Case Western Reserve University affiliates (University Hospitals, MetroHealth Medical Center, and Cleveland Clinic) in Cleveland, OH; Northwestern University in Chicago, IL; University of Wisconsin in Madison, WI; University of Minnesota in Minneapolis, MN; and University of Washington in Seattle, WA). This project was approved by the Institutional Review Boards at each clinical site. The study was registered at www.ClinicalTrials.gov Identifier: NCT: 00642486.
Protocol
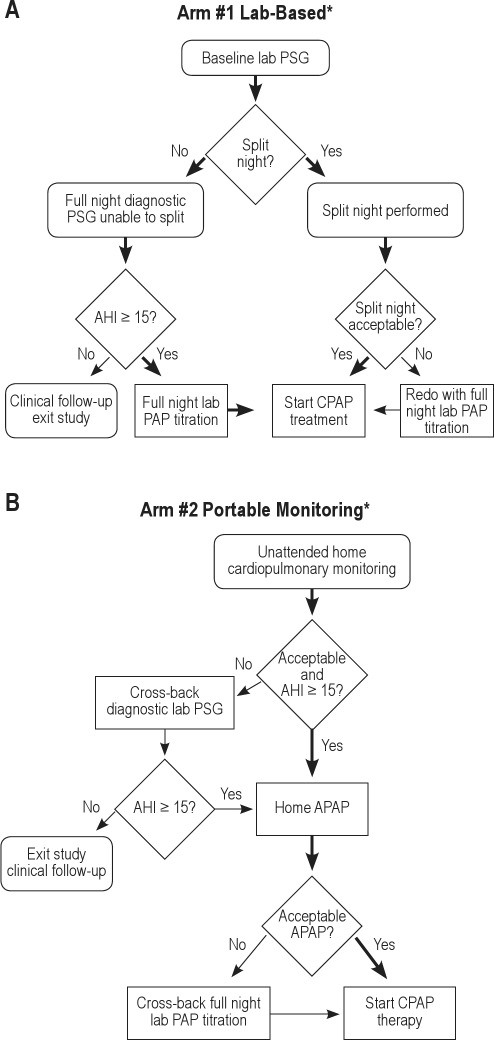
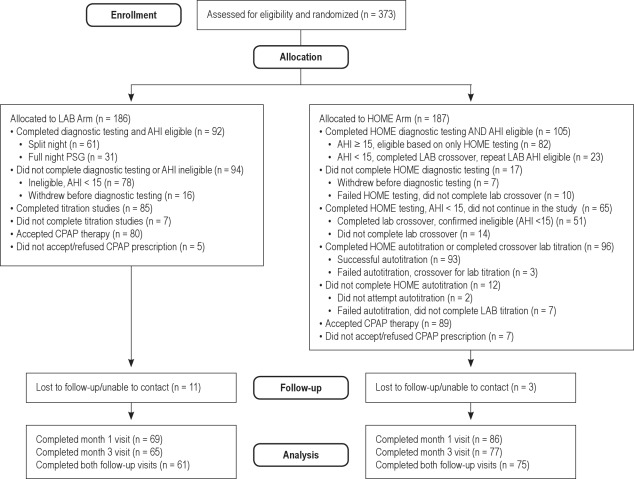
A schema of the protocol is shown in Figure 1. Potentially eligible participants signed informed consent, received standardized OSA education and mask fitting during a research clinic appointment, and then were centrally randomized to either the laboratory-based pathway (“LAB”) (A) or to home-based pathway (“HOME”) (B). At a baseline study visit, all participants had medical history information collected and a physical examination including measurement of arterial blood pressure, height, and weight from which body mass index (BMI) was calculated along with neck and waist circumferences and arterial saturation by pulse oximetry. Patients completed detailed sleep history, medical history, and other questionnaires including the ESS,6 Functional Outcomes of Sleep Questionnaire (FOSQ),7 Medical Outcomes Study 36-Item Short Form (SF-36),8 Calgary Sleep Apnea Quality of Life Index (SAQLI),9 European Health Status Questionnaire (EQ-5D),10 and Center for Epidemiologic Studies Depression scale (CESD-10).11 After the home- or laboratory-based sleep studies were performed, de-identified diagnostic and treatment sleep data from both the home-based and laboratory-based studies were securely transferred to the study's Sleep Reading Center (Case Western Reserve University) for overreading by 1 of 2 polysomnologists to confirm acceptability criteria and final eligibility by AHI. Only those participants with final eligibility, an AHI ≥ 15 on a diagnostic test (see Figure 1 and details in the next paragraphs) proceeded from the diagnostic phase to the titration phase of the study. This AHI was computed using standard AASM criteria (30% reduction in airflow associated with at least a 4% desaturation).12 Although the same scoring approach was used for respiratory event identification in the laboratory- versus home-based studies, the denominator was total sleep time for the laboratory-based studies and recording time for the home-based studies. Participants found to have an AHI < 15 events per hr exited the study after the diagnostic phase and were referred for routine clinical care from their sleep center physicians.
Figure 1.
(A) Simplified illustration of the protocol for participants randomized to the lab-based trial arm. (B) Simplified illustration of the protocol for participants randomized to the home-based trial arm. *Decisions for split, repeat, or cross-back studies per protocol for negative or unacceptable studies. AHI, apnea-hypopnea index; PSG, polysomnography; CPAP, continuous positive airway pressure; PAP positive airway pressure; APAP, autotitrating positive airway pressure.
Laboratory Arm
Participants underwent an attended PSG for diagnosis and PAP titration. If the AHI was 15 or higher during the first 2 hours of monitoring (diagnostic portion), then a split-night study was performed with the PAP titration following the diagnostic portion on the same night. If this split-night criterion was not met, then a full-night diagnostic PSG was performed. If a split study did not occur and the full-night AHI was 15 or higher, then the participant underwent a full-night PAP titration on another night. The criterion for a technically acceptable PAP titration was an AHI < 10/hr on the optimal PAP pressure. The selection of the optimal fixed titration pressure was reviewed and confirmed by the local site investigator. Participants with inadequate PAP titrations were offered a repeat titration study. Although each clinical site used their laboratories' own sensors, hardware, and software, PSG procedures including scheduling times, study montages, sensor placement, and the diagnostic PSG and PAP titration procedures were standardized to the extent possible based on recommendations from the professional sleep societies13,14 and as detailed in the study's manual of procedures.
Portable Monitoring Arm
A single night, unattended, limited channel sleep study (Embletta® X-30, Embla, Inc., Broomfield, CO) was used to confirm the OSA diagnosis, using central overreading to confirm baseline eligibility. Measurement channels included airflow by both thermistry and nasal pressure, respiratory effort (chest and abdominal movements by inductance plethysmography), oxygen saturation (SpO2), plethysmograph wave form, heart rate, electrocardiogram (ECG), and body position.
Study staff demonstrated to the participant how to apply the device before bedtime, observed a trial of self-application, gave written instructions with picture diagrams, and were available by telephone at night for questions or problems. Devices were either returned by the participant or directly retrieved by a courier service in the morning after use, depending on local preference. Home studies were downloaded, previewed for technical acceptability (at least 4 hr of recording time with quality signals for respiratory event scoring), manually scored by local study staff, then transferred to the Sleep Reading Center for overreading. Participants were asked to undergo a second home recording if the recording was technically unacceptable or if the AHI less than 15. If the second home-based diagnostic test was technically unacceptable or if the AHI was less than 15, then the participant “crossed over” for a diagnostic PSG in the sleep laboratory. If the laboratory-based AHI was confirmed to be less than 15, then the participant exited the clinical trial, but received ongoing clinical care in the sleep center. If the AHI from the laboratory-based PSG was 15 or higher, then the participant remained in the clinical trial, but “crossed back” to their original study arm assignment for a home-based titration study.
Eligible participants underwent an unattended automatically adjusting CPAP (autoCPAP) titration study at home using the REMStar® Auto-M Series (Philips-Respironics, Murraysville, PA) for 5 to 7 nights to determine their fixed CPAP prescription. At the face-to-face visit for autotitration instruction, study staff reinforced basic OSA and PAP education, reconfirmed mask fit in the supine position, determined a comfortable starting pressure, and instructed the participant on use of the autotitration device. The pressure was set to start automatically, ranging from 4 cm of water pressure (cwp) up to a maximum of 20 cwp. Study staff were in contact with the participant by telephone during the 5- to 7-day autotitration, as needed, for troubleshooting. During the home-based autoPAP, participants completed a basic sleep log indicating what time they fell asleep and what time they woke up. The recording was considered to be acceptable if the recording period in the autotitration device averaged at least 4 hr/night for at least 2 nights and large leak was absent. The optimal PAP pressure was determined by the site study investigator based on identifying the 90th percentile pressure setting in segments without large leak. The participant's automatic pressure profile also was transmitted to the Reading Center for quality control (assessing study quality and appropriateness of the pressure settings). The criteria for a successful autotitration study included at least 4 hr of recording time and a residual AHI < 10 at the optimal pressure, as measured by the device's proprietary algorithm. If the first autoCPAP study was unacceptable, then a second 5- to 7-night recording was performed. A titration failure was defined as when neither of the recordings obtained were acceptable. HOME participants with a failed autoPAP study “crossed-back” to the sleep laboratory for a full-night titration study (as part of routine care) to determine a fixed CPAP pressure for treatment.
Follow-up and Outcomes Measures for All Participants
All participants were provided with a RemStar AutoPro CPAP Unit (Philips-Respironics, Murraysville, PA). However, in-home treatment was initiated using an optimal fixed PAP pressure based on the titration study. These units were equipped with an in-line heated humidifier and the SmartCard™ system to objectively monitor CPAP adherence. Patients were reassessed by telephone contact at 3 days and 1 wk after CPAP initiation and by face-to face clinic visits at 1 and 3 mo to capture primary and secondary outcomes including CPAP adherence (daily time at pressure, percentage of days used, and percentage of days used for more than 4 hr), functional outcomes, interim clinical encounters, safety, and adverse events. Study staff was available for more frequent telephone follow-up, as needed, if problems adjusting to the mask, machine, or pressure level were reported.
Analytic Strategy
The primary outcomes were: (1) acceptance of CPAP (i.e., number of patients who accepted CPAP treatment and took the CPAP unit home; (2) objective adherence at 1 and 3 mo (average daily time in minutes at pressure; percentage of nights used, and percentage of nights with at least 4 hr of use); and (3) time in days from the diagnostic study to initiation of CPAP therapy. For those outcomes with baseline measurements, the change from baseline to 1 and 3 months was summarized. Rates and patterns in terms of differential rates of dropout over time were also summarized.
Secondary outcomes included (1) changes in subjective daytime sleepiness as measured by the ESS score; (2) change in sleep-specific quality of life, as measured by Functional Outcomes of Sleep Quality (FOSQ) and by the Sleep Apnea Quality of Life Instrument (SAQLI); (3) change in general quality of life as measured by the SF 36; (4) number of patients in the HOME arm who crossed over to the LAB arm for diagnosis or titration; (5) the percentage of patients in the HOME arm needing a laboratory-based diagnostic study or titration study outside of their assigned treatment arm; (6) percentage of patients with acceptable titration as defined by an AHI < 10. For the LAB arm, this was determined by measure of AHI at the final pressure of the attended study. For the HOME arm, this was determined by the residual AHI with pressure at the 90th percentile. Finally, the costs of each evaluation and management arms was determined by comparing within- trial differences based on current Medicare reimbursement rates for diagnostic and treatment procedures. Telephone contact time (frequency and duration) with study staff was also compared.
The study was powered for a noninferiority analysis. Sample size estimates with equivalence tests, where the portable monitoring arm was hypothesized to be no worse than the PSG arm, were run with a 1-tailed test at alpha of 0.05 and 80% power. The target level of initial CPAP acceptance was expected to be 90% and the maximum allowable acceptable difference for initial acceptance of CPAP between the 2 arms would be 8%. This analysis indicated that 372 total patients (186 participants in each arm of the study) would need to be randomized. A secondary primary outcome was an objective adherence measure of CPAP use at 1 and 3 mo. The difference between the randomized arms was expected to be no more than 0.75 hr per night. In the noninferiority setup, the null hypothesis would be that the portable monitoring arm will be less adherent than the PSG arm by at least 0.75 hr/night CPAP hours. A sample of 135 subjects per randomized arm would be needed to reject the null hypothesis with sufficient power of 80% and 170 participants for 90% power.
The primary analyses followed the intent-to-treat principle. The study protocol allowed for “cross-back” of participants between treatment arms under certain situations. The intent-to-treat principle dictates that participants be analyzed based on their randomization assignment regardless of whether they “crossed back” or not. Non-inferiority analyses were conducted for primary and secondary outcomes. Minimum clinically significant differences were identified for usage and change in functional outcomes based on literature review and expert opinion formed the basis for testing if the home arm was not inferior to the laboratory arm on these endpoints.
Baseline variables were summarized using means and standard deviations for continuous variables and counts and proportions for categoric variables. Due to the unique design of this study, where eligibility for the treatment phase was determined after randomization, variables were summarized across all participants randomized first, and then across those participants determined to be eligible. Data were also summarized within each treatment arm. For all participants randomized, as well as for all eligible participants, variables were compared across treatment arms to confirm that the randomization resulted in no clinically important group difference at baseline. Two sample independent t-tests and/or confidence intervals (CIs) were used for continuous variables and 2-sample independent portions tests and/or confidence intervals were used for dichotomous variables. Similar comparisons were made among eligible and noneligible participants to assess the effect of eligibility determined after randomization among the treatment groups. Pearson correlation coefficient or Spearman rank correlation were used to characterize the correlation among variables within each group.
RESULTS
Diagnostic Testing Completion Rates and AHI Eligibility
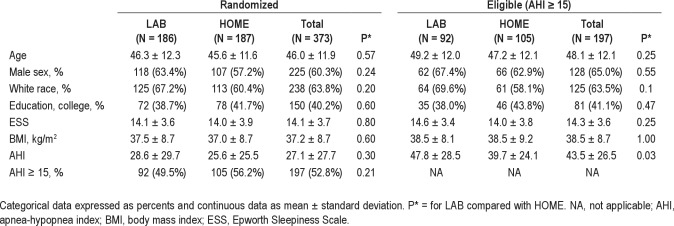
The study flow chart is presented in Figure 2. After randomization, 92 of 186 LAB participants (49%) and 105 of 187 participants (56%, 84 based on HOME test data and 23 from crossover LAB data) were confirmed to have moderate to severe (AHI ≥ 15) and remained eligible to continue in the study. Of the 92 eligible participants in the LAB arm, 61 had split-night testing and 31 had full-night PSG. Sixteen LAB participants (8%) did not complete their diagnostic testing. Of the 170 LAB participants who completed diagnostic testing, 78 (46%) were ineligible because of AHI < 15; 34 (20%) had AHI < 5, 44 (26%) had an AHI between 5-15, and 92 (54%) had AHI ≥ 15. In summary, of the 186 randomized LAB participants 78 (42%) were ineligible based on AHI < 15, and 16 (9%) did not successfully complete their study diagnostic procedures.
Figure 2.
Cohort diagram of participants randomized to the HOME versus LAB management pathways summarizing study flow. CPAP, continuous positive airway pressure, AHI, apnea-hypopnea index.
In the HOME arm (n = 187), a total of 105 HOME arm participants completed their home-based diagnostic testing and met AHI eligibility (AHI ≥ 15) to continue in the study: 82 based on their initial home diagnostic testing and 23 based on their crossover lab PSG (n = 23). There were 17 HOME participants who did not successfully complete their home-based testing: 7 withdrew before home diagnostic testing and 10 failed testing and did not complete a crossover laboratory diagnostic PSG. Of the 65 participants who completed HOME diagnostic testing and who had a home-based AHI < 15, 51 completed their crossover laboratory diagnostic PSG, which confirmed an AHI < 15, and 14 did not complete their crossover laboratory test. Of the 74 HOME participants who had technically successful home diagnostic studies with AHI < 15 and who completed laboratory crossover, 51 (69%) had a second AHI < 15 and 23 (31%) had a second AHI ≥ 15. In summary, of the 187 randomized HOME participants, 51 (27%) were ineligible based on AHI < 15 and 31 (17%) did not successfully complete their study diagnostic procedures. Among participants in the HOME arm who completed any diagnostic testing (n = 170), 40 (24%) had AHI < 5, 25 (15%) had an AHI between 5-15, and 105 (62%) had AHI ≥ 15. Among those participants with an initial home testing AHI that was less than 5, 16% had a subsequent laboratory study with an AHI ≥ 15 whereas 66% had a laboratory-based AHI that was also less than 5. If the initial home testing AHI was from 5 to 15, then 31% had a subsequent laboratory study with an AHI ≥ 15 and 31% had a laboratory-based AHI that was less than 5.
CPAP Titrations, CPAP Acceptance, and Study Visit Completion Rates
Among eligible participants in the LAB arm, 85 of 92 (92%) completed laboratory titration studies: 61 (72%) had split-night studies and 24 (28%) had full-night titration studies. Seven participants in the LAB arm did not complete their full-night titration study after their diagnostic study.
Among eligible participants in the HOME arm, 96 of 105 (91%) of eligible HOME arm participants completed titration studies, including 93 participants with successful autotitration studies and 3 participants with failed autotitration studies who crossed over to full-night laboratory titration studies. Of the 9 HOME participants who did not complete titration testing per protocol, 2 participants did not attempt their autotitration studies and 7 had failed autotitration studies, then did not complete their laboratory crossover.
Among those participants who completed their titration testing by protocol, CPAP prescription acceptance rates for a CPAP prescription were similar: 80 of 85 (94%) in the LAB arm compared with 89 of 96 (93%) in the HOME arm (P = 1.02). In the LAB arm, 69 participants (86%) completed month 1 visits, 65 (81%) completed month 3 visits, and 61 (76%) completed visits at both time points. In the HOME arm, 86 participants (97%) completed month 1 visits, 77 (87%) completed month 3 visits, and 75 (84%) completed both visits.
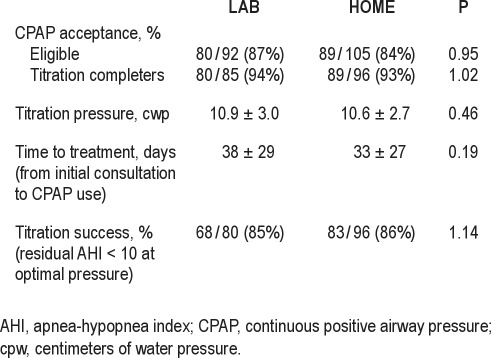
Sample Characteristics by Intervention Group
Table 1 summarizes the baseline sample characteristics by intervention group before (n = 373) and after final eligibility determination (n = 196; 91 LAB, 105 HOME). Among eligible participants (AHI ≥ 15), the sample had a mean age of 48 ± 12 yr, was obese (BMI 39 ± 9 kg/m2), and was 65% male, 62% white, and 41% college educated. The sample had moderate to severe OSA (mean AHI was 43 ± 26) and was moderately sleepy (mean ESS score 14 ± 4). There were no group differences at either randomization or at final eligibility for age, race, sex, BMI, and level of education and ESS score. The mean diagnostic AHI was similar in both groups at randomization, but lower (P = 0.03) in the eligible HOME (39.7 ± 24.1) versus eligible LAB (47.8 ± 28.5) arm, possibly because the HOME sample included individuals with lower initial AHI values (home-based AHI < 15) who then crossed over for laboratory-based studies.
Table 1.
Baseline characteristics
PAP Therapy Treatment Characteristics by Intervention Group
Characteristics of PAP therapy treatment within each intervention group are summarized in Table 2. There were no group differences in the percentage of participants who accepted CPAP therapy (more than 90%), the mean optimal fixed pressure (10 cwp), the number of days until receipt of CPAP therapy (approximately 1 mo), and number of participants with effective titrations (85% or greater).
Table 2.
PAP therapy treatment characteristics
Adherence and Functional Outcomes by Intervention Group at 1 and 3 mo
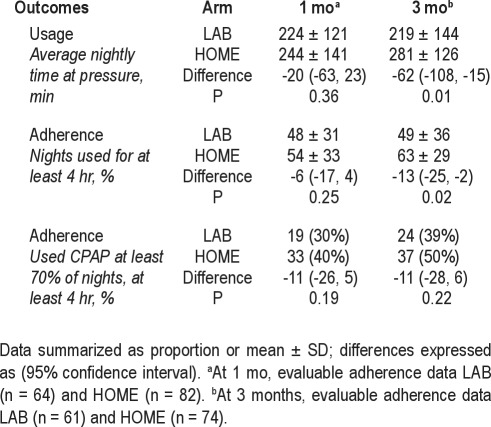
Adherence outcomes summarized in Table 3 show that PAP usage was significantly higher at 3-mo follow-up in the HOME arm for both minutes of nightly use and percentage of nights used for at least 4 hr. CPAP usage (nightly time at pressure) tended to be higher, albeit not significantly, in the HOME arm at 1 mo [244 ± 141 versus 224 ± 121 min, difference: −20 (CI: −63, 23, P = 0.36) although it was significantly higher at 3 mo ([281 ± 126 versus 219 ± 144 min, difference: −62 [CI: −108, −15], P = 0.01). Similarly, PAP adherence dichotomized as percentage of nights used at least 4 hr tended to be higher in the HOME arm at 1 mo (54 ± 33% versus 48 ± 31%, difference: −6% [CI: −17, 4] P = 0.25), but was significantly greater at 3 mo (63 ± 29% versus 49 ± 36%, difference: −13 [CI: −25, −2], P = 0.02). The percentage of patients who used CPAP therapy at least 70% of nights for at least 4 hr tended to be higher, but not significantly, in the HOME group compared with the LAB group (40% vs. 30%, difference: −11 [CI: −26, 5], P = 0.19) at 1 mo and 50% versus 39% (50% versus 39%, difference: −11 [CI: −27, 6], P = 0.22) at 3 months.
Table 3.
Adherence outcomes by intervention arm at follow-up visits
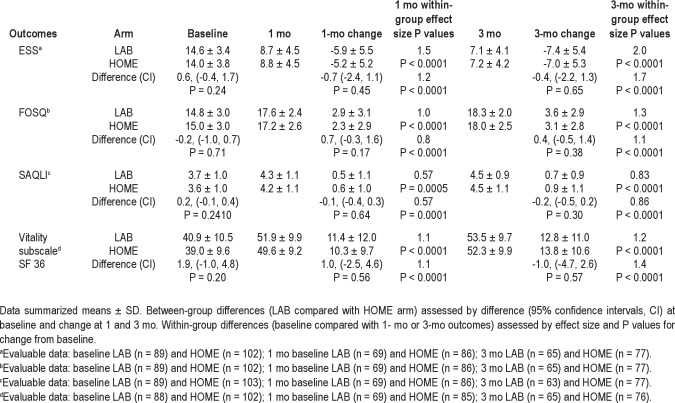
Within each group, all functional outcome measures (sleepiness measured by the ESS, disease-specific quality of life measured by the FOSQ, quality of life in response to CPAP therapy measured by the SAQLI, and the vitality subscale in generic quality of life) improved significantly from baseline to both 1- and 3-mo follow-up assessments. However, changes in functional outcomes from baseline to follow-up assessments did not differ by group (Table 4).
Table 4.
Patient-related outcomes by intervention group
Technical Failure Rates
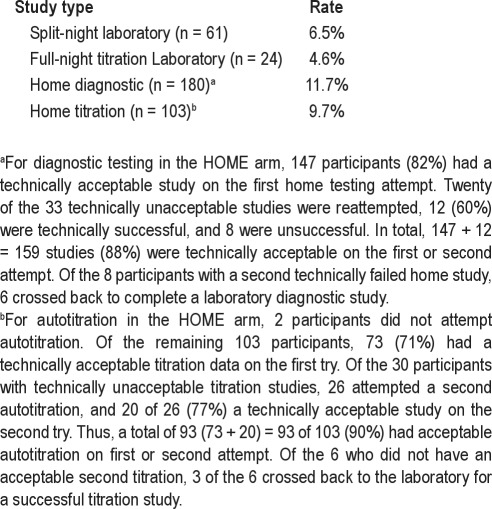
The rates of technically unsatisfactory laboratory-based studies and for home-based diagnostic or autoPAP studies as defined in the study are summarized in Table 5. In the LAB arm, unacceptable titrations requiring a full-night repeat titration study occurred in 4 of 61 (6%) of split night studies and in 1 of 24 (4%) of the completed full-night titration studies. Of the 180 HOME arm participants who attempted home diagnostic testing, 159 (88%, 147 [first try]) plus 12 [second try]) had technically acceptable studies. Of the 103 HOME arm participants who attempted autotitration, 93 (90%, 73 [first try] plus 20 [second try]) had technically acceptable studies. In summary, the technical failure rate was for the HOME arm was 21 of 180 (11.6%) for diagnostic testing and 10 of 103 (9.7%) for autotitration. In 1 participant in the HOME arm, both diagnostic and titration studies failed.
Table 5.
Rates of unacceptable studies per protocol
In-Trial Study Costs
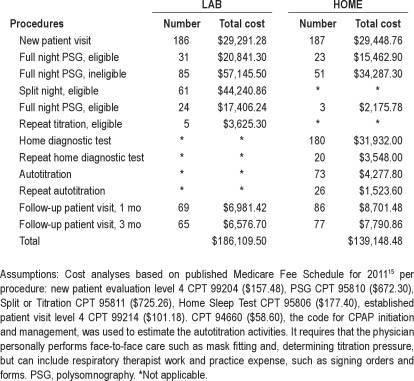
In-trial study costs were estimated based on the published Medicare Fee Schedule for 201115 for the various study procedures including office visits and diagnostic testing, taking into account the need to repeat studies (Table 6). In the absence of a current procedural terminology (CPT) code for autotitration, we estimated that cost using the current code for initiation and management of CPAP. Our analysis indicates that the HOME arm in-trial study costs were 25% less than for the LAB arm.
Table 6.
In-trial cost analysis for diagnostic and treatment procedures by study arm
Per protocol, all participants on PAP therapy received a study-related follow-up call 1 wk after starting CPAP therapy. HOME arm participants received an additional telephone call during their autotitration. Consistent with an typical ambulatory sleep center practice, participants could initiate telephone calls to staff for assistance with CPAP management, as needed. The number of telephone calls per participant and minutes spent in each telephone contact were tracked during the study. Although CPT codes exist for physician and nonphysician telephone services, no attempt was made to estimate those costs because they are currently not reimbursed by Medicare or most third-party payers. In this trial, there were more additional incoming calls from the HOME arm participants (n = 82) than the LAB arm (n = 45). However, calls from LAB participants were 4 min longer on average (23 versus 19 min). Most participants in either arm had 0 incoming calls, but some participants had numerous calls.
DISCUSSION
This randomized controlled clinical trial demonstrates that in the hands of sleep medicine specialists an integrated home-based portable monitoring strategy for both diagnosis and treatment of patients with moderate to severe OSA is not inferior to a laboratory-based strategy. The home-based strategy appears equivalent in terms of outcomes important to patients: acceptance of therapy, time to treatment, reduced hypersomnolence, improved functional outcomes (both generic and disease-specific), and treatment characteristics. Despite use of standardized approaches and strong support for enhancing adherence for all study participants, adherence was only moderately high, although it was significantly greater at 3 mo in the home-based arm in comparison with to the laboratory arm.
The findings in this study are consistent with 4 other similarly conducted studies 16–19 in patients who were (1) evaluated by physicians trained in sleep medicine, (2) at significant risk for moderate to severe OSA, and (3) excluded if they had comorbid medical conditions likely to degrade the accuracy of portable monitoring.20 Our study is unique in that it included patients and sleep specialists from multiple sleep centers across the United States with a substantial proportion of female participants (35%), and thus may provide data that can be more readily generalized to U.S. sleep medicine practices. Our findings of noninferiority are also similar to those found in a recent study a predominantly male sample from 2 Veterans Affairs (VA) hospital sites.21
Although most studies have not shown significant group differences in CPAP adherence, our study findings suggest that adherence may be better at 3 mo in patients who received home-based diagnosis and titration. Our findings of greater CPAP use in the HOME arm are similar to the Canadian study by Mulgrew et al., who also found 1 hr greater use in the ambulatory group.17 In a recent VA-based study with a protocol similar to ours, the mean hours of use in the home-based group was 0.6 hr higher than in the laboratory-based group, but the trend did not meet statistical significance.21 One theory to explain greater adherence is that psychologic and behavioral change factors known to be determinants of CPAP adherence22–24 may become activated by the portable monitoring experience.
Despite a rigorous approach for optimizing adherence, including standardized education, acclimatization, and ongoing support from dedicated sleep specialty teams, only 30% of the LAB group and 40% of the HOME group met Medicare guidelines for acceptable CPAP use at 1 mo. Although CPAP use often decreases over time, we did observe a modest improvement in PAP adherence by Medicare guidelines between 1 and 3 mo, increasing to 39% and 50.0% in the LAB and HOME groups, respectively. This finding may reflect the ongoing efforts of the team to address PAP uses throughout the intervention period. Our experience is consistent with the findings of a recent Cochrane review evaluating evidence to support the value of educational, supportive, and behavioral interventions to improve CPAP usage in adults with OSA. That review concluded that support/encouragement offered on an ongoing basis led to increased average machine usage (0.59 hr/night (95% CI 0.26 to 0.92), although there was a significant degree of variation between the results of the studies.25
In contrast with the between-group differences in adherence outcomes, there were no between-group differences observed for patient-reported functional outcomes. Despite the only moderate levels of adherence to CPAP, compared with baseline, participants in both arms reported moderately large improvements in functional outcomes including ESS, FOSQ, SAQLI, and SF-36 vital scale, consistent with data from a recent VA study.21 Given these improvements, our data raise questions concerning current reimbursement policies that restrict CPAP to patients able to adhere to CPAP. Alternatively, the relatively large improvements with PAP may reflect a Hawthorne effect from participation in a study and the lack of blinding. The selection of symptomatic patients may also have created a regression to the mean effect. Further assessments using more rigorous methods are needed to better understand the contribution of PAP and level of adherence needed to improved sleepiness and functional status.
Our study has several important limitations. Using a predefined protocol, patients were randomized before final study eligibility (AHI ≥ 15) was determined, so a substantial portion of the study sample (49% in the LAB arm and 40% in the HOME arm) exited the study. In the HOME arm, patients with initial diagnostic studies with an AHI < 15 had a second opportunity for diagnostic testing in the laboratory to determine study eligibility, without comparable provisions in the LAB arm. More than one-fourth of the patients in the HOME arm returned to that arm after their cross-back laboratory study with an AHI ≥ 15. This study feature resulted in a larger evaluable sample size and a lower mean AHI in the HOME arm. In our study, more than 30% of the HOME arm patients with AHI < 5 or with an AHI between 5 and 15 had a subsequent AHI ≥ 15 after their cross-back study in the laboratory. Night-to-night variability in respiratory events indices is well known.26–28
Our study included only patients with a documented AHI ≥ 15, so our findings may not be generalizable to symptomatic patients with milder AHI findings who would be offered PAP therapy. Of note, 26% of the LAB arm and 16% of the HOME arm patients were determined to have AHI values between 5 and 15, but were not evaluated in this study. Patients with this milder level of OSA severity were included in 2 recent studies, which also found that a laboratory-based study did not lead to superior outcomes.18,19
In our study, moderate to severe OSA was defined by respiratory events scored using the “recommended” criteria for hypopnea (30% reduction in nasal airflow and a 4% desaturation) as opposed to the “alternative” criteria for hypopnea (50% reduction in nasal pressure with either a 3% desaturation or arousal).12 A recent study compared AHI values based on the 2 hypopnea criteria and found that more patients would be identified with an AHI ≥ 15 (50% versus 38%) using the alternative versus the recommended criteria.29 Anecdotally, 1 patient in this study with an AHI of 0 derived using the recommended criteria had an AHI of 22 using the alternative criteria. We also excluded patients with severe medical comorbidities due to the current recommendations to avoid limited channel devices in patients with comorbidities,20,30 potentially reducing generalizability to patients with more severe diseases.
There was significant site-to-site variability in the initial diagnostic technical failure rate, which ranged from 1% to 42%, improved with experience, and ended up averaging almost 12% overall. Such variability underscores the challenges some sleep laboratories, even those based in academic settings, may face adopting new technologies. Given that diagnosis and treatment of OSA has been dependent on costly and limited laboratory- based PSG, this study has important implications in terms of improving access. Due to the research data collection requirements of the protocol, the time to therapy (approximately 1 mo) was equivalent in both groups and longer than ideal in a real-world setting. Future comparative effectiveness research that embeds pertinent data collection in routine clinical settings is needed to better address the efficiencies of alternative diagnostic and screening strategies.
Although the study achieved its randomization goals determined by a noninferiority analysis and sample size calculation, the number of participants who proceeded to treatment was lower than anticipated due to the large number of individuals in both arms who had an AHI < 15 despite meeting the screening criteria, reducing the study's actual power. Other analytic limitations include the absence of outcome data for participants who did not complete their diagnostic or titration studies, accept CPAP, or who were lost to follow-up before their 1- and 3-mo visits. In terms of baseline characteristics, compared with those of randomized participants who were not followed up in the study, randomized participants who were followed up in the study (i.e. had any 1- or 3-mo data) were more likely to be older, male, and white, with higher AHIs (as expected) and higher BMIs, but there were no ESS differences. There were no major differences in the percentage of participant loss by study arm at the various time points in the study.
Finally, in addition to evaluating clinically relevant outcomes, future decisions regarding the role of home versus laboratory monitoring need to account for economic costs. Specifically for OSA diagnosis and treatment, such analyses are difficult due to the ongoing changes in reimbursement. Ideally, they should include the calculation of quality-adjusted life-years, which often requires a great deal of extrapolation from limited observational data. A recent cost-effectiveness analysis by Pietzsch et al.31 concluded that full-night laboratory-based testing ends up costing the healthcare system less over time by minimizing the false-negative and false-positive tests associated with ambulatory testing.
As with all economic modeling studies, the final results are highly dependent on the underlying assumptions and the data used to populate the model. That study had to make many assumptions about failure rates, diagnostic accuracy, conditions of use, risks of long-term adverse outcomes, and costs that may be clinically uncertain or out of date.32 However, this study highlights the importance of fully addressing economic questions with a longer term perspective in terms of incremental costs and changes in quality-adjusted life-years. It serves as a reminder that caution should be taken when advocating for broad use of home studies under conditions where the frequency of false-negative and false-positive testing may be increased. Finally, it underscores the importance of randomized controlled trials with clinically robust ends points.
Although a complete cost-effectiveness analysis was beyond the scope of this study, we did estimate the in-trial study costs, taking into account the need to repeat studies. Our analysis indicates that upfront costs were approximately 25% lower in the HOME arm compared with the LAB arm. In addition to being a short-term analysis, our in-trial cost estimates have several limitations. First, true costs associated with OSA patients by portable monitoring are difficult to reliably capture in the absence of established procedure codes, fees, and reimbursement for acquisition, download, analysis archive, and professional review of autotitration data. Because Medicare does not reimburse differentially for the monthly rental of an autotitration unit with advanced diagnostic capabilities versus a simple fixed pressure units, those costs are also difficult to capture. Furthermore, the analysis does not adequately capture the reimbursement for the additional professional (physician and/or allied health professional) needed to educate the patient on how to use the portable monitoring devices for diagnosis and titration, estimated to be at least 1 hr per patient.
On the other hand, our clinical trial provides the field with more current data to populate many of the assumptions needed in a formal economic analysis in terms of diagnostic accuracy, conditions of use, failure rate, and refusal rates. One of the important assumptions in the study by Pietzsch et al. that was criticized in a commentary by Ayas et al.32 was the 22% of patients with either a technical failure or negative ambulatory study who would not return for a follow-up PSG, data obtained from an older study by Fletcher et al.33 However, we observed a very similar rate of 24% (24 of 98) in the current study. In our clinical sample, using the study's clinical algorithm that included adjusted neck circumference and ESS ≥ 12, we observed a 50% pretest probability of moderate to severe OSA. However, we also observed that 23 of 74 (31%) of HOME arm participants with home-based AHI < 15 had a subsequent AHI ≥ 15 if they underwent a full-night laboratory-based PSG. Given these observations, the study by Pietzsch et al. study serves as a reminder to assess whether home studies should automatically be considered the most cost-effective test just because the upfront costs are lower than those for laboratory-based studies.
In summary, autotitration devices have been successfully incorporated into ambulatory management pathways,16,34–36 reducing the need for laboratory-based titrations, and the technology for portable diagnostic devices continues to advance. Our findings support the use of home unattended portable monitor testing in the diagnosis and management of OSA in a highly select population with a high likelihood of OSA and without medical comorbidities. Benefits include increased access to treatment with the potential to decrease healthcare expenditures (labor, equipment, facilities) and patient burden. In the end, the essential issue is not whether ambulatory studies are better or worse than laboratory-based studies, but rather, under what conditions home studies are appropriate for use. More large, randomized trials using different diagnostic strategies in different groups of patients with collection of concomitant economic information will be required to address this important issue. It is unlikely that a single diagnostic strategy will be superior to all in different clinical scenarios.
DISCLOSURE STATEMENT
This was not an industry supported study. Embla Corporation donated the portable monitoring diagnostic devices to the study's sponsor, American Sleep Medicine Foundation (ASMF), for use in this study. Philips Respironics donated the autoCPAP devices to the ASMF along with an assortment of CPAP masks. Eligible participants used these autoCPAP devices in the fixed pressure mode during the study and were allowed to keep the device along and a CPAP mask at the end of their study participation.
Dr. Auckley has received grant support from ResMed and Cephalon and has been loaned equipment from CleveMed. Dr. Foldvary-Schaefer has received research support and has used equipment loaned CleveMed. Dr. Iber has received research support from Apnex Medical. Dr. Zee has received research support from Philips Respironics and serves on the advisory board of Sanofi-Aventis, Philips Respironics, Purdue, Merck, and Jazz Pharmaceuticals. Dr. Redline has received equipment from Philips-Respironics and ResMed Inc. and has received a research support from ResMed Inc. and Dymedic, Inc. Dr Redline is the first incumbent of an endowed professorship donated to the Harvard Medical School by Dr. Peter Farrell. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of the following individuals: Noah Andrews, RPSGT; Stella Baccaray, RN; Robyn Cox, RN; Stephanie Hirus, RPSGT; Brandon Lu, MD; Rawan Salem, RPSGT; Nancy Scott, RPSGT, EEG; Kate Sprecher, BS; Jan Steinel, RRT; Maria Sterkel, RPSGT; Kari Sveum, BS.
Support provided by American Sleep Medicine Foundation 38-PM-07 Grant: Portable Monitoring for the Diagnosis and Management of OSA.
Footnotes
A commentary on this article appears in this issue on page 735.
REFERENCES
- 1.Esparis B. Accreditation Statistics, Report to the Board of Directors, American Academy of Sleep Medicine (personal communication to JA Barrett) 2011 [Google Scholar]
- 2.AASM Special Update. Darien, Il: American Academy of Sleep Medicine; 2011. CMS Revises Sleep Medicine CPT Codes. [Google Scholar]
- 3.Rosen C, Auckley D, Benca R, et al. A multi-site randomized rrial of portable monitoring and positive airway pressure autotitration versus laboratory-based polysomnography for thediagnosis and management of obstructive sleep apnea: HomePAP study. Sleep. 2010;33(Abstract supplement):A173. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flemons WW, Whitelaw WA, Brant R, Remmers JE. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1279–85. doi: 10.1164/ajrccm.150.5.7952553. [DOI] [PubMed] [Google Scholar]
- 5.Flemons WW. Clinical practice:obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 6.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 7.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 8.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 9.Flemons WW, Reimer MA. Measurement properties of the Calgary sleep apnea quality of life index. Am J Respir Crit Care Med. 2002;165:159–64. doi: 10.1164/ajrccm.165.2.2010008. [DOI] [PubMed] [Google Scholar]
- 10.EuroQol--a new facility for the measurement of health-related quality of life: the EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 11.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. 1st ed. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 13.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 14.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services. Medicare Physician Fee Schedule Search. 2011. Available from: http://www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=1&T=4&HT=0&CT=3&H1=95806&M=5.
- 16.Whitelaw WA, Brant RF, Flemons WW. Clinical usefulness of home oximetry compared with polysomnography for assessment of sleep apnea. Am J Respir Crit Care Med. 2005;171:188–93. doi: 10.1164/rccm.200310-1360OC. [DOI] [PubMed] [Google Scholar]
- 17.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146:157–66. doi: 10.7326/0003-4819-146-3-200702060-00004. [DOI] [PubMed] [Google Scholar]
- 18.Berry RB, Hill G, Thompson L, McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31:1423–31. [PMC free article] [PubMed] [Google Scholar]
- 19.Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-based diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257–63. doi: 10.1378/chest.09-0577. [DOI] [PubMed] [Google Scholar]
- 20.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients: Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 21.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183:1238–44. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 22.Aloia MS, Arnedt JT, Stepnowsky C, Hecht J, Borrelli B. Predicting treatment adherence in obstructive sleep apnea using principles of behavior change. J Clin Sleep Med. 2005;1:346–53. [PubMed] [Google Scholar]
- 23.Stepnowsky CJ, Jr, Marler MR, Ancoli-Israel S. Determinants of nasal CPAP compliance. Sleep Med. 2002;3:239–47. doi: 10.1016/s1389-9457(01)00162-9. [DOI] [PubMed] [Google Scholar]
- 24.Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J. 2004;24:461–5. doi: 10.1183/09031936.04.00114603. [DOI] [PubMed] [Google Scholar]
- 25.Smith I, Nadig V, Lasserson TJ. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines for adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2009:CD007736. doi: 10.1002/14651858.CD007736. [DOI] [PubMed] [Google Scholar]
- 26.Dean RJ, Chaudhary BA. Negative polysomnogram in patients with obstructive sleep apnea syndrome. Chest. 1992;101:105–8. doi: 10.1378/chest.101.1.105. [DOI] [PubMed] [Google Scholar]
- 27.Le Bon O, Hoffmann G, Tecco J, et al. Mild to moderate sleep respiratory events: one negative night may not be enough. Chest. 2000;118:353–9. doi: 10.1378/chest.118.2.353. [DOI] [PubMed] [Google Scholar]
- 28.Levendowski D, Steward D, Woodson BT, Olmstead R, Popovic D, Westbrook P. The impact of obstructive sleep apnea variability measured in-lab versus in-home on sample size calculations. Int Arch Med. 2009;2:2. doi: 10.1186/1755-7682-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trikalinos TA, Ip S, Raman G, et al. Technology Assessment. Home diagnosis of obstructive sleep apnea-hypopnea syndrome. Agency for Healthcare Research and Quality Technology Assessment Program. 2007. [cited; Available from: http://www.cms.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp&where=index&tid=4&. [PubMed]
- 31.Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep. 2011;34:695–709. doi: 10.5665/SLEEP.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayas NT, Pack A, Marra C. The demise of portable monitoring to diagnose OSA? Not so fast! Sleep. 2011;34:691–2. doi: 10.5665/SLEEP.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fletcher EC, Stich J, Yang KL. Unattended home diagnosis and treatment of obstructive sleep apnea without polysomnography. Arch Fam Med. 2000;9:168–74. doi: 10.1001/archfami.9.2.168. [DOI] [PubMed] [Google Scholar]
- 34.Masa JF, Jimenez A, Duran J, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170:1218–24. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
- 35.West SD, Jones DR, Stradling JR. Comparison of three ways to determine and deliver pressure during nasal CPAP therapy for obstructive sleep apnoea. Thorax. 2006;61:226–31. doi: 10.1136/thx.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antic NA, Buchan C, Esterman A, et al. A randomized controlled trial of nurse-led care for symptomatic moderate-severe obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:501–8. doi: 10.1164/rccm.200810-1558OC. [DOI] [PubMed] [Google Scholar]