Abstract
Purpose
The affect of anterior cruciate ligament (ACL) integrity on the early postoperative stability of a collagen type-I gel scaffold was investigated. The value of fibrin glue for graft fixation in ACL deficient porcine knees over a simulated early postoperative period was also studied.
Methods
Full-thickness articular cartilage defects (11 × 6 mm) were created on the medial femoral condyle of 80 porcine knees. The ACL was left intact or completely transected in each of 40 knees. Gel plugs were tested in each group: press-fitting only in 20 specimens and press-fitting plus fibrin glue in 20 specimens. Each knee underwent 2,000 cycles in a validated ex-vivo continuous passive motion model.
Results
Press-fit-only fixation grafts in knee specimens with an intact ACL showed significantly superior stability than that in ACL deficient knees (p = 0.01). In ACL deficient knees, grafts fixed with press-fitting plus fibrin glue showed significantly superior stability than those using press-fit only fixation (p = 0.01). Press-fitting plus fibrin glue fixation showed no significant differences in worn surface area between knee specimens with intact and deficient ACL.
Conclusions
ACL deficiency led to early scaffold instability in an ex-vivo porcine knee model. Fibrin glue in ACL deficient knees led to additional graft stability. These findings indicated that cartilage regenerative techniques may give optimum results in ACL intact knees.
Introduction
Anterior cruciate ligament (ACL) rupture is one of the most common sports injuries [1] and leads to dramatically altered knee joint kinematics [2, 3]. These findings have led both clinicians and researchers to advocate ACL reconstruction.
The avascular nature of articular cartilage limits its capacity for self-repair [4]. Various surgical approaches have been used to care for patients with cartilage lesions, including debridement [5], microfracture [6], osteochondral transfer [7], and matrix-induced autologous chondrocyte implantation (MACI) [8]. However, only osteochondral transfer and MACI are able to generate hyaline-like repair [7, 9].
In clinical practice, cartilage regenerative techniques are only recommended in stable knee joints to protect the graft from shear forces and early failure [9, 10]. Partially or completely delaminated tissue engineered constructions can cause locking in the knee and subsequently poor clinical results [9, 11]. In the case of a cartilage lesion in an ACL deficient knee, a combination of ACL reconstruction and MACI in a staged procedure is an option. First, a small biopsy of articular cartilage is taken from a non-weightbearing area of the knee and sent to a lab to be processed. In a second stage, ACL reconstruction is carried out simultaneously with MACI. However, even after ACL reconstruction, the fate of the graft remains unclear because degenerative changes to knees have been reported, whether the ACL has been reconstructed or not [12, 13]. Furthermore, ACL deficiency does not always infer functional impairment and instability as confirmed by the ACL-deficient coper [14]. Despite many studies on the biomechanics of the ACL deficient knee [15, 16], the effect of ACL deficiency on early postoperative stability of tissue engineered scaffolds has not been reported in the literature.
The purpose of this pilot study was two-fold. First, the affect of ACL integrity on the early postoperative stability of a collagen type-I gel scaffold was investigated. This important information could potentially facilitate surgeons’ decisions on whether to proceed with cartilage repair procedures in ACL deficient knees. The hypothesis was that scaffolds in porcine specimens with an intact ACL show higher early postoperative stability compared with ACL deficient knees. Second, the value of fibrin glue in this scenario was evaluated. The hypothesis was that gels fixed with fibrin glue would prove to be more stable and experience less wear.
Material and methods
A custom-made, pneumatic ex-vivo continuous passive motion (CPM) device similar to the one developed for previous studies was used [17, 18]. Porcine specimens, surgical procedures and graft evaluation were identical to those in previous studies [17, 18].
Specimens
Fresh frozen (–25°C) porcine knees (aged nine months) were thawed for 16 hours at room temperature (20°C) overnight. Right and left knees were used [19]. Eighty porcine knees were used for testing. The specimens were divided into two groups of 40 specimens: (1) intact ACL, and (2) completely transected ACL. The stability of the stifle joints was examined before testing, as carried out in clinical settings. In each group, gel plugs were tested in 20 specimens with press-fitting only and in 20 specimens with press-fitting plus fibrin glue. To evaluate the effect of the integrity of the ACL on graft stability, group 1 was compared to group 2. To assess the value of fibrin glue on the stability of the grafts in ACL-deficient knees, press-fitting only and press-fitting plus fibrin glue were compared.
Acellular collagen type-I gel
The three-dimensional cell-free gel plugs (CaRes-1S®, Arthro Kinetics, Krems/Donau, Austria) consisted of 4.8 mg/mL collagen type-I from the tails of rats. The plugs used in this study measured 11 mm in diameter and 6 mm in depth. They were stored in phosphate-buffered saline solution and preserved at 4°C until use. This collagen gel is in clinical use as a cell carrier for MACI [20].
Creation of chondral lesions
The femur and tibia were osteotomised approximately 10 cm from the joint line. Through a medial parapatellar arthrotomy the cartilage of the medial femoral condyle was exposed and a standardised full-thickness chondral lesion (diameter, 11 mm; depth, 6 mm) was created in the weightbearing area using proprietary devices (Arthro Kinetics, Krems/Donau, Austria).
Ex-vivo CPM protocol
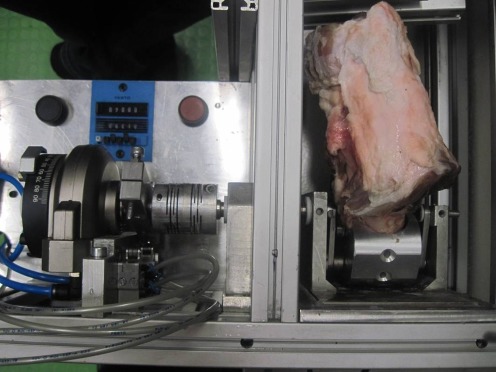
After fixation of the knee specimens in a vertical position into the ex-vivo CPM device, test cycles through the whole range of motion were undertaken to align the mechanical axis of the stifle joints with the mechanical axis of the ex-vivo CPM device (Fig. 1). Most rehabilitation programs recommend non-weightbearing in the early phase after cartilage restoration surgery [21, 22], so the tests were done unloaded. Using press-fit-only fixation, the graft was implanted into the defect without additional material. For the press-fitting plus fibrin glue fixation, the area towards the bone and the surrounding cartilage rim was covered with 0.3 mL of Tissucol Duo (Baxter, Unterschleißheim, Germany). Plugs were considered to be sufficient if complete congruity with the surrounding cartilage rim was achieved and fitted exactly into the prepared defect. To moisten the articular surface and lower the friction, 5 mL of 0.9% sodium chloride solution was instilled into the intra-articular environment. Immediately after fixation, and again after 2,000 motion cycles, standardised digital photographs were taken for analyses.
Fig. 1.
Test setup using the ex-vivo CPM device. The porcine knee specimen is fixed in a vertical position
Graft evaluation
Gross stability of the graft was graded as “intact”, “marginally detached”, “partially detached”, or “completely displaced” [23]. Marginal detachment was scored if a fissure was visible between the graft and host cartilage rim. Partial detachment was scored if the graft covered only a part of the prepared defect. In case of a completely empty defect zone, this was graded as graft displacement. Digital photographs obtained after 0 motion cycles and 2,000 motion cycles were transferred using image-processing software (QUIPS, Leica, Wetzlar, Germany). To further assess the effect of ACL integrity upon graft stability as well as the value of fibrin glue in an ACL-deficient knee, objective worn surface analyses were carried out using an edge detection algorithm in an interactive procedure. The worn surface area and the total area of the defect were measured in pixels; the percentage of worn surface was calculated from the ratio of the two measurements. The percentage of worn surface area represents graft stability, with the least worn surface area indicating superior graft stability. The areas of interest were traced by two independent observers.
Statistical analyses
The method of Blad and Altman was used to calculate inter-observer correlation. Differences in graft stability per percentage of worn surface area were analysed by two-way ANOVA and post-hoc analyses. P < 0.05 was considered significant. Additionally, the corresponding 95% family-wise confidence intervals (CI) were calculated. All statistical analyses were carried out with R software (Foundation for Statistical Computing, Vienna, Austria, version 2.12.1).
Results
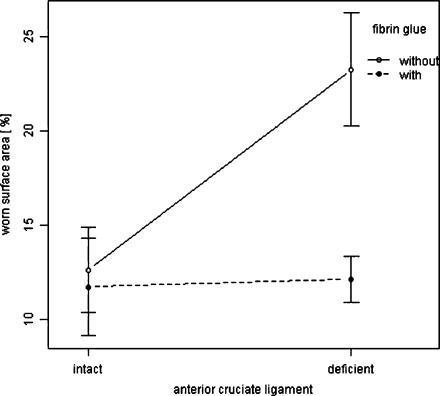
Good agreement between the two independent observers was found as indicated by 95% confidence intervals (CIs) ranging from –0.97 to 0.89. Therefore, the measured data from both observers were averaged. In specimens with an intact ACL, the mean worn surface area in specimens with an intact ACL (12.6%) was significantly less than ACL deficient specimens (23.2%) if using press-fit-only fixation, indicating superior stability (p = 0.01; 95% CI 1.85–19.43%). No significant differences in mean worn surface area between intact (11.4%) and ACL deficient knees (12.1%) could be observed if using the press-fit plus fibrin glue technique (p = 1.0; 95% CI –8.3% to 9.2%). In specimens of ACL-deficient knees, press-fit plus fibrin glue (12.1%) revealed significantly superior graft stability when compared with press-fit only fixation (23.2%) (p = 0.01; 95% CI 2.3–19.9%). The mean worn surface area as a percentage is shown in Fig. 2. Representative photographs of the gross stability of cell-free collagen type-I gel are shown in Fig. 3. The gross stability in each group is presented in Table 1.
Fig. 2.
Mean worn surface area of the grafts (%) with 95% confidence intervals after 2,000 motion cycles using press-fit only vs. press-fit + fibrin glue fixation in knee specimens with intact ACLs vs. ACL-deficient knees
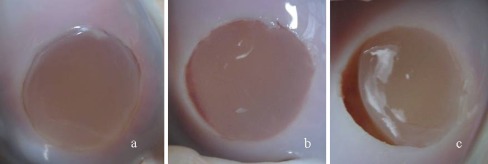
Fig. 3.
Gross stability of the cell-free collagen type-I gel after 2,000 motion cycles graded as (a) intact, (b) marginally detached, and (c) partially detached
Table 1.
Summary of gross stability of scaffolds after 2,000 motion cycles in each group with fixation procedures and integrity of the anterior cruciate ligament
| Gross stability | ACL intact | ACL deficient | ||
|---|---|---|---|---|
| Type of fixation | ||||
| Press-fit only | Press-fit + fibrin glue | Press-fit only | Press-fit + fibrin glue | |
| Intact | 9 | 10 | 0 | 6 |
| Marginal | 7 | 7 | 3 | 8 |
| Partial | 4 | 3 | 17 | 6 |
Discussion
In this study, the effect of the integrity of the ACL on the stability of a collagen type-I gel plug was tested. The results showed that ACL deficiency had a detrimental effect upon the early postoperative stability of a type-I collagen gel in terms of gross stability and mean surface area wear. However, these effects could (at least in part) be neutralised by the addition of fibrin glue into the graft fixation procedure. This addition reduced the degree of graft instability and surface area wear to levels similar to that observed in ACL-intact knees.
ACL rupture leads to altered kinematics of the knee joint and may compromise knee stability, resulting in chronic instability, recurrent injury and intra-articular disease [24, 25]. Biomechanical analyses have revealed a maximum load to failure of 2160 ± 157 N for the ACL [26]. However, in healthy joints forces up to this amount can be largely compensated by an intact ACL and menisci. ACL deficiency may change the static and dynamic loading of the knee, resulting in increased forces on the cartilage [27, 28]. In the case of a cartilage repair procedure in an ACL deficient knee, these forces may be transmitted to the repair tissue, and early postoperative graft stability could be affected. The results of our ex-vivo study showed that ACL deficiency leads to a greater worn surface area of the grafts compared with knee specimens with an intact ACL in short-term follow-up, and may ultimately produce detrimental long-term outcomes. These findings echo the recommendation that cartilage-repair procedures should be done only in stable knee joints to protect grafts from shear forces [25]. Surprisingly, the integrity of the ACL did not affect graft stability if press-fit plus fibrin glue fixation was used. In ACL-deficient knees, the latter procedure showed significantly higher stability, with 11.1% less worn surface area. One may speculate that fibrin glue provides additional stability to collagen gels, going some way to compensate for the deficient ACL. However, one cannot conclude that collagen type-I gel plugs should be used in ACL deficient knee joints, even if using additional fibrin glue for graft fixation. Chondral lesions suitable for tissue-engineered constructions diagnosed before ligament reconstruction can be treated in two stages: undertaking chondrocyte biopsy via arthroscopic means and carrying out combined surgery some weeks later. For smaller chondral lesions, bone-marrow stimulation procedures may be carried out simultaneously with ACL reconstruction in a single stage.
The coincidence of ACL rupture and cartilage lesions is well known, whereas cartilage damage can occur at the same time as injury or secondary to chronic instability. Articular cartilage lesions were found in 16–46% of patients with acute ACL injuries [29, 30]. In chronic ACL deficiency it has been reported to be as high as 77% [31].
Some patients with cartilage lesions are asymptomatic with ACL deficiency. This indicates that ACL deficiency does not always infer functional impairment and instability as confirmed by the ACL-deficient coper [14]. Even in these patients, when treating the cartilage defect, there should be an indication for ligament reconstruction to protect the grafts [9, 10].
The main purpose of ACL reconstruction is to: (i) reconstruct the kinematics; (ii) provide joint stability; (iii) prevent or delay joint degeneration. Even if ACL reconstruction can restore knee mechanics, an ACL-reconstructed knee is not a normal knee [32, 33]. The fate of the ACL graft on long-term follow-up remains unclear. Some data show that ACL-injured knees are at risk for degenerative changes at long-term follow-up whether the ligament is reconstructed or not [34, 35]. Biomechanical markers of turnover of bone and cartilage have been investigated in blood and urine to identify focal cartilage lesions from an early stage of osteoarthritis after injury of the human ACL [36]. Degeneration seems to be caused by intra-articular processes induced at the time of ACL injury, combined with long-term dynamic joint loading changes [37].
We could not evaluate the effect of ACL deficiency upon long-term scaffold stability. We cannot say what happens with scaffolds with worn surfaces over time. The relevance of worn surface area is controversial. A peripheral worn surface has been reported after certain cartilage-repair procedures in stable knees [38, 39], showing increased vertical shear stresses between repair and native cartilage with consequent micromotion and degenerative changes [40, 41]. However, worn surfaces alone do not have statistical effects upon functional outcome in short-term follow-up [42]. Nevertheless, it has been suggested that long-term results are dependent upon sufficient joint surface congruity [43]. Partially or completely delaminated tissue engineered constructions can cause locking in the knee and poor outcomes [9, 11]. However, a comprehensive treatment to repairing cartilage lesions and ACL reconstruction hopefully will prolong optimal knee function [10]
This study has some limitations. First, the availability of young human knees for test models is limited. Therefore, it is reasonable to use porcine test specimens [44]. Second, for mechanical testing, the model described here involved simulated uniplanar motion. Compared with the clinical situation, no force was applied by the muscles. The ex-vivo CPM device may therefore only partially simulate typical clinical applications. Even if ex-vivo models are important for assessing the effect of the integrity of the ACL on graft stability, in-vivo conditions might be even more important. For example, various studies have shown that several fluid components are believed to inhibit the integration of a cartilage construction into adjacent tissue [45, 46]. However, in-vivo evaluation is difficult. Third, no compressive load was applied to the test specimens. We are aware that this may have influenced the final results. However, the vertical orientation, the corresponding tibial cartilage, and the intra-articular fluid should have generated friction forces and shear forces on the gel that corresponded with the early postoperative period. Finally, with this experimental protocol, only short-term outcomes could be assessed. It would be better to evaluate the effect of ACL deficiency upon graft stability by long-term follow-up. However, these limitations can be seen as fruitful avenues for future research. Ex-vivo investigation may allow greater insight into the effect of ACL deficiency upon cartilage-repair procedures in vivo.
The study supports the importance of an intact ACL if using tissue engineered scaffolds for cartilage repair. Fibrin glue was shown to provide additional stability to the grafts compared to the press-fit technique in ACL deficient knees. However, due to the limitations of this ex-vivo study, we would recommend that tissue engineered scaffolds only be used in stable knee joints.
Acknowledgments
Conflict of interest
TE, TJH and MDS are consultants to Smith&Nephew, Arthroscopy, Germany. No benefits or funds were received in support of this study.
References
- 1.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: A 10-year study. Knee. 2006;13(3):184–188. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Velde SK, Bingham JT, Hosseini A, Kozanek M, DeFrate LE, Gill TJ, Li G. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60(12):3693–3702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88(8):1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 4.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460–466. [PubMed] [Google Scholar]
- 5.Hubbard MJ. Articular debridement versus washout for degeneration of the medial femoral condyle. A five-year study. J Bone Joint Surg Br. 1996;78(2):217–219. [PubMed] [Google Scholar]
- 6.Negrin L, Kutscha-Lissberg F, Gartlehner G, Vecsei V (2011) Clinical outcome after microfracture of the knee: a meta-analysis of before/after-data of controlled studies. Int Orthop. Oct 4 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 7.Hangody L, Vasarhelyi G, Hangody LR, Sukosd Z, Tibay G, Bartha L, Bodo G. Autologous osteochondral grafting—technique and long-term results. Injury. 2008;39(Suppl 1):S32–39. doi: 10.1016/j.injury.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 8.Iwasa J, Engebretsen L, Shima Y, Ochi M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol Arthrosc. 2009;17(6):561–577. doi: 10.1007/s00167-008-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Levy AS, Meier SW. Approach to cartilage injury in the anterior cruciate ligament-deficient knee. Orthop Clin North Am. 2003;34(1):149–167. doi: 10.1016/S0030-5898(02)00065-2. [DOI] [PubMed] [Google Scholar]
- 11.Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149–162. doi: 10.1097/00003086-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 13.Neuman P, Kostogiannis I, Friden T, Roos H, Dahlberg LE, Englund M. Patellofemoral osteoarthritis 15 years after anterior cruciate ligament injury—a prospective cohort study. Osteoarthritis Cartilage. 2009;17(3):284–290. doi: 10.1016/j.joca.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Herrington L, Fowler E. A systematic literature review to investigate if we identify those patients who can cope with anterior cruciate ligament deficiency. Knee. 2006;13(4):260–265. doi: 10.1016/j.knee.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Yoo JD, Papannagari R, Park SE, DeFrate LE, Gill TJ, Li G. The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. Am J Sports Med. 2005;33(2):240–246. doi: 10.1177/0363546504267806. [DOI] [PubMed] [Google Scholar]
- 16.Mannel H, Marin F, Claes L, Durselen L. Anterior cruciate ligament rupture translates the axes of motion within the knee. Clin Biomech (Bristol, Avon) 2004;19(2):130–135. doi: 10.1016/j.clinbiomech.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Efe T, Fuglein A, Heyse TJ, Stein T, Timmesfeld N, Fuchs-Winkelmann S, Schmitt J, Paletta JR, Schofer MD (2011) Fibrin glue does not improve the fixation of press-fitted cell-free collagen gel plugs in an ex vivo cartilage repair model. Knee Surg Sports Traumatol Arthrosc. Jun 9. [Epub ahead of print] [DOI] [PubMed]
- 18.Efe T, Schofer MD, Fuglein A, Timmesfeld N, Fuchs-Winkelmann S, Stein T, El-Zayat BF, Paletta JR, Heyse TJ. An ex vivo continuous passive motion model in a porcine knee for assessing primary stability of cell-free collagen gel plugs. BMC Musculoskelet Disord. 2010;11:283–289. doi: 10.1186/1471-2474-11-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9(3):330–340. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 20.Andereya S, Maus U, Gavenis K, Muller-Rath R, Miltner O, Mumme T, Schneider U. First clinical experiences with a novel 3D-collagen gel (CaReS) for the treatment of focal cartilage defects in the knee. Z Orthop Ihre Grenzgeb. 2006;144(3):272–280. doi: 10.1055/s-2006-933445. [DOI] [PubMed] [Google Scholar]
- 21.Irrgang JJ, Pezzullo D. Rehabilitation following surgical procedures to address articular cartilage lesions in the knee. J Orthop Sports Phys Ther. 1998;28(4):232–240. doi: 10.2519/jospt.1998.28.4.232. [DOI] [PubMed] [Google Scholar]
- 22.Reinold MM, Wilk KE, Macrina LC, Dugas JR, Cain EL. Current concepts in the rehabilitation following articular cartilage repair procedures in the knee. J Orthop Sports Phys Ther. 2006;36(10):774–794. doi: 10.2519/jospt.2006.2228. [DOI] [PubMed] [Google Scholar]
- 23.Marlovits S, Striessnig G, Kutscha-Lissberg F, Resinger C, Aldrian SM, Vecsei V, Trattnig S. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2005;13(6):451–457. doi: 10.1007/s00167-004-0535-3. [DOI] [PubMed] [Google Scholar]
- 24.Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech. 2005;38(2):293–298. doi: 10.1016/j.jbiomech.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31(1):75–79. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 26.Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 27.Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;325:10–18. doi: 10.1097/00003086-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/B:ABME.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 29.Engebretsen L, Arendt E, Fritts HM. Osteochondral lesions and cruciate ligament injuries. MRI in 18 knees. Acta Orthop Scand. 1993;64(4):434–436. doi: 10.3109/17453679308993661. [DOI] [PubMed] [Google Scholar]
- 30.Joseph C, Pathak SS, Aravinda M, Rajan D. Is ACL reconstruction only for athletes? A study of the incidence of meniscal and cartilage injuries in an ACL-deficient athlete and non-athlete population: an Indian experience. Int Orthop. 2008;32(1):57–61. doi: 10.1007/s00264-006-0273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobic V. Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction: a preliminary clinical study. Knee Surg Sports Traumatol Arthrosc. 1996;3(4):262–264. doi: 10.1007/BF01466630. [DOI] [PubMed] [Google Scholar]
- 32.Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34(12):2006–2012. doi: 10.1177/0363546506290403. [DOI] [PubMed] [Google Scholar]
- 33.Patel RR, Hurwitz DE, Bush-Joseph CA, Bach BR, Jr, Andriacchi TP. Comparison of clinical and dynamic knee function in patients with anterior cruciate ligament deficiency. Am J Sports Med. 2003;31(1):68–74. doi: 10.1177/03635465030310012301. [DOI] [PubMed] [Google Scholar]
- 34.Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 35.Struewer J, Frangen TM, Ishaque B, Bliemel C, Efe T, Ruchholtz S, Ziring E (2011) Knee function and prevalence of osteoarthritis after isolated anterior cruciate ligament reconstruction using bone-patellar tendon-bone graft: long-term follow-up. Int Orthop. Sep 7 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 36.Streich NA, Zimmermann D, Schmitt H, Bode G. Biochemical markers in the diagnosis of chondral defects following anterior cruciate ligament insufficiency. Int Orthop. 2011;35(11):1633–1637. doi: 10.1007/s00264-010-1191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 38.Kish G, Modis L, Hangody L. Osteochondral mosaicplasty for the treatment of focal chondral and osteochondral lesions of the knee and talus in the athlete. Rationale, indications, techniques, and results. Clin Sports Med. 1999;18(1):45–66. doi: 10.1016/S0278-5919(05)70129-0. [DOI] [PubMed] [Google Scholar]
- 39.Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Newman AP. Articular cartilage repair. Am J Sports Med. 1998;26(2):309–324. doi: 10.1177/03635465980260022701. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911–1920. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 43.Sittinger M, Perka C, Schultz O, Haupl T, Burmester GR. Joint cartilage regeneration by tissue engineering. Z Rheumatol. 1999;58(3):130–135. doi: 10.1007/s003930050162. [DOI] [PubMed] [Google Scholar]
- 44.Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage. 2009;17(6):705–713. doi: 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Englert C, McGowan KB, Klein TJ, Giurea A, Schumacher BL, Sah RL. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum. 2005;52(4):1091–1099. doi: 10.1002/art.20986. [DOI] [PubMed] [Google Scholar]
- 46.Schaefer DB, Wendt D, Moretti M, Jakob M, Jay GD, Heberer M, Martin I. Lubricin reduces cartilage–cartilage integration. Biorheology. 2004;41(3–4):503–508. [PubMed] [Google Scholar]