Abstract
Purpose
Infection of a total hip replacement is potentially a devastating complication. Statistical process control methods have been generating interest as a means of improving the quality of healthcare, and we report our experience with the implementation of such a method to monitor the one year infection rate after primary total hip replacement.
Method
Infection was defined as the growth of the same organism in cultures of at least two aspirates or intra-operative specimens, or growth of one pathogen in a patient with local signs of infection such as erythema, abscess or draining sinus tract. The cumulative summation test (CUSUM test) was used to continuously monitor the one year postoperative infection rate. The target performance was 0.5% and the test was set to detect twice that rate.
Results
Over the three year study period, 2006 primary total hip replacements were performed. Infection developed within one year after surgery in eight (0.4%) hips. The CUSUM test generated no alarms during the study period, indicating that there was no evidence that the process was out of control.
Conclusion
The one year infection rate after primary total hip replacement was in control. The CUSUM test is a useful method to continuously ensure that performance is maintained at an adequate level.
Introduction
Total hip replacement (THR) is one of the most successful elective orthopaedic procedures [19]. Approximately 250,000 THRs are performed each year in the United States, and these figures are increasing dramatically [18]. Over time, one patient in five will undergo revision of the prosthesis, usually for aseptic loosening, infection, instability or a mechanical complication [6, 9]. Infection rates after hip replacement surgery vary from 0.4% to 1.4%, with most infections occurring during the first year [8, 12, 23, 24, 26]. Infection of a total hip prosthesis is a potentially catastrophic event associated with an increased risk of postoperative complications and with significant impairment in function and quality of life [7, 22, 25]. Moreover, the cost of treating patients with infected total hip prostheses is high [17]. Therefore, strategies for preventing infections have received considerable attention from the orthopaedic community. Numerous measures at various steps of the process of care have been implemented, including pre-operative optimisation of the patient’s condition, improved peri-operative management to decrease the risk of contamination and routine prophylactic antibiotic therapy [16].
Statistical process control has been generating interest as a means of improving the quality of healthcare since the occurrence of dramatic events such as high mortality rates among children undergoing heart surgery at the Bristol Royal Infirmary [10] and the publication of new data of results over time of single surgeons performing complex cardiac surgery [11]. Statistical control charts are designed to detect unexpected variations in the performance of a process. The process under scrutiny may be a simple process, such as a surgical procedure [3, 4, 11], or a more complex process, such as an influenza outbreak in a large area [29]. Control charts, such as the cumulative summation (CUSUM) test, were developed to monitor time-to-event data, such as time to failure of a prosthesis or solid organ transplant [5, 13, 27]. These methods are critical in maintaining an adequate level of performance over time and in ensuring the prompt detection of lapses in performance [28].
Our objective was to report our experience with statistical process control used to monitor the one year infection rate after primary THR in an orthopaedic department.
Patients and methods
Centre, surgeons and patients
The study took place at a coordinating centre for patients with complex musculoskeletal infections in the Paris conurbation, France. This hospital has a high volume of primary hip replacements and complex hip revisions. All operations are performed by high-volume operating staff surgeons. Microbiologists subspecialised in musculoskeletal infections work in close collaboration with the surgeons. Patients were included in the study if they underwent primary THR between 1 January 2008 and 31 December 2010. Patients undergoing revision procedures were not included. The local ethics board approved the study.
Process under scrutiny
All patients were evaluated for risk factors for postoperative infection, such as diabetes, anaemia and potential sources of haematogenous infection. When possible, identified risk factors were controlled pre-operatively. Smokers were asked to stop smoking at least three weeks before surgery, but their procedure was performed as scheduled if they did not comply with this request. Screening for methicillin-resistant Staphylococcus aureus (MRSA) was not performed routinely. Patients were admitted to the ward on the day before surgery to allow shaving and showering with a povidone–iodine solution. Patients took another shower on the morning of surgery and were sent to the operating room wearing only a clean hospital gown, with none of their personal belongings. Surgical staff used alcohol-based solutions for hand washing. A first- generation cephalosporin was administered intravenously 30 min before the incision then eight and 16 h after surgery; for long procedures, this antibiotic was also given every four hours during surgery. Vancomycin was substituted for cephalosporin in patients with beta-lactam allergy. All operating rooms were equipped with ISO 5 multidirectional flow; body-exhaust suits were not used. The surgical site was prepped by the scrub nurse using a povidone–iodine solution then by the surgeon before draping; chlorhexidine gluconate solution was used in patients who were allergic to iodine. Drains were inserted routinely then removed after 48 h during the first dressing change.
Diagnosis of prosthesis infection
Infection was defined as isolation of the same micro-organism from two cultures of joint aspirate and/or intra-operative tissue specimen associated with at least one of the following criteria: a sinus tract communicating with the prosthesis; purulence of the synovial fluid at the time of arthrocentesis or during surgery; a synovial leukocyte count >1,700/ml and a differential of >65% neutrophils; or clinical (local inflammatory signs including erythema, swelling, warmth, pain), biologic [elevated C-reactive protein (CRP)] and radiologic signs (periosteal bone formation, subchondral osteolysis) of prosthetic joint infection (PHI).
Methods
A database (Microsoft Access ® ) was designed specifically for the study. Access to the database was made available in the hospital offices and outpatient clinic to allow continuous recording of relevant information after each inclusion and during the follow-up visits three and 12 months after surgery. The patient identifier, age, sex, date of surgery, date of clinic visit and date of infection, if any, were entered into the database. Charts of study patients were flagged using coloured stickers to facilitate complete data collection. Meetings about study progress were held yearly. A CUSUM test [21] was constructed to monitor infection rates after primary THR. After each new observation, the CUSUM tests the hypothesis that the process is in control, i.e. that the observed infection rate is equal to, or lower than, the target infection rate, versus the hypothesis that the process is out of control, i.e. that the observed infection rate is higher than the target infection rate. We reached a consensus in our orthopaedic surgery department that the target infection rate should be set at 0.5% (λ0) and the out-of-control infection rate at 1%. Based on computer simulations (10,000 samples), we determined a limit h that yielded a false discovery rate of 9.9% and a true discovery rate of 75%, for a rate ratio of two over five years of monitoring. A detailed explanation of the CUSUM test for time-to-event data is given in the “Appendix”. Graphically, the interpretation of CUSUM tests is easy and intuitive. The CUSUM score, St, is plotted over time, and the curve rises when an infection is reported and declines otherwise. If the curve crosses the predefined limit h, an alarm is triggered and the infection rate is considered to be out of control. As long as it remains below the limit h, the infection rate is considered to be in control.
Results
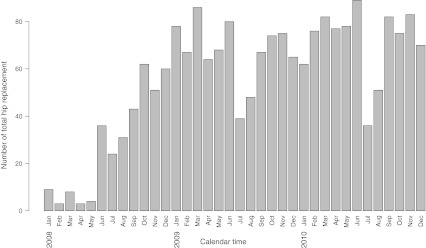
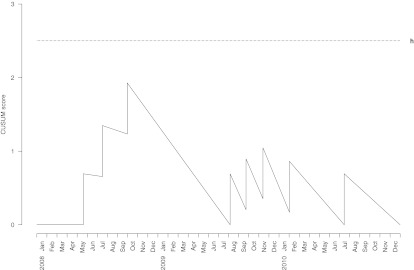
Over the three year study period, 2,006 primary THRs were performed in 1,882 patients. The distribution of the number of hip replacements over time is shown in Fig. 1. Infection developed within one year after surgery in eight (0.4%) hips. The CUSUM test generated no alarms during the study period (Fig. 2), indicating that there was no evidence that the process was out of control. As shown in the figure, the curve rose sharply when each infection occurred and declined slowly between infections. By crossing our monitoring data with administrative data, we were able to determine that 50% of procedures were monitored the first year, 97% the second year and 95% the third year; after a run-in period of a few months, the mean number of procedures included was >95% per year.
Fig. 1.
Histogram showing the number of total hip replacements performed per month over the three year study period
Fig. 2.
Cumulative summation (CUSUM) test for monitoring the 1-year infection rate after primary total hip replacement. The target rate (λ0 ) was set at 0.5%, the rate ratio (RR) at 2, and the predefined discovery rate limit (h) at 2.5
Four patients experienced early wound complications. Wound inflammation occurred during the second or third postoperative week in two patients. Hip aspiration was performed in both cases and grew a group G Streptococcus in one and a Propionibacterium acnes and S. capitis in the other. A thorough washout was performed, and appropriate antibiotics were given for four weeks. At last follow-up (29 and 14 months, respectively), the infection had not recurred. One patient underwent irrigation and drainage of a hematoma 11 days postoperatively. The specimens collected during drainage were sterile. The hematoma recurred a week later, requiring repeat irrigation; the specimen grew Pseudomonas aeruginosa, Enterobacter cloacae, Escherichia coli and Enterococcus faecalis. Antibiotic treatment failed to improve the clinical or laboratory abnormalities. Single-stage revision was performed five weeks after the initial replacement procedure. At last follow-up (seven months), the infection had not recurred. The last patient was seen three weeks postoperatively with wound discharge, a fever, and CRP elevation. A hip aspirate was positive for MRSA. Single-stage revision was performed. Signs of septic shock developed on the first postoperative day, followed rapidly by multiorgan failure. The patient was transferred to the intensive care unit and died on the second day after the revision.
Three patients reported increasing discomfort and pain around the hip, one after six weeks and two after three months. CRP level was high in all three patients, and radiographs showed a subtle periosteal reaction in two patients. The hip aspirate grew a coagulase-negative Staphylococcus in one patient and a P. acnes in two patients. All three patients were treated with single-stage revision and appropriate antibiotic therapy for three to four months in all. At last follow-up (29, 18 and 20 months), the infection had not recurred.
One patient experienced early instability with one episode of dislocation and several episodes of subluxation. Revision surgery was performed two weeks after the initial procedure to improve stability by increasing the length of the femoral neck. A periarticular hematoma was found during the procedure and was washed out. The specimen grew a S. aureus. Appropriate antibiotic therapy was given for three weeks. At last follow-up (three months), the infection had not recurred.
Discussion
Surgical site infection is a major concern for surgeons in all specialties [30]. Infection after THR is rare but potentially devastating. Consequently, prompt detection of an increased infection rate is crucial, as an investigation can then be conducted to identify the causes of the increase. Statistical process control is well suited to monitoring infection rates and rapidly detects changes in performance. In addition, data from statistical process control can be used to inform patients about specific risks at a particular institution.
Continuous monitoring of performance using statistical process control provides surgeons with real-time information about the process of care that they are part of and sometimes dependent upon. Moreover, healthcare authorities are increasingly recommending the use of statistical process control, in particular because of cost considerations [1, 14, 15]. In 2000, the Institute of Medicine reported that the performance of today’s healthcare system is far inferior than it could and should be and encouraged healthcare organisations “to develop a culture of safety and create systems for continuously monitoring patient safety” [14]. In a recent review of preventing deep infection in joint replacement surgery, Jamsen et al. [16] reported that monitoring infection rates on a local, national and even international scale is an essential part of quality control and is necessary in order to be able to identify weaknesses in infection prevention practices. However, to date, to the best of our knowledge, no such monitoring system has been described in orthopaedic surgery.
Infection rates after primary THR performed with contemporary aseptic conditions have varied from 0.4% to 1.4% in case series ranging from 575 to 9,245 patients, with approximately two thirds of these infections occurring during the first year following surgery [8, 12, 23, 24, 26]. Based on this information and on a consensus within our department, we set the target at 0.5% of THR procedures during the first postoperative year. In our study, the infection rate remained on target throughout the monitoring period. The one year infection rate of 0.4% during the study period compares favourably with previously published data [8, 12, 23, 24, 26].
Most hospitals currently track the yearly infection rate. However, this has significant drawbacks, which the CUSUM test overcomes. First, if the infection rate increases at the beginning of a year, it will only be detected the following year after numerous patients have been through the flawed process. On the other hand, the CUSUM test allows real-time monitoring with almost immediate corrective actions. Second, measuring yearly infection rates is sensitive to the timing of infections. For instance, if two infections occur in December and two more in January the following year, this increase in infection rate may not to be apparent. The CUSUM test is not affected by such considerations. Third, when the observed infection rate increases, care providers may not be able to determine whether this increase is due to random variation or to a significant shift in the performance of the process. For instance, say a centre sets a target at 0.5% of patients with a postoperative infection and that the yearly infection rate is 1%. How are they to decide whether this is just some expected variation around the target, and accordingly wait one year until the next report, or that this is the sign of inadequate performance and take immediate actions? The CUSUM is a test that allows decisions with controlled type I and type II errors over time.
The CUSUM test is also efficient in detecting small persistent changes in performance that would otherwise remain unknown for a longer period of time [20]. In 2001, the Institute of Medicine proposed six key points for establishing the healthcare system of the twenty-first century and bridging the quality chasm. “Timely” was one of these key points, and the report emphasised the need for “reducing waits and sometimes harmful delays for both those who receive and those who give care” [15]. At the Bristol Royal Infirmary, abnormally high mortality rates occurred in children undergoing heart surgery between 1984 and 1995 [10]. The mortality rate following open heart surgery in patients younger than one year old was twice the national average and did not follow the downward trend seen in the rest of the country. It took years for that poor performance to be detected and corrected. A retrospective review showed that using statistical process control would have detected the inadequate performance much earlier and saved lives [28]. The case of La Clinique du Sport, a private hospital in Paris, France, is another example of the need for quality-control procedures. At this centre, 58 patients who underwent spinal surgery between 1988 and 1993 developed Mycobacterium xenopi infection [2]. Only in September 1997 did the Ministry of Health order an enquiry. The enquiry established that patient contamination with M. xenopi was due to inadequate sterilisation of surgical instruments. Again, the use of a statistical process control to monitor infection rates after spinal surgery would have ensured early detection of the decrease in performance, thereby preventing the contamination of numerous patients. These examples also emphasise the dependence of surgeons upon the process of care to which they contribute: even when a surgeon feels confident that he or she is providing high-quality care, other parts of the process may compromise quality. Consequently, the performance of the process should be monitored.
Monitoring seeks to provide an accurate measure of quality in space and in time. Surgeons often estimate their outcomes based on reports from other centres and surgeons, sometimes located in other countries, published years ago, and observed in very different settings. Some surgeons simply estimate their current performance based on personal information that is no longer up to date. The rate of postoperative infection after joint replacement ranges from 0.4% to 1.4%, a 3.5-fold increase [8, 12, 23, 24, 26]. This level of variability is problematic when asking patients for their consent to a procedure and informing them of the risk for infection. Statistical process control provides accurate real-time information on the performance of a centre or a surgeon. This information can be reported to patients and healthcare authorities.
Our study has several limitations. First, monitoring systems are designed for surveillance and not for epidemiological studies or data collection. Therefore, only minimal information is collected, and the generation of an alarm requires an audit. Few exploratory analyses can be conducted based on data collected for monitoring. Second, the performance of statistical process control is limited when the monitored event is rare. Although a rate ratio of two represents an important relative increase in the probability of infection, the absolute difference in infection rates between the null and the alternative hypotheses is small (0.5%). Developing a method capable of detecting such small changes in performance is challenging. Previous evaluations of this issue in the context of the Poisson distribution suggest that the CUSUM test and the sets methods are the most efficient method [31, 32]. Third, the monitoring system can only be successful if patients are included and targeted events reported. During the first few months of our study, patient inclusion was incomplete (Fig. 1 and Table 1). However, with time, completeness of inclusion improved. This problem emphasises the difficulties and time necessary to implement such a surveillance system. It requires that the system be embedded in the usual pattern of work of physicians, nurses, clerks and operating-room staff. Similarly, a bias may be introduced if infections are not reported by the surgeons. In our study, infections were identified both by having the surgeons report cases online during patient follow-up and by searching a separate database where all musculoskeletal infections are collected. Cross-referencing multiple sources of information helps control and improve the quality of data used.
Table 1.
Proportion of total hip replacement monitored
| Period | 2008 | 2009 | 2010 |
|---|---|---|---|
| January | 15% | 94% | 78% |
| February | 6% | 100% | 94% |
| March | 14% | 100% | 96% |
| April | 5% | 98% | 95% |
| May | 7% | 99% | 95% |
| June | 55% | 100% | 92% |
| July | 75% | 83% | 92% |
| August | 100% | 98% | 100% |
| September | 84% | 97% | 100% |
| October | 73% | 86% | 90% |
| November | 98% | 100% | 100% |
| December | 91% | 94% | 99% |
| Total | 50% | 97% | 95% |
In conclusion, we show that the one year postoperative infection rate after primary THR at this coordinating centre was in control over a three year period. Statistical process control methods can help physicians monitor rare events and should therefore be used more often.
Acknowledgements
We are grateful to Annie Vincensini and Michel Droniou-Cassaro for their dedicated administrative and IT help.
Appendix
The CUSUM test1 (cumulative summation test) was used to monitor the infection rate after primary total hip replacement (THR). The CUSUM sequentially tests after each observation Xt(t > 0) the following hypothesis H0: λ = λ0, i.e. the process is in control, versus H1: λ≠ λ0, i.e. the process is out of control. The value λ0 is referred to as the target infection rate in this report. The test is based on the statistic St computed after each observation Xt as:
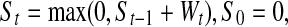 |
1 |
where the sample weight Wt depends on the observation Xt, λ0, and rate ratio (RR) (see Eq. 2). The test statistic St is compared with a predefined limit, h. If St equals or exceeds h, the null hypothesis is rejected. In quality control wording, the CUSUM test is said to emit an alarm indicating that the process is out of control. As long as St remains below h, the null hypothesis cannot be rejected, and monitoring continues under the assumption that the process is in control. In this report, a CUSUM for time to event data based on a Poisson distribution was chosen, with the following sample weight:
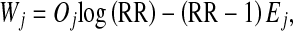 |
2 |
where Oj represents the number of THR infection observed on interval j, Ej (see Eq. 3) represents the number of THR infection expected on interval j under the null hypothesis of an infection rate of λ0, and RR represents the rate ratio defining the smallest unacceptable increase in the infection rate relative to the target that one wants to detect.
Ej is defined as:
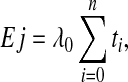 |
3 |
where ti is the length of time that a patient i remains infection free during the interval before censoring.
The CUSUM test has a particular feature: it has a holding barrier at zero and can never accept the null hypothesis. Therefore, theoretically, regardless the true performance of the process under observation, type I and type II errors of the test are 100% and 0%, respectively, and performances of CUSUM tests are expressed differently, namely, with the true and false discovery rates (TDR and FDR). These rates correspond to the probability of an alarm to be emitted under the alternative and null hypotheses, respectively, within a defined number of observations2. Also, because of that holding barrier at 0, the score St cannot deviate too far from the decision limit over long periods without any infection, and it remains responsive at all times to a sudden increase in the infection rate.
In our study, the target infection rate (process in control) chosen was λ0 = 0.5%, and the RR to detect was two; the out-of-control infection rate was therefore 1%. A limit, h = 2.5 ,was determined based on computer simulations (10,000 samples) to yield a false discovery rate of 9.9% and a true discovery rate of 75% over five years of monitoring.
References
1. Page ES. Continuous inspection schemes. Biometrika 1954;41: 100–115.
2. Marshall C, Best N, Bottle A, Aylin P. Statistical issues in the prospective monitoring of health outcomes across multiple units. J Roy Stat Soc A 2004;167: 541–559.
References
- 1.Arias KM. Mandatory reporting and pay for performance: health care infections in the limelight. AORN J. 2008;87:750–758. doi: 10.1016/j.aorn.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Astagneau P, Desplaces N, Vincent V, Chicheportiche V, Botherel A, Maugat S, Lebascle K, Leonard P, Desenclos J, Grosset J, Ziza J, Brucker G. Mycobacterium xenopi spinal infections after discovertebral surgery: investigation and screening of a large outbreak. Lancet. 2001;358:747–751. doi: 10.1016/S0140-6736(01)05843-3. [DOI] [PubMed] [Google Scholar]
- 3.Biau DJ, Milet A, Thevenin F, Anract P, Porcher R. Monitoring surgical performance: an application to total hip replacement. J Eval Clin Pract. 2009;15:420–424. doi: 10.1111/j.1365-2753.2008.01029.x. [DOI] [PubMed] [Google Scholar]
- 4.Biau DJ, Landreau P, Graveleau N. Monitoring surgical performance: an application of industrial quality process control to anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18:1263–1268. doi: 10.1007/s00167-010-1157-6. [DOI] [PubMed] [Google Scholar]
- 5.Biswas P, Kalbfleisch JD. A risk-adjusted CUSUM in continuous time based on the Cox model. Stat Med. 2008;27:3382–3406. doi: 10.1002/sim.3216. [DOI] [PubMed] [Google Scholar]
- 6.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF (2002) Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. 84-A:171–177 [DOI] [PubMed]
- 7.Biring GS, Kostamp T, Garbuz DS, Masri BA, Duncan CP. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer. J Bone Joint Surg Br. 2009;91-B:1431–1437. doi: 10.1302/0301-620X.91B11.22026. [DOI] [PubMed] [Google Scholar]
- 8.Blom AW, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total hip arthroplasty. The Avon experience. J Bone Joint Surg Br. 2003;85:956–959. doi: 10.1302/0301-620X.85B7.14095. [DOI] [PubMed] [Google Scholar]
- 9.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 10.The report of the public inquiry into children’s heart surgery at the bristol royal infirmay 1984-1995 (2001) http://www.bristol-inquiry.org.uk/. [DOI] [PubMed]
- 11.Leval MR, Francois K, Bull C, Brawn W, Spiegelhalter D. Analysis of a cluster of surgical failures. Application to a series of neonatal arterial switch operations. J Thorac Cardiovasc Surg. 1994;107:914–923. [PubMed] [Google Scholar]
- 12.Fender D, Harper WM, Gregg PJ. Outcome of Charnley total hip replacement across a single health region in England: the results at five years from a regional hip register. J Bone Joint Surg Br. 1999;81:577–581. doi: 10.1302/0301-620X.81B4.9859. [DOI] [PubMed] [Google Scholar]
- 13.Hardoon SL, Lewsey JD, Meulen JH. Continuous monitoring of long-term outcomes with application to hip prostheses. Stat Med. 2007;26:5081–5099. doi: 10.1002/sim.2900. [DOI] [PubMed] [Google Scholar]
- 14.To Err Is Human: Building a Safer Health System. Washington, DC: National academy press; 2000. [PubMed] [Google Scholar]
- 15.Crossing the quality chasm: a new health care system for the 21st century. Washington, DC: National academy press; 2001. [PubMed] [Google Scholar]
- 16.Jamsen E, Furnes O, Engesaeter LB, Konttinen YT, Odgaard A, Stefansdottir A, Lid-gren L. Prevention of deep infection in joint replacement surgery. Acta Orthop. 2010;81:660–666. doi: 10.3109/17453674.2010.537805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klouche S, Sariali E, Mamoudy P. Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res. 2010;96:124–132. doi: 10.1016/j.otsr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz SM, Ong K, Lau E, Mowat F, Halpern M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 19.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery DC. Introduction to statistical quality control. 5. New York: John Wiley and Sons; 2005. [Google Scholar]
- 21.Page ES. Continuous inspection schemes. Biometrika. 1954;41:100–115. [Google Scholar]
- 22.Park YS, Moon YW, Lim SJ, Oh I, Lim JS. Prognostic factors influencing the functional outcome of total hip arthroplasty for hip infection sequelae. J Arthroplasty. 2005;20:608–613. doi: 10.1016/j.arth.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 24.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romano CL, Romano D, Logoluso N, Meani E. Septic versus aseptic hip revision: how different? J Orthop Traumatol. 2010;11:167–174. doi: 10.1007/s10195-010-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schutzer SF, Harris WH. Deep-wound infection after total hip replacement under contemporary aseptic conditions. J Bone Joint Surg Am. 1988;70:724–727. [PubMed] [Google Scholar]
- 27.Sego LH, Reynolds MR, Woodall WH. Risk-adjusted monitoring of survival times. Stat Med. 2009;28:1386–1401. doi: 10.1002/sim.3546. [DOI] [PubMed] [Google Scholar]
- 28.Spiegelhalter D, Grigg O, Kinsman R, Treasure T. Risk-adjusted sequential probability ratio tests: applications to Bristol, Shipman and adult cardiac surgery. Int J Qual Health Care. 2003;15:7–13. doi: 10.1093/intqhc/15.1.7. [DOI] [PubMed] [Google Scholar]
- 29.Tillett HE, Spencer IL. Influenza surveillance in England and Wales using routine statistics. Development of ’cusum’ graphs to compare 12 previous winters and to monitor the 1980/81 winter. J Hyg (Lond) 1982;88:83–94. doi: 10.1017/S0022172400069928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenzel RP. Minimizing surgical-site infections. N Engl J Med. 2010;362:75–77. doi: 10.1056/NEJMe0908753. [DOI] [PubMed] [Google Scholar]
- 31.Barbujani G, Calzolari E. Comparison of two statistical techniques for the surveillance of birth defects through a Monte Carlo simulation. Stat Med. 1984;3:239–247. doi: 10.1002/sim.4780030306. [DOI] [PubMed] [Google Scholar]
- 32.Chen R. The relative efficiency of the sets and the cusum techniques in monitoring the occurrence of a rare event. Stat Med. 1987;6:517–525. doi: 10.1002/sim.4780060410. [DOI] [PubMed] [Google Scholar]