Abstract
Background:
Correct diagnosis of rapid eye movement sleep behavior disorder (RBD) is important because it can be the first manifestation of a neurodegenerative disease, it may lead to serious injury, and it is a well-treatable disorder. We evaluated the electromyographic (EMG) activity in the Sleep Innsbruck Barcelona (SINBAR) montage (mentalis, flexor digitorum superficialis, extensor digitorum brevis) and other muscles to obtain normative values for the correct diagnosis of RBD for clinical practice.
Setting:
Two university hospital sleep disorder centers.
Participants:
Thirty RBD patients (15 idiopathic [iRBD], 15 with Parkinson disease [PD]) and 30 matched controls recruited from patients with effectively treated sleep related breathing disorders.
Interventions:
Not applicable.
Methods and Results:
Participants underwent video-polysomnography, including registration of 11 body muscles. Tonic, phasic, and “any” (any type of EMG activity, irrespective of whether it consisted of tonic, phasic or a combination of both) EMG activity was blindly quantified for each muscle. When choosing a specificity of 100%, the 3-sec miniepoch cutoff for a diagnosis of RBD was 18% for “any” EMG activity in the mentalis muscle (area under the curve [AUC] 0.990). Discriminative power was higher in upper limb (100% specificity, AUC 0.987–9.997) than in lower limb muscles (100% specificity, AUC 0.813–0.852). The combination of “any” EMG activity in the mentalis muscle with both phasic flexor digitorum superficialis muscles yielded a cutoff of 32% (AUC 0.998) for patients with iRBD and with PD-RBD.
Conclusion:
For the diagnosis of iRBD and RBD associated with PD, we recommend a polysomnographic montage quantifying “any” (any type of EMG activity, irrespective of whether it consisted of tonic, phasic or a combination of both) EMG activity in the mentalis muscle and phasic EMG activity in the right and left flexor digitorum superficialis muscles in the upper limbs with a cutoff of 32%, when using 3-sec miniepochs.
Citation:
Frauscher B; Iranzo A; Gaig C; Gschliesser V; Guaita M; Raffelseder V; Ehrmann L; Sola N; Salamero M; Tolosa E; Poewe W; Santamaria J; Högl B. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. SLEEP 2012;35(6):835-847.
Keywords: SINBAR EMG montage, normal values, cutoff, EMG activity, quantification, movement disorders
INTRODUCTION
Rapid eye movement sleep behavior disorder (RBD) is a parasomnia characterized by excessive electromyographic (EMG) activity during rapid eye movement (REM) sleep and acting out of dreams, leading to injurious or potentially injurious behaviors to the patient or bedmate.1,2 Available data indicate that in many patients, idiopathic RBD (iRBD) is an early sign of an evolving synucleinopathy such as Parkinson disease (PD).3–6 In addition, in patients with established neurodegenerative disorders such as PD, recognition of RBD is important because it may require specific treatment.2 A correct diagnosis of RBD is therefore of the utmost importance. Only video-polysomnography (PSG) allows for a sufficiently sensitive and specific diagnosis of RBD. Sleep history alone may lead to false-positive results because of RBD mimics7–10 and false-negative results because of the patient's potential unawareness of his or her dream-enacting behaviors,11 lack of an attentive bedmate, and mild and uncommon manifestations.12,13 One big step in improving accuracy of RBD diagnosis was the revision of the International Classification of Sleep Disorders (ICSD-2), which now includes PSG as mandatory for RBD diagnosis.14 However, until now, ICSD-2 criteria did not provide cutoff values for the definition of “excessive amounts of phasic or tonic EMG activity“ nor did they clearly indicate which muscles should be investigated in patients with suspected RBD. In iRBD patients and those with RBD associated with PD, we previously performed a quantitative EMG analysis in 13 different muscles of the body to investigate which combination of muscles provided the highest rates of phasic EMG activity by a still-acceptable number of EMG channels.15 Highest rates of phasic EMG activity were found in the mentalis muscle, the flexor digitorum superficialis (FDS) muscle in the upper limb muscles, and the extensor digitorum brevis (EDB) muscle in the lower limb muscles in what we termed the SINBAR EMG montage. Phasic EMG activity differed significantly according to the evaluated muscles. In a second study, we performed a combined video-PSG analysis in patients with iRBD to investigate if the characteristic clinical motor (e.g., jerking, punching, etc.) and vocal (e.g., moaning, shouting, etc.) manifestations of RBD were captured using this muscle combination.16 Using this combination of muscles, EMG activity was present in 95% of all motor and vocal manifestations, whereas using the mentalis muscle alone, the common practice in many centers involved in RBD research, EMG activity was only present in 35% of these behaviors. Following these 2 studies we felt the need to establish the degree of EMG activity that can be considered within normal limits and which degree exceeds this limit and should be considered abnormal. Thus, in the current study we sought to evaluate in detail the EMG activity in the SINBAR EMG montage and other muscles in patients with RBD to obtain normative EMG values with calculated cutoffs for establishing the diagnosis of RBD in clinical practice.
METHODS
Patient Selection
Thirty consecutive patients from the sleep laboratories of the Departments of Neurology of Innsbruck Medical University, Austria (n = 15), and Hospital Clinic de Barcelona, Spain (n = 15) with the diagnosis of iRBD (n = 15) and secondary RBD associated with PD (n = 15) were included. Diagnosis of RBD required a history of dream-enacting behaviors and nocturnal video-PSG demonstrating prominent tonic and/or phasic EMG activity associated with abnormal behaviors and absence of electroencephalographic epileptiform activity during REM sleep.14
Diagnosis of iRBD required absence of cognitive and motor complaints, and normal neurologic examination. The diagnosis of PD was made according to the United Kingdom PD Society Brain Bank criteria.17
Controls were 30 consecutive sex- and age-matched individuals recruited from patients with sleep related breathing disorders under effective treatment with either nasal continuous positive airway pressure (CPAP) therapy (n = 28) or an intraoral device (n = 1) for at least 6 months and who did not have current sleep complaints; absence of RBD was confirmed by PSG. None of the controls reported dream-enactment behaviors before or after treatment initiation with CPAP therapy or oral devices. One control patient was a healthy volunteer without any sleep complaint and without sleep disordered breathing in PSG. Exclusion criteria for RBD patients and controls were an apnea-hypopnea index (AHI) > 10 per hr of total sleep, and an AHI > 10 per hr of REM sleep, history of bruxism, presence of snoring despite treatment with CPAP or an oral device, history suggestive of a clinically relevant sleep disorder, and treatment with clonazepam or melatonin. In patients with iRBD and controls, current or past use of any central nervous system (CNS) active medication was a further exclusion criterion. In patients with PD, CNS active medications except clonazepam and melatonin were accepted. All participants granted written informed consent according to the Declaration of Helsinki.
Nocturnal PSG
Nocturnal PSG was performed with a digital polygraph (Innsbruck: Schwarzer Brainlab, software version 4.00, Munich, Germany; Barcelona: Deltamed, software version 2007 Paris, France) and consisted of electrooculography, electroencephalography (F4-A1, C4-A1, O2-A1), surface EMG of 11 muscles in different localizations of the body, electrocardiography, nasal and oral air flow, thoracic and abdominal respiratory effort, oxygen saturation, and microphone and digitally synchronized videography. Sleep stages were scored according to American Academy of Sleep Medicine (AASM) criteria18 with allowance to score REM sleep despite excessive EMG activity in the mentalis muscle channel.19 The occurrence of the first REM in the electrooculographic channel was used to determine the onset of REM sleep period. The end of the REM sleep period was determined when either no REMs were detected in 3 consecutive minutes or an awakening, K complexes, or spindles were observed. A new REM sleep episode was considered when it occurred 30 min or more after the previous episode. Otherwise it was considered part of the same REM sleep episode. Scoring of sleep stages and quantification of phasic and tonic EMG activity in REM sleep was done by different investigators who were blinded to the patient's category. Because of ongoing collaboration and exchange between both participating centers, the interrater reliability is high, with a mean kappa coefficient of 0.872.15
Investigated Muscles
Surface EMG activity of 11 different body localizations was quantified during REM sleep. We evaluated the mentalis muscle and bilateral EMG activity from sternocleidomastoid, biceps brachii, FDS, anterior tibialis, and EDB muscles. These muscles were chosen according to our experience in RBD because they had either the higher rates of phasic EMG activity in REM sleep (mentalis, FDS, EDB, and sternocleidomastoid) or in the presence of medium rates of phasic EMG activity because they are proximal limb muscles (biceps brachii) or commonly recorded in routine PSG (anterior tibialis).15 For the sake of simplicity, we did not include the abductor pollicis brevis muscle because it shows rates of phasic EMG activity similar to that of the FDS muscle and a high number of artifacts according to our experience.15
Two surface electrodes per muscle were attached to the skin with each electrode spaced 2 to 3 cm apart. The EMG activity was analyzed in all channels using amplification at 5 μV/mm, low-frequency filter at 10 Hz, high-frequency filter at 100 Hz, and a sampling rate of 256 Hz. Impedances of surface EMG electrodes had to be lower than 10 kΩ.
Analysis of EMG Activity
In each participant, quantification of tonic, phasic, and “any” (either tonic or phasic) EMG activity was performed manually during REM sleep. EMG activity was not measured during non-REM sleep. Before the analysis of EMG activity, REM sleep was carefully examined for artifacts (e.g., snoring) and increases in EMG tone due to arousals from respiratory events. All artifacts and increases in EMG tone due to arousals from respiratory events were excluded from the quantitative scoring of REM sleep related EMG activity. Scorers were blinded to the patient's category (control, iRBD, PD-RBD).
Tonic EMG activity was scored only in the mentalis muscle channel using 30-sec epochs. Each epoch was scored as “tonic” when the increased sustained EMG activity was present in more than 50% of the total 30-sec epoch duration with an amplitude of at least twice the background EMG muscle tone or more than 10 μV. The periods of PSG shorter than a full 30-sec epoch of sleep, mainly occurring at the end of each REM sleep episode, were not included in this analysis.
For scoring phasic EMG activity, the recording was divided into 3-sec miniepochs. Each 3-sec miniepoch was scored as having or not having “phasic” EMG activity. Phasic EMG activity was defined as any burst of EMG activity lasting between 0.1 and 5.0 sec with an amplitude exceeding twice the background EMG activity irrespective of its morphology. We defined the end of each phasic EMG burst when there was an identifiable return to the baseline or an interburst interval of more than 250 msec. When analyzing the tonic EMG in the mentalis muscle channel it was not unusual to see bursts of phasic activity superimposed on a background of tonic activity. To identify a burst of phasic EMG activity during a 3-sec miniepoch with sustained tonic activity, it was required that the burst of phasic EMG had at least twice the amplitude of the background tonic EMG activity within that same 3-sec miniepoch, resulting in a waxing and waning morphology as opposed to the sustained isomorphic aspect of tonic EMG epochs not containing phasic activity. For analysis of phasic EMG activity, each EMG channel was scored separately on the computer screen. After finishing the scoring of 1 EMG channel, the process was repeated in the remaining EMG channels. Every 3-sec miniepoch in each EMG channel was scored as “0” when phasic EMG activity was not present, “1” when phasic EMG activity was present, or “3” when an artifact prevented optimal scoring. Miniepochs scored as artifacts (“3”) were excluded from analysis.
To simplify the scoring system and to include periods of phasic EMG activity between 5 and 15 sec that are not measured with current scoring systems, we also scored each 3-sec miniepoch as having or not having “any” EMG activity, irrespective of whether it contained tonic, phasic, or a combination of both EMG activities. Finally, we also calculated the percentage of 30-sec epochs containing 5 or more 3-sec miniepochs with phasic EMG activity, following the suggestion of the last edition of the AASM Manual for the Scoring of Sleep and associated events.18
Periodic leg movements during sleep (PLMS) were scored according to World Association of Sleep Medicine criteria.20 A PLMS index was calculated independently in both right and left anterior tibialis and right and left EDB. PLMS during REM sleep were distinguished from phasic EMG activity by their characteristic periodic EMG and videographic appearance. Once PLMS were identified in REM sleep, they were excluded from the quantitative analysis of RBD-related phasic EMG activity.
Statistical Analysis
Descriptive demographic, clinical, and PSG data are given as mean ± standard deviations, numbers, and frequencies. Demographic, clinical, and PSG variables were compared among RBD patients and controls with a t test for quantitative variables and with a chi-square test for qualitative variables. The same analyses were performed to compare patients with iRBD and RBD associated with PD. We used the t test for comparison of rates of EMG activity in the different muscles and combinations of muscles among RBD patients and controls, and among patients with iRBD and RBD associated with PD. We computed the rates of phasic EMG activity in different combinations of muscles, including those most commonly used in routine practice and other combinations of the mentalis muscle with upper or lower limb muscles to determine the best combination to distinguish RBD patients from controls. In this study, a 3-sec miniepoch was computed as having phasic EMG activity when at least 1 of the muscles evaluated in a specific combination had phasic EMG activity, and as not having phasic EMG activity when none of the muscles in the combination had phasic EMG activity. A similar analysis was performed for the “any” EMG activity measure. Receiver operator characteristic (ROC) curves were computed for tonic, phasic, and “any” EMG activity in all the different muscles and combinations of muscles. ROC analyses were performed among RBD patients and controls, and among patients with iRBD and RBD associated with PD. In all analyses, the area under the curve (AUC) was calculated, and sensitivity and specificity values for different cutoff points were obtained. The 95% confidence intervals were computed with 2,000 stratified bootstrap replicates.21 Due to the implications of diagnosing iRBD in a patient, we decided to look for cutoff scores that yielded 100% specificity. Finally, rates of isolated and simultaneous phasic EMG activity in different combinations of the recorded muscles were calculated.
RESULTS
Demographic, Clinical, and PSG Characteristics
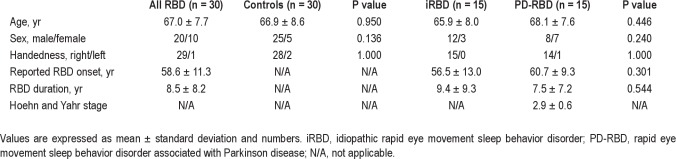
The RBD group consisted of 30 patients (20 men, 10 women) with a mean age of 67.0 ± 7.7 yr; more specifically, 15 patients had iRBD (12 men, 3 women) and 15 patients had RBD associated with PD (8 men, 7 women). Mean RBD duration was 8.5 ± 8.2 yr. With 1 exception, all patients were right-handed. Patients with iRBD were not on CNS active medication. All patients with PD were on antiparkinsonian drugs (12 on levodopa/carbidopa, 7 on pramipexole, 5 on rasagiline, 3 on ropinirole, 4 on entacapone, 2 on amantadine, and 1 on selegiline), 5 on antidepressants (2 on mirtazapine, and 1 each on citalopram, venlafaxine, and sertraline), 1 on neuroleptic agents (olanzapine), and 1 on hypnotic agents (diazepam). Mean Hoehn and Yahr score in patients with PD was 2.9 ± 0.6 (range, 1.5–4). There was no difference in sex, age, handedness, and RBD duration between the iRBD and the PD-RBD groups. RBD patients were compared with 30 CNS drug-naïve controls (25 men, 5 women) with a mean age of 66.9 ± 8.6 yr. Age and sex did not differ among RBD patients and controls (Table 1).
Table 1.
Demographic and clinical characteristics of RBD patients and controls
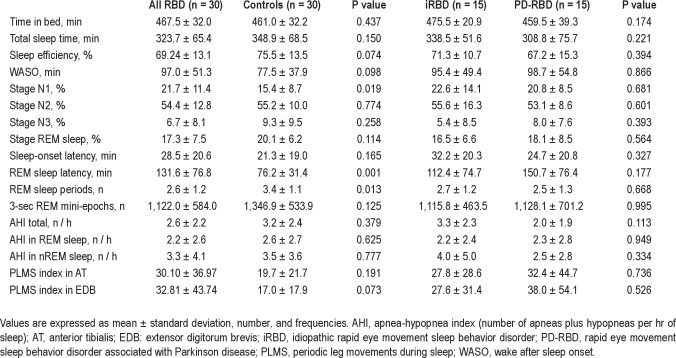
PSG showed that patients with RBD had significantly higher amounts of stage N1 sleep, longer REM sleep latency, and fewer REM episodes than controls (P < 0.05) (Table 2). All other sleep parameters did not differ among RBD patients and controls. In particular, PLMS indices were higher in RBD patients than in controls in both anterior tibialis and EDB muscles but did not reach significant differences. There were no significant differences in sleep parameters among patients with iRBD and PD-RBD (Table 2). AHI was not different among RBD patients and controls, and among patients with iRBD and PD-RBD (Table 2).
Table 2.
Polysomnographic variables in RBD patients and controls
Analysis of EMG Activity
We manually analyzed 74,066 3-sec miniepochs of REM sleep for each of the 11 muscles recorded in the 60 patients included in the study. This corresponds to a total of 814,726 3-sec miniepochs of REM sleep. The mean number of 3-sec miniepochs analyzed per patient was 1,234.4 ± 566.2 (range, 145–2,925) for each muscle. We also computed EMG activity during 7,125 full 30-sec REM sleep epochs in each of the 11 muscles recorded in the study population, with a mean of 118.8 ± 55.5 30-sec epochs (range, 11- 287) per patient.
Analysis of EMG Activity Using 3-Sec Miniepochs
EMG activity in the mentalis muscle
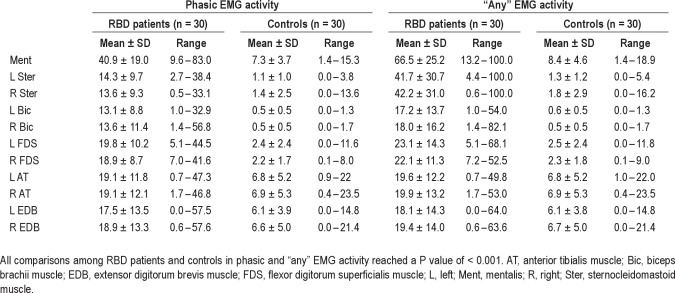
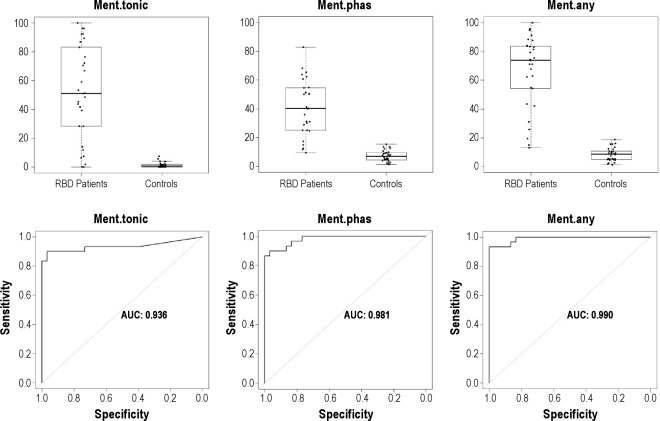
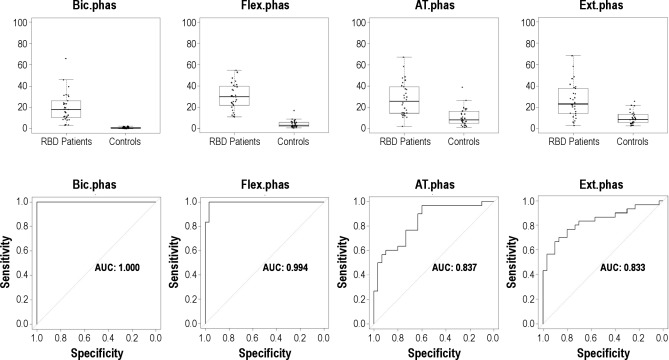
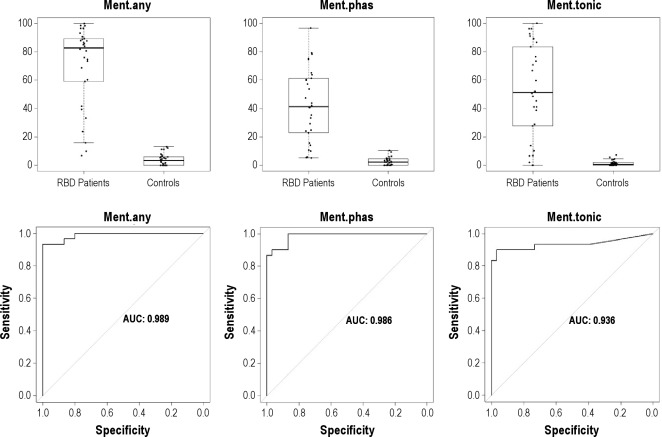
The mean rates of mentalis muscle phasic, tonic, and “any” EMG activity were significantly higher in patients with RBD than in controls (all P values were < 0.001). Mean percentage of tonic EMG activity was 51.8 ± 32.8 (range, 0–100) in patients with RBD and 1.4 ± 1.9 (range, 0–7.5) in controls. Mean percentage of phasic EMG activity was 40.9 ± 19.0 (range, 9.6–83.0) in RBD patients and 7.3 ± 3.7 (range, 1.4–15.3) in controls. Mean percentage of “any” EMG activity was 66.5 ± 25.2 (range, 13.2–100) in patients with RBD and 8.4 ± 4.6 (range, 1.4–18.9) in controls (Table 3). There were patients with RBD who had rates of mentalis muscle EMG activity that were clearly within the range of control values with an area of overlap, no matter which EMG metrics we used (Figure 1). “Any” EMG activity had the highest AUC value in the ROC analysis (0.990), followed by phasic (0.981) and tonic (0.936) EMG activity.
Table 3.
Rates of 3-sec miniepochs with phasic and “any” EMG activity
Figure 1.
Boxplot representation of the percentages of different types of mentalis muscle EMG activity (tonic, phasic, “any”) measured in 3-sec miniepochs in patients with RBD and controls, and corresponding receiver operating characteristic curves. AUC, area under the curve; Ment, mentalis muscle; Phas, phasic EMG activity.
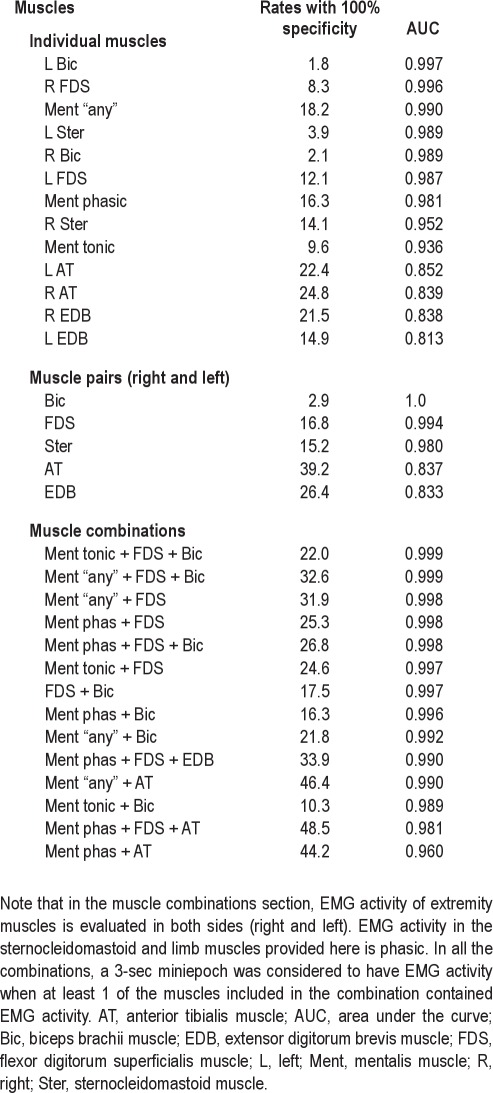
Phasic and “any” EMG activity in the sternocleidomastoid and limb muscles
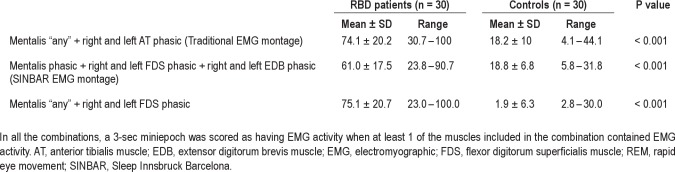
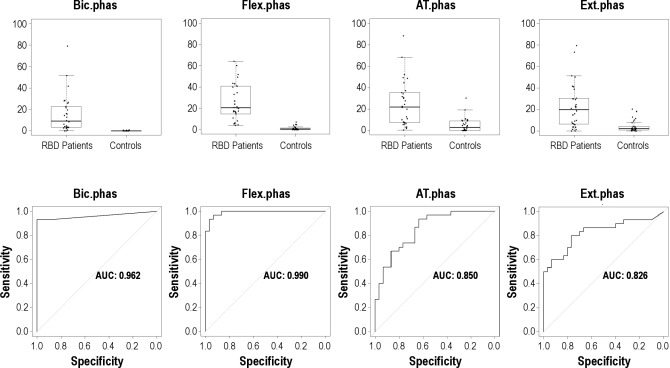
Sternocleidomastoid and limb muscles were both evaluated bilaterally. The mean rates of both phasic and “any” EMG activity in REM sleep were significantly higher in patients with RBD than in controls in all muscles evaluated (all comparisons had a P value < 0.001). Interestingly, phasic EMG activity in controls was more frequent in lower limbs than in the mentalis muscle and than in the upper limbs. Upper limb muscles had a much greater AUC than the lower limb muscles (Figure 2). Bilateral biceps brachii muscle had the highest AUC (1.000), followed by the FDS (0.994), the anterior tibialis (0.837), and the EDB muscles (0.833). Lower limb muscles had rates of phasic EMG activity with an important overlap among RBD patients and controls (Figure 2).
Figure 2.
Boxplot representation of the percentages of different types of phasic EMG activity measured in 3-sec miniepochs in the 4 bilateral limb muscles of the upper and lower limbs in patients with RBD and controls, and corresponding receiver operating characteristic curves. All muscles were evaluated bilaterally. AT, anterior tibialis muscle; AUC, area under the curve; Bic, biceps brachii muscle; Ext, extensor digitorum brevis muscle; Flex, flexor digitorum superficialis muscle.
Combination of muscles
In addition to the analysis of EMG activity in single muscles, we computed the rates of EMG activity in REM sleep in different combinations of muscles, including the mentalis with 1 of the upper limb muscles, the mentalis with 1 of the lower limb muscles, and finally the mentalis together with an upper and a lower limb muscle as in the SINBAR EMG montage (mentalis + FDS + EDB). In these combinations, limb muscles were evaluated bilaterally. In all the combinations, a 3-sec miniepoch was considered to have EMG activity when at least 1 of the muscles included in the combination contained EMG activity. All muscle combinations showed significantly higher rates of EMG activity in RBD than in controls (P < 0.001). For the sake of simplicity, the results of only 3 of these combinations are presented in Table 4.
Table 4.
Three-sec miniepochs of EMG activity in REM sleep in different muscle combinations
Cutoff values using 3-sec miniepochs
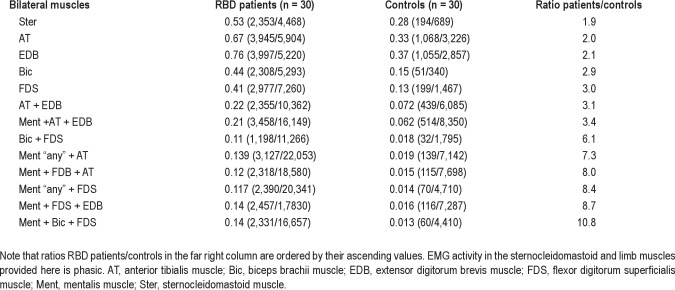
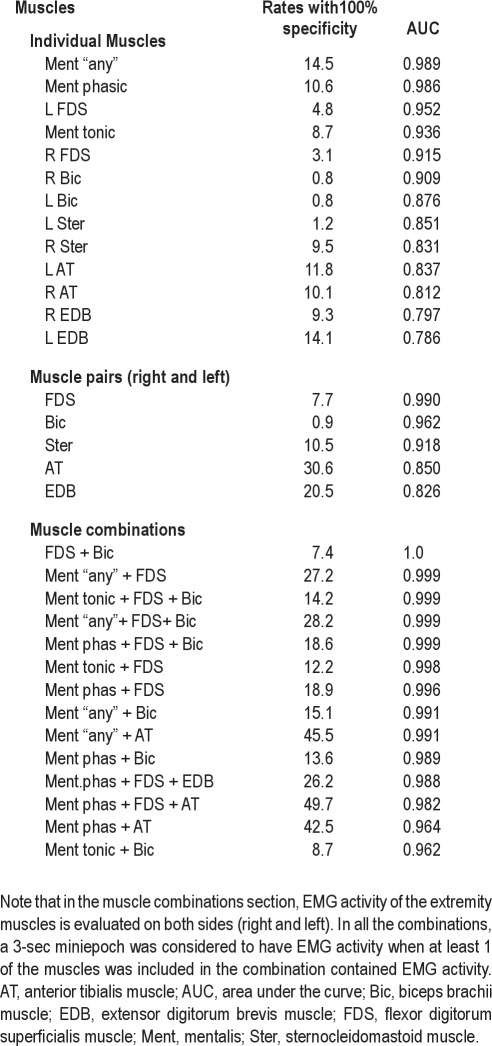
We explored the cutoff values, based on the percentage of 3-sec miniepochs of a full PSG night containing EMG activity, yielding 100% specificity for the diagnosis of RBD in individual muscles as well as in different combinations of muscles using tonic, phasic, and “any” EMG activity in the mentalis muscle, and phasic and “any” EMG acitivity in the rest of the muscles evaluated in this study (Table 5). In the mentalis muscle, the cutoff was 16.3% for phasic EMG activity (AUC 0.981) and 9.6% for tonic EMG activity (AUC 0.936).
Table 5.
Cutoff values for rates of EMG activity with 100% specificity for RBD in individual muscles and combinations of muscles ordered from highest to lowest AUC values in 3-sec miniepochs
The highest AUC value was found in both biceps brachii muscles (AUC 1.000), followed by the combination of the mentalis muscle (tonic or “any”) with both biceps brachii and both FDS muscles (AUC 0.999), the combination of the mentalis muscle (tonic or “any”) with both FDS muscles (AUC 0.997–0.998), and the combination of the mentalis muscle (phasic) with both FDS and both biceps brachii muscles (AUC 0.998). The left biceps brachii and the right FDS were the individual muscles with highest AUC values. Lower limb muscles, individually or in combination with any other muscle or combination of muscles, tended to decrease the AUC values, showing a lower discriminative power. The combination of the mentalis muscle (“any”) with both anterior tibialis muscles (the standard EMG montage used in routine PSG) had an AUC of 0.990. Discriminative power was higher in upper limbs (100% specificity, AUC 0.987–9.997) than in lower limb muscles (100% specificity, AUC 0.813- 0.852).
Simultaneous and isolated phasic EMG activity
We measured the ratio simultaneous/isolated phasic EMG activity in the 5 muscles recorded bilaterally as well as in different combination of muscles, including the 4 muscles of the upper limbs (right and left biceps brachii, right and left FDS), the 4 muscles of the lower limbs (right and left anterior tibialis, right and left EDB), and combinations of the mentalis muscle with upper and lower limb muscles (Table 6). We found that RBD patients had twice as frequent 3-sec miniepochs with simultaneous phasic EMG activity than controls, and this number was higher in muscles of the upper limbs than of the lower limbs. Simultaneous activation of the mentalis and bilateral FDS muscles was 8 times more frequent in RBD patients than in controls.
Table 6.
Ratios of 3-sec miniepochs with simultaneous activity/total number of 3-sec miniepochs with EMG activity in the 5 muscles recorded bilaterally and in combinations of muscles in RBD patients and controls
Comparison between iRBD and PD associated with RBD
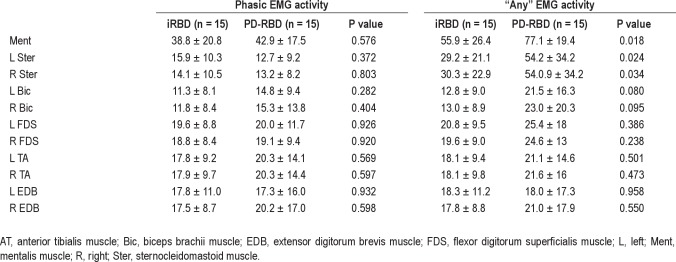
Tonic EMG activity in the mentalis muscle was significantly higher in PD-RBD patients (66.8 ± 30.4) than in iRBD patients (36.8 ± 28.7) (P = 0.009). There were no significant differences in phasic EMG activity in the 11 muscles recorded between the 2 groups. The only significant differences in the “any” EMG activity measure were found in the mentalis muscle (P = 0.018) and in the left and right sternocleidomastoid muscles (P = 0.024 and 0.034, respectively), probably because these muscles have more tonic EMG activity. The cutoff of 31.9 (AUC 0.998) for the combination between “any” mentalis muscle EMG activity and bilateral FDS was the same for both iRBD and PD-RBD groups (Table 7).
Table 7.
Rates of phasic EMG activity and “any” EMG activity in iRBD and RBD patients with PD
EMG Analysis Using 30-Sec Epochs
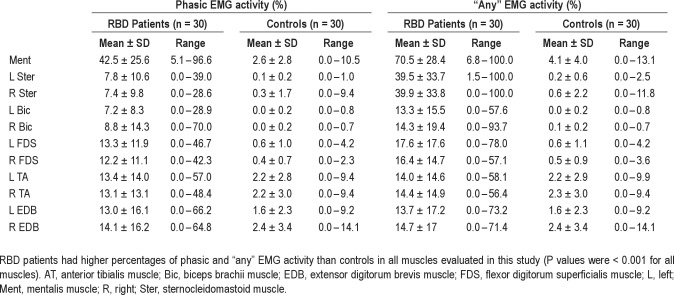
We also computed the rates of phasic EMG activity and “any” EMG activity in 30-sec epochs. For this analysis, a 30-sec epoch containing 5 or more 3-sec miniepochs with phasic EMG activity was considered a “phasic” epoch. A similar approach was made for the category “any” EMG activity. Using these metrics, RBD patients had higher percentages of phasic and “any” EMG activity than controls in all muscles evaluated in this study (P values were < 0.001 for all muscles) (Table 8). In patients with RBD and in controls, the percentages of 30-sec epochs with phasic EMG activity in all muscles evaluated were similar to those found when using the 3-sec miniepochs (Figures 3 and 4).
Table 8.
Rates of 30-sec epochs with phasic and “any” EMG activity in RBD patients and controls
Figure 3.
Boxplot representation and the corresponding receiver operating characteristic curves of the mentalis muscle EMG activity values measured in 30-sec epochs. AUC, area under the curve; Ment, mentalis muscle; Phas, phasic EMG activity.
Figure 4.
Boxplot representation of the percentages of different types of 30-sec epochs with phasic EMG activity in the 4 muscles of the upper and lower limbs in RBD patients and controls and corresponding receiver operating characteristic curves. Muscles are presented bilaterally. AT, anterior tibialis muscle; AUC, area under the curve; Bic, biceps brachii muscle; Flex, flexor digitorum superficialis muscle; phas, phasic EMG activity.
Using the 30-sec approach, the AUC values for distinction among patients with RBD and controls remained very similar to the 3-sec miniepoch approach. The AUC for mentalis muscle “any” EMG activity is 0.989 in the 30-sec approach and 0.990 in the 3-sec miniepoch approach. The AUC for the combination of mentalis muscle “any” EMG activity with both FDS muscles is 0.999 in the 30-sec approach and also 0.998 in the 3-sec miniepoch approach (Tables 8 and 9).
Table 9.
Cutoff values for rates of EMG activity with 100% specificity for RBD in individual muscles and combinations of muscles using 30-sec epochs
DISCUSSION
To the best of our knowledge, this is the first study that investigates different body muscles and types of EMG activity for best discriminating RBD from non-RBD individuals. We chose this approach because isolated use of the mentalis muscle is prone to breathing and snoring artifacts, it shows low correlation with most behaviors that patients with RBD display during REM sleep,16 and the differentiation into phasic and tonic EMG activity can be challenging. Previous studies by our group aiming to develop a suitable EMG montage for RBD detection demonstrated that a combination of the mentalis, the FDS, and the EDB muscles (SINBAR EMG montage) showed the highest rates of phasic EMG activity in RBD.15 In a consecutive video-PSG study, this montage was found to be useful for capturing more than 94% of the RBD-related motor and vocal behaviors.16 The aim of the current study was to investigate different types of EMG activity (tonic, phasic, “any”) in the SINBAR EMG montage and other muscles in patients with RBD to calculate normative values for the routine diagnosis of RBD in sleep centers.
Evaluation of the Mentalis Muscle for RBD Detection
Our data demonstrated that the mentalis muscle is useful to discriminate patients with RBD from controls. When choosing a specificity of 100%, the cutoff for RBD diagnosis was 16.3% for phasic EMG activity (AUC 0.981) and 9.6% for tonic EMG activity (AUC 0.936). Good discriminative power of the mentalis muscle was also demonstrated in a recent study.22 In that study, 2-sec miniepochs were used for evaluating phasic EMG activity and 20-sec epochs for evaluating tonic EMG activity, instead of 3-sec miniepochs and 30-sec epochs, as we used. In addition, definition of phasic EMG activity was selected differently with an amplitude of 4 times the background EMG activity lasting between 0.1 and 10 sec. A total correct classification of 84% for phasic EMG activity of at least 15% was found. Interestingly, although criteria for phasic EMG activity were different and 2-sec miniepochs were applied, this cutoff is very similar to that obtained in our study. In contrast, the EMG cutoff for tonic activity was much higher at 30% (correct classification rate 82%). Whether this discrepancy may be explained by the total epoch duration (20 vs. 30 sec) remains speculative.
In addition to traditional evaluation of phasic and tonic EMG activity, we also evaluated the measure “any” (either phasic or tonic) EMG activity. This evaluation was performed only in the mentalis muscle because it is the only muscle with considerable tonic EMG activity in RBD when compared with the limb muscles. Another study23 combined tonic and phasic EMG measurements to generate a “PSG RBD score”, which was a post hoc average of the RBD measure for the “proportion of epochs containing elevated muscle tone” and of the RBD measure for the “proportion of miniepochs containing burst activity”. Our approach was different. “Any” EMG activity was defined to include any muscle activity exceeding 0.1 sec with an amplitude of more than twice the background EMG activity. We found that this simplified approach resulted in excellent discriminative power to distinguish patients with RBD from controls. When choosing a specificity of 100%, the EMG cutoff for RBD diagnosis was 18.2 % for “any” mentalis muscle EMG activity. Its AUC of 0.990 is similar to that resulting from differentation into phasic and tonic EMG activity. Based on our data, it might be assumed that for clinical routine the “any” EMG measure in the mentalis muscle is sufficient for RBD diagnosis because it greatly decreases the complexity of the scoring process and saves time to score EMG activity. In addition, phasic and tonic EMG activity is sometimes difficult to distinguish. Of note, both the mentalis and the sternocleidomastoid, which are both cranial nerve innervated muscles, had much lower rates of phasic than of “any” EMG activity, whereas the limb muscles had similar rates of phasic and “any” EMG activities. The explanation for this difference is most probably due to the high rates of tonic EMG activity in both the mentalis and sternocleidomastoid muscles, which is not present in the evaluated limb muscles.
Quantification of “any” EMG activity in the mentalis muscle would facilitate the task of those computer programs under development for the automatic diagnosis of RBD, because they will not have to distinguish phasic from tonic EMG activity. However, this automatic quantification would only be feasible after visual inspection of the mentalis muscle channel showing the absence of artifacts from, for example, snoring, arousals associated with sleep disordered breathing, and electrical noise.
Evaluation of Upper Limb Muscles for RBD Detection
Upper limb muscles showed the highest discriminative power for RBD diagnosis, even higher than that of the mentalis muscle. Phasic EMG activity in bilateral FDS muscle showed a very high AUC of 0.994 (cutoff 16.8%). The AUC in bilateral biceps brachii muscles was even higher, at 1.000 (cutoff 2.9 %). Based on this finding it might be concluded that if a patient has EMG activity in the biceps brachii muscle during REM sleep, he or she is very likely to have RBD. However, patients with RBD showed a low percentage of EMG activity in this muscle of only 13%. This means that there could be a lack of discriminative power in case of artifacts if EMG activity is quantified only in the biceps brachii muscle for the routine diagnosis of RBD. Therefore, the FDS muscle, which also has a high rate of EMG activity in patients with RBD, seems preferable for RBD detection. Advantages of upper extremity muscles are that they are easy to record, show few artifacts, and have no overlap with tonic EMG activity. According to our data, using a bilateral muscle of the upper limbs, preferably the FDS muscles, is highly recommended for RBD detection.
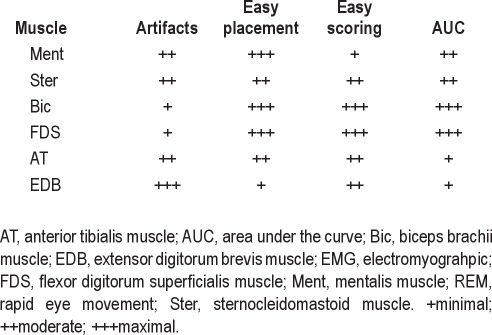
The major advantage of using the mentalis muscle for RBD detection is that it is the muscle used routinely in standard PSG to score sleep stages. However, its isolated use implies important drawbacks due to its vulnerability to breathing and snoring artifacts, its low correlation with most behaviors that patients with RBD display during REM sleep,16 and the challenging differentiation into phasic and tonic EMG activity. In contrast, according to our experience, the upper limb muscles such as the FDS are less prone to show artifacts, are easy to locate in the arms, and are also easier to be scored without tonic activity interferring the scoring (Table 10).
Table 10.
Clinical experience on practical issues of the evaluated body muscles while scoring EMG activity in REM sleep
Evaluation of Lower Limb Muscles for RBD Detection
Lower limb muscles showed an important overlap in EMG activity among patients with RBD and controls. Phasic EMG activity in both anterior tibialis muscles showed an AUC of 0.837 (cutoff 39.2%). The AUC in EDB muscles was similar, at 0.833 (cutoff 26.4%). Although discriminative power was still good, it was less than in the mentalis muscle and than in the upper limb muscles. The explanation for this finding may be due to significant overlap with other phasic phenomena such as fragmentary myoclonus or PLMS.24 This finding is even more striking because in our study we did not count apparent PLMS as phasic EMG activity, admitting that this was in a few cases challenging. EMG quantification in the lower limb muscles, therefore, seems to offer no additional advantage to differentiate patients with RBD from controls when comparing the upper limb muscles and the mentalis muscle. In addition, our experience recording muscle activity in the EDB indicates that artifacts are common in this muscle as appropriate impedances below 5 kΩ are difficult to obtain and derivations seem to be unstable. The combination of mentalis muscle “any” with bilateral anterior tibialis (the montage used in routine PSG for stage scoring and detection of PLMS) had an AUC of 0.990 (cutoff 46.4%), which is the same AUC as the mentalis muscle “any” isolated but lower than the AUC obtained in the upper limbs (Table 5).
Evaluation of Single Muscles and a Combination of Muscles for RBD Detection
Our data demonstrated that evaluation of single muscles for RBD identification shows very high AUC values. For example, the AUC value for “any” EMG activity in the mentalis muscle is 0.990. When the mentalis muscle “any” is combined with bilateral upper extremity muscles such as the FDS, the AUC value increases up to 0.998 (cutoff 32%). In contrast, adding a lower limb muscle to the mentalis or to any upper limb muscle had no further benefit. For example, the combination of mentalis “phasic” with both FDS and with both EDB (SINBAR EMG montage) showed an AUC of 0.990 (cutoff 34 %). Besides AUC values, clinical experience (RBD behaviors mostly involve the extremities) as well as practical issues (Table 10) favor the use of a combination of muscles for the detection of RBD rather than evaluating a single muscle.
We think that the combination of mentalis muscle “any” EMG activity with bilateral FDS phasic EMG activity is a feasible combination for the diagnosis of RBD in routine clinical practice because it provides a very high AUC (0.998); provides the quantification of the metric “any” EMG activity in the mentalis muscle, which eliminates the difficulty of distinguishing phasic from tonic EMG activity; involves the muscle with highest phasic and tonic EMG activity seen in RBD (mentalis muscle)15; involves limb muscles where clinical motor manifestations of RBD are typical and frequent; involves the FDS, which is the muscle with the highest phasic EMG activity in the limbs15; and involves bilateral evaluation of the upper limbs given the fact that in RBD bilateral movements are more frequent than unilateral movements.
In the current proposed EMG montage, the exclusion of lower limb muscles (EDB) from the original SINBAR EMG montage15 does not limit the discriminative power to distinguish RBD patients from controls as we have shown. Although patients with RBD may show isolated lower limb movements, these are rarely the only type of abnormal movements displayed during the PSG study in RBD, and usually are accompanied by movements in the upper limbs. Also, not evaluating phasic EMG in the lower limbs for the diagnosis of RBD eliminates the problem of having to distinguish between PLMS and phasic EMG activity during REM sleep.
Conventional 3-Sec Miniepoch Analysis and 30-Sec Epoch AASM Approach
In addition to analysis of phasic EMG activity in 3-sec miniepochs, we analyzed our data according to the recent AASM recommendation for scoring of phasic EMG activity. According to these criteria, a 30-sec epoch was classified as “phasic” when more than 5 3-sec miniepochs contained phasic EMG activity.18 Our data demonstrated that by using this approach, the AUC values for distinction among patients with RBD and controls remained very similar to the 3-sec miniepoch approach. The AUC for mentalis muscle “any” EMG activity is 0.989 in the 30-sec approach and 0.990 in the 3-sec miniepoch approach. The AUC for the combination of mentalis muscle “any” EMG activity with both FDS muscles is also 0.999 in the 30-sec approach and 0.998 in the 3-sec miniepoch approach.
Comparison Between iRBD and RBD in Patients with PD
In this study, we included both iRBD and PD patients with RBD to establish diagnostic cutoff values that are representative for RBD in general. The combination of “any” mentalis muscle EMG activity with phasic EMG activity in both FDS muscles provided the same cutoff of 32% in iRBD and RBD associated with PD for the correct diagnosis of RBD. Rates of phasic EMG activity were similarly distributed between both groups. In contrast, tonic EMG activity in the mentalis muscle was more prominent in the PD group. This may be explained by the fact that some patients with PD were taking antidepressants at the time of PSG. These medications are known to increase tonic but not phasic EMG activity in the mentalis muscle.25 This finding is in agreement with an earlier study showing that tonic EMG activity in the mentalis muscle is not different among patients with iRBD and patients with PD not taking antidepressants.11
Evaluation of Simultaneous EMG Activation
Of note, RBD patients had twice as frequent 3-sec miniepochs with simultaneous activation of different muscles than controls, and the ratio simultaneous/isolated phasic EMG activity was higher in muscles of the upper limbs than of the lower limbs. Simultaneous activation of the combination mentalis muscle “any” with both FDS muscles was 8 times more frequent in RBD patients than in controls. This pattern of EMG activity is consistent with video-PSG data in patients with RBD compared with controls, showing that the major difference between physiologic and pathologic REM sleep is reflected in the amount and the extent of movements during REM sleep.26,27 This favors the use of bilateral rather than unilateral EMG recording in the limbs for the diagnosis of RBD. In a future project it might be worthwhile to study if the position of the patients alters the scoring of EMG activity in the limbs.
Our study has some potential limitations. First, in clinical practice we usually tend to identify specific patterns of movements that are highly suggestive of RBD on video. These movements usually go along with activation of the limbs and consist of typically scenic or violent behavioral manifestations.13,26 In the current study we exclusively focused on EMG measures for the establishment of RBD diagnostic cutoff values without including video analysis. However, in a previous study16 we showed that most RBD clinical manifestations seen in the video are detected when evaluating the EMG activity in the mentalis muscle and limbs.16 Second, the metrics using 30-sec epochs were derived from previously analyzed 3-sec miniepochs rather than from an initial scoring of the full epochs. Because of this approach, we assume that this 30-sec epoch analysis is by far more accurate than any analysis performed initially in full 30-sec epochs. Third, patients included were affected by different forms of RBD (iRBD and RBD associated with PD) and were using different medications. We selected such a heterogeneous sample because we aimed to evaluate the habitual patient presenting to a sleep center to confirm the diagnosis of RBD ranging from the CNS active drug-naïve iRBD individual to the patient with PD taking CNS active medication, including those that may change EMG activity during REM sleep (e.g., antidepressants). Most of the controls were recruited from patients with effectively treated sleep related breathing disorders. Fourth, patients with RBD and controls were selected on a 1:1 ratio, which does not reflect the real-life prevalence of RBD in the general population.28,29 To prove the underlying utility of EMG case identification, a future study is needed to investigate a population not selected for disease and determine specificity and positive predictive value in such a population. The current study may best be viewed as a prelude to future population-based studies that might eventually lead to data that allow the use of sleep and diagnostic PSG as important screening tools for incident neurodegenerative disease.
CONCLUSION
For the diagnosis of RBD in clinical pratice, we recommend using a PSG montage quantifying “any” (either tonic or phasic) EMG activity in the mentalis muscle and phasic EMG activity in right and left flexor digitorum brevis muscles in the upper limbs with a cutoff of 32%, using 3-sec miniepochs. This cutoff value is the same for the idiopathic form of RBD and RBD in the setting of PD. To this montage, the addition of bilateral anterior tibialis EMG can be useful for the traditional detection of PLMS.20
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Frauscher has received support for speaking engagements from UCB and has served as a consultant for Pfizer, Mundipharma. Dr. Iranzo has received support for speaking engagements and serves on the advisory board of UCB. Dr. Poewe has received consultancy and lecture fees from Astra Zeneca, Teva, Novartis, GSK, Boehringer-Ingelheim, UCB, Orion Pharma, Merck, Serono, and Solvay-Abbot. Dr. Högl has received a grant from UCB; has received support for speaking engagements and/or serves on the advisory board of GSK, BI, UCB, Pfizer, Cephalon, Jazz, Sanofi, Lundbeck, and Merz. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Work for this study was performed at Department of Neurology, Innsbruck, Medical University, Innsbruck, Austria and Neurology Service, Hospital Clinic de Barcelona, Barcelona, Spain.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AT
anterior tibialis muscle
- AHI
apnea hypopnea index
- AUC
area under the curve
- Bic
biceps brachii muscle
- CNS
central nervous system
- CPAP
continuous positive airway pressure
- EDB
extensor digitorum brevis muscle
- EMG
electromyographic
- FDS
flexor digitorum superficialis muscle
- ICSD-2
International Classification of Sleep Disorders, 2nd revision
- IRBD
idiopathic REM sleep behavior disorder
- Ment
mentalis muscle
- PLMS
periodic limb movements in sleep
- PD
Parkinson's disease
- Phas
phasic
- PSG
polysomnography
- RBD
REM sleep behavior disorder
- REM
rapid eye movement
- ROC
receiver operator characteristic
- SINBAR
sleep Innsbruck Barcelona
- Ster
sternocleidomastoid muscle
- WASO
wake after sleep onset
Footnotes
A commentary on this article appears in this issue on page 743.
REFERENCES
- 1.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Iranzo A, Santamaria L, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009;13:385–401. doi: 10.1016/j.smrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38 % of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–93. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 4.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disease: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 5.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–9. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalamalapu U, Goldberg R, DiPhillipo M, Fry JM. Behaviors simulating REM behavior disorder in patients with severe obstructive sleep apnea. Sleep Res. 1996;25:311. [Google Scholar]
- 8.D'Cruz OF, Vaughn BV. Nocturnal seizures mimic REM sleep behavior disorder. Am J Electroneurodiagn Technol. 1997;37:258–64. [Google Scholar]
- 9.Guilleminault C, Leger D, Philip P, Ohayon MM. Nocturnal wandering and violence: review of a sleep clinic population. J Forensic Sci. 1998;43:158–63. [PubMed] [Google Scholar]
- 10.Iranzo A, Santamaría J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–6. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 11.Iranzo A, Santamaría J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65:247–52. doi: 10.1212/01.wnl.0000168864.97813.e0. [DOI] [PubMed] [Google Scholar]
- 12.Eisensehr I, v Lindeiner H, Jäger M, Noachtar S. REM sleep behavior disorder in sleep-disordered patients with versus without Parkinson's disease: is there a need for polysomnography? J Neurol Sci. 2001;186:7–11. doi: 10.1016/s0022-510x(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 13.Frauscher B, Gschliesser V, Brandauer E, et al. REM sleep behavior disorder in 703 sleep-disorder patients: the importance of eliciting a comprehensive sleep history. Sleep Med. 2010;11:167–71. doi: 10.1016/j.sleep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 14.The international classification of sleep disorders. 2nd ed. Vol. 2005. Westchester, IL: American Academy of Sleep Medicine; REM sleep behavior disorder; pp. 148–52. [Google Scholar]
- 15.Frauscher B, Iranzo A, Högl B, et al. Quantification of EMG activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31:724–31. doi: 10.1093/sleep/31.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iranzo A, Frauscher B, Santos H, et al. Usefulness of the SINBAR EMG montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med. 2011;12:284–8. doi: 10.1016/j.sleep.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 19.Mahowald MW, Schenck CH. REM sleep parasomnias. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier Saunders Company; 2005. pp. 897–916. [Google Scholar]
- 20.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010;25:2044–51. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 23.Consens FV, Chervin RD, Koeppe RA, et al. Validation of a polysomnographic score for REM sleep behavior disorder. Sleep. 2005;28:993–7. doi: 10.1093/sleep/28.8.993. [DOI] [PubMed] [Google Scholar]
- 24.Bliwise DL, He L, Ansari FP, Rye DB. Quantification of EMG activity during sleep: a phasic EMG metric. J Clin Neurophysiol. 2006;23:59–67. doi: 10.1097/01.wnp.0000192303.14946.fc. [DOI] [PubMed] [Google Scholar]
- 25.Winkelman JW, James L. Serotonergic antidepressants are associated with REM sleep without atonia. Sleep. 2004;27:317–21. doi: 10.1093/sleep/27.2.317. [DOI] [PubMed] [Google Scholar]
- 26.Frauscher B, Gschliesser V, Brandauer E, et al. Video analysis of motor events in REM sleep behavior disorder. Mov Disord. 2007;22:1464–70. doi: 10.1002/mds.21561. [DOI] [PubMed] [Google Scholar]
- 27.Frauscher B, Gschliesser V, Brandauer E, Ulmer H, Poewe W, Högl B. The relation between abnormal behaviors and REM sleep microstructure in patients with REM sleep behavior disorder. Sleep Med. 2009;10:174–81. doi: 10.1016/j.sleep.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Ohayon MM, Caulet M, Priest RG. Violent behavior during sleep. J Clin Psychiatry. 1997;58:369–376. [PubMed] [Google Scholar]
- 29.Chiu HF, Wing YK, Lam LC, et al. Sleep-related injury in the elderly - an epidemiological study in Hong Kong. Sleep. 2000;23:513–17. [PubMed] [Google Scholar]