Abstract
Objectives:
This study examined whether the 5-HTTLPR polymorphism in the SLC6A4 gene is associated with self-reported symptoms of depressed mood in first-year university students with a persistent pattern of short sleep.
Design:
Students provided DNA samples and completed on-line sleep diaries and a mood scale during the first semester. A priori phenotypes for nocturnal sleep and mood scores were compared for the distribution of genotypes.
Setting:
Brown University, Providence, Rhode Island.
Participants:
A sample of 135 first-year students, 54 male, 71 Caucasian, mean age 18.1 (± 0.5) yr.
Interventions:
None.
Measurements:
Students completed on-line sleep diaries daily across the first term (21-64 days; mean = 51 days ± 11) and Center for Epidemiologic Studies-Depression (CES-D) mood scale after 8 wk. DNA was genotyped for the triallelic 5-HTTLPR polymorphism. Low-expressing S and LGpolymorphisms were designated S′, and high-expressing LA was designated L′. Phenotype groups were identified from a combination of CES-D (median split: high > 12; low < 13) and mean nocturnal total sleep time (TST) from diaries: (shorter ≤ 7 hr; longer ≥ 7.5 hr). Three genotypes were identified (S′S′, S′L′, L′L′); the S′S′ genotype was present in a higher proportion of Asian than non-Asian students.
Results:
Four phenotype groups were compared: 40 students with shorter TST/high CES-D; 34 with shorter TST/low CES-D; 29 with longer TST/high CES-D; 32 with longer TST/low CES-D. Female:male distribution did not vary across phenotype groups (chi-square = 1.39; df = 3; P = 0.71). S′S′ participants (n = 23) were overrepresented in the shorter TST/high CES-D group (chi- square = 15.04; df = 6; P < 0.02). This association was sustained after removing participants with preexisting evidence of depressed mood (chi-square = 12.90; df = 6; P = 0.045).
Conclusion:
These data indicate that young adults who reported shorter nocturnal sleep and higher depressed mood are more likely than others to carry a variant of the SLC6A4 gene associated with low expression of the serotonin transporter.
Citation:
Carskadon MA; Sharkey KM; Knopik VS; McGeary JE. Short sleep as an environmental exposure: a preliminary study associating 5-HTTLPR genotype to self-reported sleep duration and depressed mood in first-year university students. SLEEP 2012;35(6):791-796.
Keywords: Sleep, serotonin, depression, genetics, 5-HTTLPR, undergraduate students
INTRODUCTION
Depression has been linked to a polymorphism in the promoter region of the serotonin transporter gene (5-HTTLPR in SLC6A4). Nevertheless, the association is indirect and findings have been mixed.1 Caspi and colleagues2 indicate that environmental moderators play a significant role in the link between genetic background and symptom development; that is, exposure to stressful life events constitute a moderating influence on the association between 5-HTTLPR genotype and depression.2–4 The mechanism(s) by which stressful life events may influence the development of mood disorders in genetically susceptible individuals have not been elucidated.
A link between insomnia and depression is well known and considered a bidirectional process.5–7 Thus, for example, sleep difficulty is a prominent feature of depressive illness, including trouble falling asleep, staying asleep, waking too early in the morning, or, in some, the atypical manifestation of hypersomnolence.8,9 On the other hand, epidemiologic studies show that past insomnia predicts future development of depression in older humans7 as well as young adults.10 Short sleep per se has been associated with mood problems,11 and a recent study found that teens reporting short sleep or extended sleep were at highest risk of suicidal tendencies.12
The serotonin neurotransmitter system is a central actor in depression, most notably in depression pharmacotherapy, which features drugs that affect serotonin reuptake.13 Serotonin also plays a prominent role in sleep neuroregulation: activity in serotonin-rich neurons of the raphé nucleus inhibits the expression of rapid eye movement (REM) sleep.14–17 The interaction of REM sleep systems and depressed mood has been identified for decades. For example, short REM sleep latency is a feature common to a subgroup of patients with major depressive disorder,18,19 and suppression of REM sleep either operationally or pharmacologically improves mood of depressed patients.20,21
The current research was informed by this literature; however, the specific stimulus was a 2005 report that chronic (8-day) sleep restriction in rats produced blunting of the serotonin 1A (5-HT1A) receptor system.22 Furthermore, these authors demonstrated that reversal of this blunting required a comparable amount of daily recovery sleep. These data led us to speculate that exposure to chronic sleep restriction in humans may result in similar changes to serotonin neurotransmission systems and that such changes are a proximal cause of mood dysregulation in susceptible individuals.
The emergence of depressed mood has a well-known association with such major life transitions as birth, death, job loss/gain, and geographic relocation.23 We selected the transition to university status at a residential college as a model system to examine the development of depressed mood because this life transition is often associated with stress.24,25 Furthermore, this transition is often accompanied by a change in sleep patterns26,27 and therefore is a good target to examine the relation of sleep and mood. We hypothesized that first-year university students who carry a susceptible genetic background in the promoter region of the serotonin transporter gene would be more likely to manifest symptoms of depressed mood at this transition in the context of a chronic sleep reduction. This prospective study sample included students assessed before entering university and subsequently through the first 8-9 wk after the start of their first semester.
METHODS
First-year students who accepted admission to Brown University in April 2009 and April 2010 were invited by e-mail to join the project. The 2009 invited cohort included all incoming students (n = 1,498); the 2010 cohort included only students age 18 yr and older (n = 1,340). Parental permission was sought for participants younger than 18 yr, a procedure exclusive to the 2009 cohort. All procedures were approved by the Rhode Island Hospital/Lifespan Institutional Review Board for the Protection of Human Subjects. Participants were compensated as described in the following paragraphs.
After accepting admission, 787 participants completed an initial survey in May, June, or August (with differences in timing between the 2009 and 2010 cohorts) before enrolling in September. Students in both cohorts who completed a survey were invited by e-mail to take part in phase 2 of the study, which included attending a brief session to provide consent and a tissue sample for genotyping.28 Two hundred fifty-three students provided DNA samples and were asked to complete daily on-line sleep diaries starting with the first day of classes and continuing for approximately 58 days (2009) to 61 days (2010) before the outcome survey. Diaries were available to complete each day from early evening until early the next morning, and access was restricted by requiring students to log in with their identification number and a password. Participants were incentivized to complete diaries each day with a $1.00 payment and small bonuses for completing 3 and 7 consecutive days. The outcome survey was presented on-line with an additional incentive of $18.00. This outcome survey included questions about sleep and behaviors, as well as our primary mood outcome measure, the Centers for Epidemiology Studies-Depression (CES-D) scale. All on-line data were collected and stored on a secure server using DatStat Illume 4.5 (DatStat, Inc., Seattle, WA).
Genomic DNA was isolated from buccal cells using a modification of published methods.29 The cheeks and gums were rubbed for 20 sec with 3 sterile, cotton-tipped wooden swabs. The swabs were placed in a 50-ml capped polyethylene tube containing lysis bugger (500 μl of 1 M Tris − HCl; 200 mM disodium ethylene diaminetetraacetic acid, pH 8.0; 500 μl 10% sodium docecyl sulfate and 100 μl 5 M sodium chloride). Participants then rinsed out the mouth vigorously with 10 ml of distilled water for 20 sec and this solution was added to the 50-ml tube. The tubes were stored at 4°C until the DNA was extracted using an ethanol precipitation method. The assay is a modification of the method of Lesch et al.30 The primer sequences were: forward, 5′ -GGCGTTGCCGCTCTGAATGC-3′ (fluorescently labeled) and reverse, 5′ -GAGGGACTGAGCTGGACAACCAC-3′. These primer sequences yielded products of 484 or 528 base pairs (bp). Allele sizes were scored by two investigators independently, and inconsistencies were reviewed and rerun when necessary. To distinguish between the S, LA, and LG fragments, the polymerase chain reaction fragment was digested with MspI according to the methods reported by Wigg et al.31 The resulting polymorphic fragments were separated using an ABI 3130xl DNA sequencer (S: 297, 127, and 62 bp; LA: 340, 127, and 62 bp; LG: 174, 166, 127, and 62 bp). Consistent with previous research,32 3 genotype groups were formed: S′S′ = participants with 2 copies of the lower expressing alleles (SS, SLG, or LGLG), S′L′ = participants with 1 copy of a lower expressing allele (SLA or LGLA), and L′L′ = participants homozygous for the higher expressing LA allele (LALA).
Phenotypes were determined a priori based on a median split of the CES-D outcome score (> 12 = high depressed mood; ≤ 12 = low depressed mood) and by the average total nighttime sleep (total sleep time, TST) across all sleep diary days completed. The CES-D median split was selected because we did not expect students to become clinically depressed, yet we wished to examine for differences in endorsement of depressive symptoms within the sample. Daily TST values were computed as the interval from the time of lights out to the time of lights on minus the minutes of estimated sleep latency. Shorter sleep was defined as an average TST of < 7 hr and longer sleep as > 7.5 hr, based on tertiles of the TST distribution in the sample. Four phenotype groups were identified from the combination of CES-D and TST.
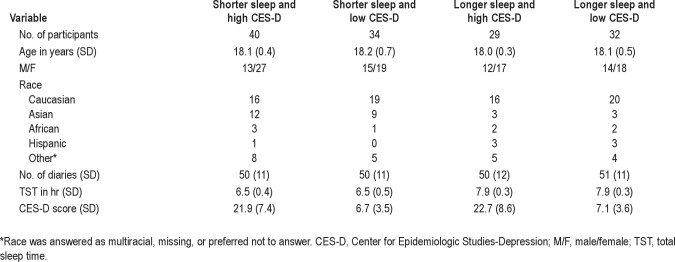
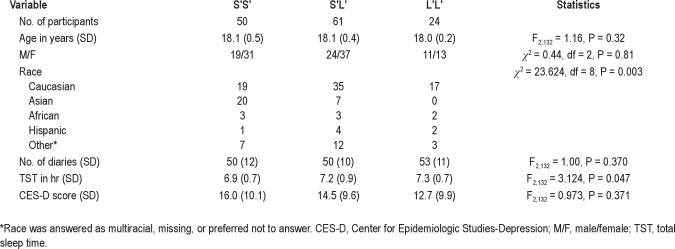
Of the 253 participants who provided DNA samples, 195 completed at least 21 daily diaries and the final CES-D and had successful DNA extraction. Our analyses excluded 60 participants whose average TST was more than 7 hr or less than 7.5 hr. Thus, 135 participants are included in the final dataset. Table 1 provides descriptive data for the phenotype groups, and Table 2 presents descriptive data for the genotype groups.
Table 1.
Phenotype group demographics
Table 2.
Genotype group demographics
Phenotype and genotype groups were examined using analysis of variance (ANOVA) or the chi-square test as appropriate for differences in demographic variables. Fisher least significant differences (LSD) test was used for post hoc analyses after ANOVA. The distribution of genotypes across phenotype groups was examined for association using chi-square testing. Analyses were performed using SPSS version 19 statistical package (IBM, Armonk, NY).
RESULTS
As shown in Table 1, phenotype groups did not differ with respect to sex distribution (chi-square = 1.39; df = 3; P = 0.71), age (F3,131 = 0.67, P = 0.57), racial distribution (chi-square = 14.1; df = 12; P = 0.295), or number of diary nights (F3,131 = 0.04, P = 0.99). As designed, TST (F3,131 = 125.87, P < 0.001) and CES-D (F3,131 = 69.48, P < 0.001) showed significant group differences driven by the phenotype grouping parameters, although Fisher LSD demonstrated that the average TSTs did not differ between the 2 shorter TST groups or between the 2 longer TST phenotype groups. Furthermore, the CES-D scores did not differ between the 2 high CES-D groups and between the 2 low CES-D groups. Table 2 shows significant differences in racial distribution and TST among the 3 genotype groups: the S′S′ group had a significantly higher proportion of Asian students, i.e., 74% of Asian students were S′S′. In addition, Fisher LSD indicates that students in the S′S′ group had shorter TSTs than those in the S′L′ and L′L′ groups. Sex distribution, age, number of diary nights, and CES-D scores did not differ by genotype group.
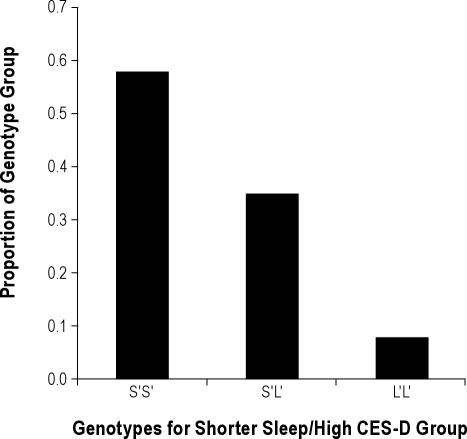
Table 3 provides the distribution of genotypes for each of the phenotypic groups. Results of an exact test for Hardy-Weinberg proportions using Markov chain–Monte Carlo implementation33 indicate that our observed genotype frequencies do not differ from Hardy- Weinberg equilibrium (P = 0.598). A significant overrepresentation of the S′S′ genotype for the 5-HTTLPR was seen for participants who reported shorter sleep and high CES-D scores relative to other groups (chi-square = 15.04; df = 6; P = 0.02). Figure 1 illustrates the proportion of participants carrying the genotypes who were shorter sleepers with high CES-D scores: almost one-half of the S′S′ group showed this phenotype, whereas approximately one-fourth with the S′L′ genotype and only one-eighth of the L′L′ genotype had this phenotype.
Table 3.
Distribution (n) of genotypes within phenotype groups
Figure 1.
Each bar represents the proportion of participants in the high CES-D plus shorter sleep phenotype group who carried the 5-HTTLPR phenotypes.
The bidirectional nature of the sleep and depressed mood association, i.e., that depressed mood can predict poor sleep and vice versa, raises concern that preexisting depression might be the determining factor in the observed genetic association. Thus, to exclude the possibility that the shorter sleep times observed in the daily diaries were biased by ongoing depressive symptoms that preceded the transition to university, we repeated this analysis after removing participants whose CES-D scores from a precollegiate prospective survey were above a clinically meaningful cutoff score of 16.34 Although 32 participants were excluded on this basis, the shorter sleep/high CES-D phenotype remained distinguished from the others by overrepresentation of S′S′ genotype (chi-square = 12.90; df = 6; P = 0.045).
DISCUSSION
Our findings indicate that 1st-year university students who carry 2 alleles of the low-expressing polymorphism of the serotonin transporter gene reported more depressed mood in the presence of a persistent pattern of short nocturnal sleep. Sleep difficulties and depression have been linked in the clinical pantheon since the earliest descriptions of mood disorders (e.g., Hippocrates, 4th century BC). Yet, not all who suffer from sleep disturbance or short sleep are depressed. We propose that a short sleep pattern—whatever the source (insomnia, stress, lifestyle choices)—constitutes an “environmental exposure” that interacts with genetic vulnerability (5-HTTLPR S′S′ genotype), leading to increased likelihood of depressed mood in susceptible individuals. This model nests well within the current theories on 5-HTTPLR and its role in biased attention for emotionally salient cues35 and plasticity to environmental influences.36 For example, a short sleep pattern might reduce the cognitive resources required to shift attention away from negative stimuli. This reduced ability to disengage from negative stimuli would suggest that negative environmental influences might have a more profound effect on mood in relatively sleep-deprived individuals who tend to have biased attention for such exposures. Although our outcome in this analysis was mood, we expect to see a similar association of 5-HTTLPR and chronic short sleep exposure with other domains such as hostility, attention bias, anxiety, fear, suicidality, and so forth.
Although polysomnographic aspects of sleep have been associated with psychopathology, the role of sleep in our model is based on chronic exposure to longer or shorter sleep rather than sleep electroencephalographic features. Thus, the quantity of sleep over an extended time frame is a central factor that can bias outcome depending on genetic background. A key strength of our approach, therefore, was to capture sleep exposure on a night-to-night basis for an average of 50 nights, rather than to ask for a retrospective estimate or a few nights of polysomnography. This approach enhances the conclusion that shorter sleep exposure over time is associated with symptoms of depressed mood in susceptible individuals. The conclusion is further strengthened by the prospective nature of the study, which allowed us to test the hypothesis and confirm the findings after excluding participants with prior evidence of depressed mood.
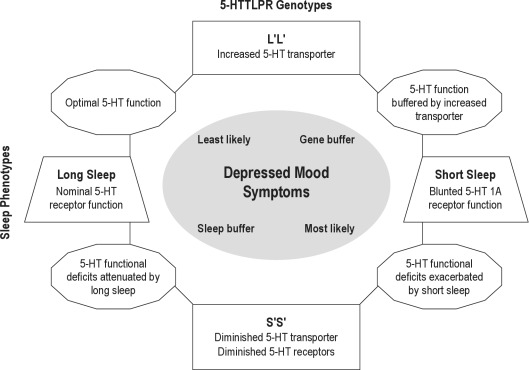
Another advantage of our approach is a clear mechanistic pathway from the environmental exposure, i.e., chronic short sleep, to the outcome, i.e., depressed mood. Thus, the physiology of 5-HT1A receptors and 5-HTTLPR— and the interplay between these 2 components of the serotonin system—suggest a possible mechanism by which exposure to short sleep can manifest as increased depressive symptoms in genetically vulnerable individuals. Central 5-HT1A receptors are present in the dorsal raphé nuclei as presynaptic autoreceptors where they inhibit cell firing and decrease serotonin release.37 In addition, postsynaptic 5-HT1A receptors occur in numerous brain regions that are implicated in mood regulation, e.g., the hippocampus, lateral septum, and cingulate and entorhinal cortex, as well as the raphé nuclei.37 Decreased serotonergic neurotransmission through 5-HT1A has been implicated in depression and suicide,38 and Roman and colleagues observed decreased 5-HT1A neurotransmission after 8 days of sleep restriction in the rat.22 In addition, evidence that low expression of 5-HTTLPR can further reduce 5-HT1A neurotransmission has been shown in 5-HT transporter knockout mice in whom density and expression of 5-HT1A receptors is low.39 Similarly, a positron emission tomography study in human adults showed that individuals with at least 1 S′ allele for the serotonin transporter have decreased 5-HT1A receptor binding.40 Thus, exposure to short sleep duration in the presence of S′ alleles of 5-HTTLPR may represent an additional insult to the serotonin system, leaving those with the 5-HTTLPR short alleles most vulnerable to depressed mood from shortened sleep. Figure 2 provides a schematic model of these effects.
Figure 2.
Illustration of a mechanistic model linking sleep length (horizontal) and genotype (vertical) in the expression of depressed mood. The 5-HTTLPR polymorphisms (S′S′ and L′L′) are associated with differences in transporter expression30 and number of presynaptic and postsynaptic 5-HT1A receptors.39 Shorter sleep is associated with blunting of 5-HT1A receptor function.22 The synergy between these 2 influences may place those with S′S′ genotype at greater risk for depressed mood symptoms under circumstances of prolonged short sleep, as illustrated by the diagram in the lower right region in which short sleep blunting of 5-HT1A receptors is noted as exacerbating the 5-HT deficits.
We recognize that our findings are limited by a relatively small sample size; however, the project is ongoing and future analyses will examine this finding with more participants. In addition, we plan to use a larger sample to examine whether cumulative or aggregate genetic scores across several genes may help to identify other pathways that affect the association of sleep length to mood disorders, and whether genes that are known to affect sleep “need” and sleep length act as moderator factors in response to the shorter or longer sleep exposures. As with all genetic association studies, we acknowledge a risk of unmeasured third variables accounting for the results, including the possibility of population stratification or linkage disequilibrium between measured and causal variants. A larger sample will allow us to examine these issues.
We note that other outcomes such as weight gain,41 cardiovascular consequences,42 risk-taking behavior,43 substance abuse,44 and impulsivity 45 have been linked to short sleep, though with inconsistent expression. As with depressed mood, individual differences may be understood better by using a gene by “environmental” exposure (G × E) approach. In other words, exposure to chronic levels of insufficient or disrupted sleep may manifest a preexisting vulnerability in genetically susceptible individuals, whereas exposure to longer, less disrupted sleep may lead to improved outcomes.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Tifenn Raffray, MD, and Tamara Bond, PhD, for assisting with the design of the surveys, Brandy Roane, PhD, for assistance with data collection and extraction, Caroline Gredvig-Ardetto for data management, Michelle Loxley for Illume programming, Kayla Beaucage for DNA assays, and the staff of the Sleep for Science Research Laboratory for assisting with sample collection. This research was supported by the Sleep Research Society Foundation Elliot D. Weitzman, MD, Research Grant and National Institute of Mental Health grant, MH079179, awarded to MAC, 1S10RR023457-01A1 and Shared equipment grants (ShEEP) from the Medical Research Service of the Department of Veteran Affairs, awarded to JEM, and K23MH086689 awarded to KMS.
Footnotes
A commentary on this article appears in this issue on page 739.
REFERENCES
- 1.Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression. JAMA. 2009;301:2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm K, Mitchell PB, Niven H, et al. Life events, first depression onset and the serotonin transporter gene. Br J Psychiatry. 2006;188:210–15. doi: 10.1192/bjp.bp.105.009522. [DOI] [PubMed] [Google Scholar]
- 4.Cervilla JA, Molina E, Rivera M, et al. The risk for depression conferred by stressful life events is modified by variation at the serotonin transporter 5HTTLPR genotype: evidence from the Spanish PREDICT-Gene cohort. Mol Psychiatry. 2007;12:748–55. doi: 10.1038/sj.mp.4001981. [DOI] [PubMed] [Google Scholar]
- 5.Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42:209–12. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 6.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. discussion 69-70. [DOI] [PubMed] [Google Scholar]
- 7.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 8.Benca RM, Okawa M, Uchiyama M, et al. Sleep and mood disorders. Sleep Med Rev. 1997;1:45–56. doi: 10.1016/s1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins DR, Taub JM, Van de Castle RL. Extended sleep (hypersomnia) in young depressed patients. Am J Psychiatry. 1985;142:905–10. doi: 10.1176/ajp.142.8.905. [DOI] [PubMed] [Google Scholar]
- 10.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 11.National Sleep Foundation. Sleep in America Poll - Teens and Sleep. 2006. [cited 2011 September 29]. Available from: http://www.sleepfoundation.org/article/sleep-america-polls/2006-teens-and-sleep.
- 12.Fitzgerald CT, Messias E, Buysse DJ. Teen sleep and suicidality: results from the youth risk behavior surveys of 2007 and 2009. J Clin Sleep Med. 2011;7:351–6. doi: 10.5664/JCSM.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller RW, Wong DT. Effects of antidepressants on uptake and receptor systems in the brain. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:485–90. doi: 10.1016/0278-5846(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 14.Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. 2011;15:269–81. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Monti JM. The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev. 2010;14:319–27. doi: 10.1016/j.smrv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Lydic R, McCarley RW, Hobson JA. Serotonin neurons and sleep. II. Time course of dorsal raphe discharge, PGO waves, and behavioral states. Arch Ital Biol. 1987;126:1–28. [PubMed] [Google Scholar]
- 17.Lydic R, McCarley RW, Hobson JA. Serotonin neurons and sleep. I. Long term recordings of dorsal raphe discharge frequency and PGO waves. Arch Ital Biol. 1987;125:317–43. [PubMed] [Google Scholar]
- 18.Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2009;44:242–52. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Kupfer DJ. REM latency: a psychobiologic marker for primary depressive disease. Biol Psychiatry. 1976;11:159–74. [PubMed] [Google Scholar]
- 20.Vogel GW, Traub AC, Ben-Horin P, Meyers GM. REM deprivation. II. The effects on depressed patients. Arch Gen Psychiatry. 1968;18:301–11. doi: 10.1001/archpsyc.1968.01740030045006. [DOI] [PubMed] [Google Scholar]
- 21.Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–77. [PubMed] [Google Scholar]
- 22.Roman V, Walstra I, Luiten PG, Meerlo P. Too little sleep gradually desensitizes the serotonin 1A receptor system. Sleep. 2005;28:1505–10. [PubMed] [Google Scholar]
- 23.Paykel ES. Life events and affective disorders. Acta Psychiatr Scand Suppl. 2003:61–6. doi: 10.1034/j.1600-0447.108.s418.13.x. [DOI] [PubMed] [Google Scholar]
- 24.Hicks T, Heastie S. High school to college transition: a profile of the stressors, physical and psychological health issues that affect the first-year on-campus college student. J Cult Divers. 2008;15:143–7. [PubMed] [Google Scholar]
- 25.Dyson R, Renk K. Freshmen adaptation to university life: depressive symptoms, stress, and coping. J Clin Psychol. 2006;62:1231–44. doi: 10.1002/jclp.20295. [DOI] [PubMed] [Google Scholar]
- 26.Levine B, Roehrs T, Zorick F, Roth T. Daytime sleepiness in young adults. Sleep. 1988;11:39–46. [PubMed] [Google Scholar]
- 27.Carskadon M, Davis S. Sleep-wake patterns in the high school-to-college transition: preliminary data. Sleep Research. 1989;18:113. [Google Scholar]
- 28.Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;1:1356–8. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- 29.Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet. 1997;27:251–7. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- 30.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 31.Wigg KG, Takhar A, Ickowicz A, et al. Gene for the serotonin transporter and ADHD: no association with two functional polymorphisms. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:566–70. doi: 10.1002/ajmg.b.30247. [DOI] [PubMed] [Google Scholar]
- 32.Zalsman G, Huang YY, Oquendo MA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–93. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 33.Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–72. [PubMed] [Google Scholar]
- 34.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 35.Gibb B, Beevers C, McGeary J. Towards an integration of cognitive and genetic models of risk for depression. Cognit Emot. doi: 10.1080/02699931.2012.712950. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 37.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Lemonde S, Turecki G, Bakish D, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–99. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7888–95. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David SP, Murthy NV, Rabiner EA, et al. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci. 2005;25:2586–90. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: a systematic review. Sleep Med Rev. 2011 doi: 10.1016/j.smrv.2011.05.005. Epub ahead of print, PMID:21784678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 43.Yen CF, King BH, Tang TC. The association between short and long nocturnal sleep durations and risky behaviours and the moderating factors in Taiwanese adolescents. Psychiatry Res. 2010;179:69–74. doi: 10.1016/j.psychres.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 44.John U, Meyer C, Rumpf HJ, Hapke U. Relationships of psychiatric disorders with sleep duration in an adult general population sample. J Psychiatr Res. 2005;39:577–83. doi: 10.1016/j.jpsychires.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Paavonen EJ, Raikkonen K, Lahti J, et al. Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics. 2009;123:e857–64. doi: 10.1542/peds.2008-2164. [DOI] [PubMed] [Google Scholar]