Abstract
There is paucity of data from Asian women on the association between serum estrogens and osteoporotic hip fracture risk. We conducted a case-control study nested within a population-based prospective cohort, The Singapore Chinese Health Study, to evaluate serum estrogens levels, ERα-mediated estrogenic activity and hip fracture risk in postmenopausal Asian women. Among 35,298 women who were recruited between 1993 and 1998, 15,410 women donated blood for research between 1999 and 2004. From this subcohort, we identified 140 cases who subsequently suffered hip fracture after blood donation, and 278 age-matched controls. Serum levels of total estrone, estradiol and sex hormone binding globulin levels were measured in a blinded fashion among cases and controls. ERα-mediated estrogenic activity of serum samples was quantified using a sensitive ERα-driven cell bioassay. Women with hip fracture had lower serum estrogens than control women. Compared to the lowest quintile, women in the highest quintile of free estradiol exhibited a statistically significant 57% reduction in risk of hip fracture (95% confidence interval (CI), 6%–80%), with a dose-dependent relationship (p for trend = 0.021). High levels of ERα-mediated estrogenic activity was also associated with decreased risk of hip fracture (p for trend=0.048). Overall, women with relatively high levels of both free estradiol and ERα-mediated estrogenic activity had a 55% reduction in hip fracture risk (95% CI, 17%–76%) compared to women with low levels of both. High levels of free estradiol and ERα-mediated estrogen activity in sera were associated with reduced hip fracture risk in Chinese postmenopausal women.
Keywords: Estrogens, Hip Fracture, ERα-mediated estrogenic activity, Population-based, Asian women
Introduction
Hip fractures from osteoporosis are a significant cause of mortality and morbidity in elderly women [1]. Risk of osteoporotic fractures increases exponentially after menopause, and this observation leads to the development of a unitary model for estrogen deficiency as a cause for bone loss and consequent fractures [2]. Supporting this model for the central role of estrogens was an early epidemiological study indicating that women with detectable estrogens in the blood have a lower risk of osteoporotic hip fractures [3]. However, a later study involving US women indicated that the effect of free estradiol on fracture risk was attenuated when adjusted for testosterone and sex hormone binding globulin (SHBG) levels and no longer evident when further adjusted for body mass index (BMI) [4]. Similarly, a study in a French population showed that the association of higher free estradiol with a lower risk of hip fracture was no longer significant after adjustment for weight [5]. Recent evidence in the last decade has led to a significant shift from an estrogen-centric view as a cause of osteoporotic fractures to one of aging and oxidative stress [6]. Supporting the view that aging is the pivotal determinant of bone mass and strength is the finding that age-related bone loss begins in the third decade in estrogen-replete women [7]. Independent of estrogens, factors such as changes in collagen quality [8], reactive oxygen species [6], progesterone deficiency [9] and polymorphisms of genes of the receptor activator of NFκB ligand signaling system [10] have been put forth as critical determinants of bone strength. Thus, the role of estrogens at physiological levels in postmenopausal hip fracture risk remains controversial. In particular there is a lack of studies on rapidly aging Asian populations, who have different estrogenic profiles compared to Caucasians [11].
Experimental studies suggest that estrogenic compounds exert their effects by binding and stimulating estrogen receptors (ER) to prolong survival of osteoblasts and induce apoptosis in osteoclasts, thereby reducing bone resorption and promoting bone health [12]. Estrogen receptors may be stimulated by endogenous estrogens such as estrone and estradiol, or inhibited by their metabolites such as 2-hydroxyestrone [13]. Exogenous estrogens, including dietary soy phytoestrogens, genistein/daidzein and endocrine disrupting compounds [14, 15], also contribute to overall estrogenic activity. An added layer of complexity is that about 98–99% of estradiol in the blood is bound to SHBG and albumin, and only about 1–2% is freely accessible to target tissues and cells [16]. Ultimately, biologically active estrogenic ligands bind to and activate (or inhibit) ERα and ERβ, the two main subtypes of ER with 59% homology in their ligand binding domains [17]. ERα is the predominant subtype in post-menopausal bone specimens [18] and there is evidence that mice with normal ERα, but non-functional ERβ (βERKO), have normal bone mineral density and trabecular structure [17]. Treatment with ERα, but not ERβ, -selective agonists can prevent bone loss in ovariectomised rats [19, 20], indicating the critical role of ERα in bone homeostasis. Ligand-activated ER dimerizes, and binds to estrogen response elements in the promoters of target genes, where it recruits co-regulator complexes to regulate transcription in the nucleus of bone cells.
Methods to measure serum estrogenic-mediated activity include the rodent uterotrophic assay, E-screen based on proliferation of estrogen sensitive MCF-7 cells, receptor binding competitive affinity assays, induction of estrogen regulated genes such as PS2 or progesterone receptor, and reporter gene assays based on yeast or human cell lines [21]. Among these modalities, reporter genes driven by ER-responsive promoters in human cell lines provide good quantitative data and scalability. Such ER-driven reporter gene assays are widely used to evaluate environmental samples [15] and have been validated for measuring low serum levels of estrogens in pre-pubertal girls [22] and post-menopausal women [23]. Such cell-based assays have the advantage of measuring the global activity of all estrogenic compounds (known and unknown, agonist and antagonist, exogenous and endogenous) in blood plasma, which can act on a relevant physiological endpoint, i.e., ERα-driven transcriptional activity [24, 25]. We aimed to determine the contribution of blood estrogens to hip fracture risk in a case-control study nested within the Singapore Chinese Health Study. Using prospectively collected serum samples, hip fracture risk was correlated with two independent measurements of estrogenic activities – free estradiol and ERα-mediated estrogenic activity by a cell bioassay.
Methods
Study population
The subjects were participants of the Singapore Chinese Health Study, a population-based, prospective cohort of 63,257 Chinese subjects (including 35,298 women). Subjects, aged 45–74 years, were recruited between April 1993 and December 1998 from residents of government-subsidized housing schemes, in which the majority (86%) of Singaporeans resided at the time of recruitment [26]. Study subjects were restricted to the two major Chinese dialect groups: Hokkien and Cantonese, who originated from two contiguous prefectures in southern China. The Institutional Review Boards at the National University of Singapore and the University of Minnesota approved this study.
At recruitment, subjects were interviewed in-person using a structured questionnaire that asked for information including demographics, use of tobacco, menstrual (including menopausal status) and reproductive (including use of menopausal hormonetherapy) histories (women only), medical history, as well as a dietary component assessing current intake patterns. Respondents were asked to choose from predefined frequency and portion size categories for each of the 165 listed food/beverage items that he/she consumed during the past 12 months. We used the Singapore Food Composition Table to estimate average daily intake of 96 nutrient and non-nutrient compounds for each study subject, including soy isoflavones and calcium intakes [26]. Between April 1994 and December 1999, blood and single-void urine specimens were collected from a random 3% sample of study enrollees. Details of the biospecimen collection, processing and storage procedures have been described previously [27]. Between January 2000 and April 2005, we extended our biospecimen collection to all surviving cohort members and collected biospecimens from 32,543 subjects, representing a consent rate of about 60% of surviving cohort participants at that time. Only women without prior history of hip fracture at recruitment were eligible as cases and controls for this study.
Case ascertainment
Women in the Singapore Chinese Health Study who donated blood samples prior to hip fracture were eligible for the study. Incident hip fracture cases were identified through the nationwide hospital discharge claims system, the MediClaims Database. This database was set up in 1990 to comprehensively capture inpatient discharge information (including diagnosis) from all hospitals in Singapore [28]. After excluding 57 prevalent cases of hip fracture among female cohort participants, 871 incident cases of hip fracture were identified as of 31 December 2008. The diagnoses of hip fracture cases were verified by cross-checking with records of the appropriate surgical procedures or manual review of medical records. Cases of traumatic fractures from road traffic accident or pathological fractures due to malignant metastasis to the femur were excluded. As mentioned earlier, our blood collection was conducted after recruitment into the cohort, and majority between years 2000 and 2005. Among cases of hip fracture in the cohort, 188 women donated blood samples for research. For this study, we included 140 cases who had donated blood samples before their fractures occurred, thus excluding the 48 women whose blood was collected after their hip fractures had occurred.
Control selection
For each of the 140 cases, 2 control subjects were randomly selected among all female cohort participants who had donated blood samples, and who were alive and free of hip fracture history at the time of hip fracture of their index case. The chosen controls were matched to the index case on age at study enrollment (±3 years), dialect group (Hokkien, Cantonese) and date of study enrollment (±2 year) and date of biospecimen collection (±6 months). There were two cases where only 1 eligible control was found for each of them. Hence, the study consists of 140 cases and 278 controls.
Blood analysis
Serum samples of a given matched set (containing the samples from the case and one or two matched controls) were arranged in random order, identified only by unique codes, and tested in the same laboratory batch for all measurements. Laboratory personnel were blinded to case or control status of the samples. Serum samples were thawed at 4°C and individually filtered via 0.22 µm sterile cartridges. Filtered sera were collected in aliquots for measurements of estrone, estradiol, SHBG, and ERα-mediated estrogenic activity.
Serum estrone and estradiol were measured with liquid chromatography tandem mass spectrometer using d4-estrone and d5-estradiol as internal standards, as reported previously [29]. The intra and inter-assay variabilities for estradiol (10–1,000 pM) and for estrone (25–1,000 pM) were less than 16%.
Serum SHBG concentrations were measured using a time-resolved immunofluorometric assay [30]. The intra- and inter-assay CVs were less than 8% for 6–200 nM SHBG. Percent free estradiol was calculated from serum SHBG levels based on the following regression model: Percent free estradiol=−0.01533 × serum SHBG + 2.921 [31]. Free estradiol level for each subject was then computed as the product of percent free estradiol and total estradiol level.
Biological activity of estrogens in serum was assessed using a validated estrogen-driven recombinant cell bioassay [25]. In brief, human uterine cervical HeLa cells stably expressing recombinant ERα protein were exposed to test sera. Estrogenic ligands in serum activated or inhibited an ERα-driven luciferase reporter gene. The calibration curve for each sample was constructed by adding incremental amounts of estradiol to human sera stripped of steroids. Total estrogen-mediated activity in this assay was expressed as pM estradiol equivalent. The detection limit for this assay was 8.45 pM estradiol. The intra- and inter-assay CVs were 6% and 14%, respectively.
Statistical Analysis
The chi-square test and the Student’s t-test were used to compare the distributions of selected demographic, lifestyle and dietary factors between cases and controls. The distributions of all biomarkers measured were markedly skewed with a long tail toward high values, which were corrected, to a large extent, by transforming the original values to logarithmic values. Therefore, formal statistical test was performed on logarithmically transformed values, and geometric (as opposed to arithmetic) means are presented. The analysis of covariance (ANCOVA) method was used to examine the differences in the concentrations of serum biomarkers between hip fracture cases and control subjects. The conditional logistic regression method was used to examine the associations between serum biomarkers measured and risk of hip fracture. Study subjects were grouped into quintiles of individual serum parameters based on their distributions among control subjects (see the quintile cut-off values in Appendix 1 in Supplemental Data). The magnitude of the association was assessed by odds ratio (OR) and its corresponding 95% confidence interval (CI) and P value. The following covariates (potential cofounders) were included in all ANCOVA and regression models: age at blood draw, level of education (no formal education, primary, secondary or higher); weekly vigorous work or strenuous sports (yes, no), BMI (kg/m2), number of cigarettes smoked per day (never smoker, 1–12, 13–22, or ≥23), number of years of smoking (never smokers, 1–19, 20–39 or ≥40), self-reported histories of stroke or diabetes mellitus at baseline (yes, no), total calcium intake from food and supplement (quartiles), total isoflavone intake from soy food (quartiles), and use of menopausal hormone therapy (yes, no).
Statistical computing was carried out using the SAS version 9.1software. All P values quoted were two-sided. The statistical significance level was set at two-sided P value of 0.05.
Results
Patients with hip fracture and control subjects were comparable in terms of mean age at blood sampling, BMI, dialect group and levels of education (Table 1). The mean time interval from blood draw to the occurrence of fracture was 4.4 (SD 2.5) years and the mean age of cases at the time of fracture occurrence was 73.7 (SD 7.1) years. As reported previously using data from the entire cohort of women [32, 33], hip fracture cases were more likely to be current smokers, spend less time on physical activity, and have lower dietary intake of soy isoflavones and higher dietary intake of calcium, although these differences did not reach statistical significance in this substudy. Cases were also more likely to have a history of stroke or diabetes mellitus at baseline compared to controls. Few women used menopausal hormone therapy in this study (Table 1). All the women reported that they were postmenopausal at the time of blood draw with the exception of one case and one control. However, these two women were 54 and 51 years old, respectively, at the time of blood draw and their low serum estrogen levels indicated that they were perimenopausal at that time. When we repeated our statistical analysis with the exclusions of these two women, all the results remained the same.
Table 1.
Baseline characteristics of cases and controls of hip fracture [mean (standard deviation) or number (percent)]
| Cases (n=140) |
Controls (n =278) |
P-value | |
|---|---|---|---|
| Age (years) at blood taken | 69.3 (7.2) | 68.8 (7.0) | 0.498 |
| Body mass index (kg/m2) | 23.4 (3.5) | 23.5 (3.7) | 0.948 |
| Dialect (%) | 0.939 | ||
| Cantonese | 77 (55.0) | 154 (55.4) | |
| Hokkien | 63 (45.0) | 124 (44.6) | |
| Level of education (%) | 0.872 | ||
| No formal education | 63 (45.0) | 127 (45.7) | |
| Primary school | 58 (41.4) | 109 (39.2) | |
| Secondary and above | 19 (13.6) | 42 (15.1) | |
| History of stroke (%) | 0.027 | ||
| No | 136 (97.1) | 277 (99.6) | |
| Yes | 4 (2.9) | 1 (0.4) | |
| History of diabetes mellitus (%) | 0.020 | ||
| No | 112 (80.0) | 246 (88.5) | |
| Yes | 28 (20.0) | 32 (11.5) | |
| Cigarette smoking (%) | 0.829 | ||
| Never smokers | 122 (87.1) | 242 (87.1) | |
| Former smoker | 5 (3.6) | 13 (4.7) | |
| Current smokers | 13 (9.3) | 23 (8.3) | |
| Weekly moderate activity (%) | 0.814 | ||
| No | 107 (76.4) | 208 (74.8) | |
| 0.5–3 hours/week | 16 (11.4) | 30 (10.8) | |
| 4+ hours/week | 17 (12.1) | 40 (14.4) | |
| Soy isoflavones (mg/1000kcal/day) | 11.6 (8.4) | 12.4 (10.3) | 0.727 |
| Calcium (mg/1000 kcal/day) | 293.9 (122.9) | 292.3 (122.6) | 0.776 |
| Use of menopausal hormone therapy (%) | 2 (1.4) | 6 (2.2) | 0.607 |
Hip fracture cases tended to have lower circulating estrone, estradiol, free estradiol and ERα-mediated estrogenic activity, but higher serum SHBG levels than controls (Table 2). The difference in the concentration of serum free estradiol between cases and controls was statistically significant (P=0.042). The relationships between decreasing hip fracture risk and increasing free estradiol (p for trend = 0.021) and ERα-mediated estrogenic activity (p for trend = 0.048) were statistically significant (Table 3). Conversely, there was no clear association between serum estrone and SHBG levels and risk of hip fracture. The risk estimates for the association between free estradiol and hip fracture risk remained essentially the same after adjustment for SHBG; compared to the lowest quintile, the OR (95% CI) of hip fracture risk for the highest quintile of free estradiol was 0.44 (0.19–1.01) (p for trend = 0.034). We also performed further analysis by including both free estradiol and ERα-mediated estrogenic activity as covariates in the same model to check if there was significant confounding effect between these two factors. The results showed that the risk estimates of hip fracture associated with these two factors across quintile levels remained essentially the same. Compared to the lowest quintile, the OR (95% CI) of hip fracture risk for the highest quintile of free estradiol was 0.43 (0.19–0.95). For ERα-mediated estrogenic activity, compared to the lowest quintile, the OR (95% CI) of hip fracture risk for the highest quintile was 0.53 (0.24–1.18).
Table 2.
Geometric means of blood parameters in hip fracture cases and control subjects
| Hip fracture Cases (n=140) |
Controls (n=278) |
2-sided P* | |
|---|---|---|---|
| Estrone (pM) | 134.28 | 136.55 | 0.819 |
| Estradiol (pM) | 49.27 | 55.25 | 0.096 |
| SHBG (nM) | 34.79 | 33.21 | 0.420 |
| Free estradiol (pM) | 1.10 | 1.27 | 0.042 |
| ERα-mediated estrogenic activity (pM estradiol equivalent) |
14.56 | 14.71 | 0.379 |
Based on the analysis of covariance (ANCOVA) models that also included age at blood-taking, level of education (no formal education, primary, secondary or higher), weekly vigorous work or strenuous sports (yes, no), BMI (kg/m2), number of cigarettes smoked per day (never smoker, 1–12, 13–22, or ≥23), number of years of smoking (never smokers, 1–19, 20–39 or ≥40), total calcium intake from food and supplement (mg/1000 kcal/day) (quartiles), self-reported stroke and diabetes at baseline, total isoflavone intake from soy food (quartiles), and use of menopausal hormone therapy (yes, no)
Table 3.
Serum estrogenic parameters and risk of hip fracture.
| Odds Ratio (95% confidence interval)* |
||||||
|---|---|---|---|---|---|---|
| Serum parameters | Q1 | Q2 | Q3 | Q4 | Q5 | P for trend |
| Estrone | 1.00 | 0.78 (0.38–1.59) | 1.06 (0.52–2.17) | 1.21 (0.60–2.44) | 0.68 (0.31–1.50) | 0.823 |
| Estradiol | 1.00 | 1.19 (0.59–2.40) | 1.14 (0.55–2.35) | 0.94 (0.46–1.92) | 0.56 (0.25–1.25) | 0.128 |
| SHBG | 1.00 | 0.85 (0.40–1.80) | 0.93 (0.46–1.89) | 1.52 (0.74–3.09) | 1.34 (0.59–3.08) | 0.219 |
| Free estradiol | 1.00 | 0.80 (0.41–1.57) | 0.52 (0.25–1.08) | 0.59 (0.28–1.21) | 0.43 (0.20–0.94) | 0.021 |
| ERα-mediated estrogenic activity |
1.00 | 0.82 (0.43–1.59) | 0.60 (0.30–1.19) | 0.58 (0.28–1.17) | 0.50 (0.23–1.11) | 0.048 |
Conditional logistic regression; adjusted for age at blood taken, level of education (no formal education, primary, secondary or higher), weekly vigorous work or strenuous sports (yes, no); BMI (kg/m2), number of cigarettes smoked per day (never smoker, 1–12, 13–22, or ≥23), number of years of smoking (never smokers, 1–19, 20–39 or ≥40), total calcium intake from food and supplement (mg/1000 kcal/day) (quartiles), self-reported stroke and diabetes at baseline, and total isoflavone intake from soy food (quartiles), and use of menopausal hormone therapy (yes, no)
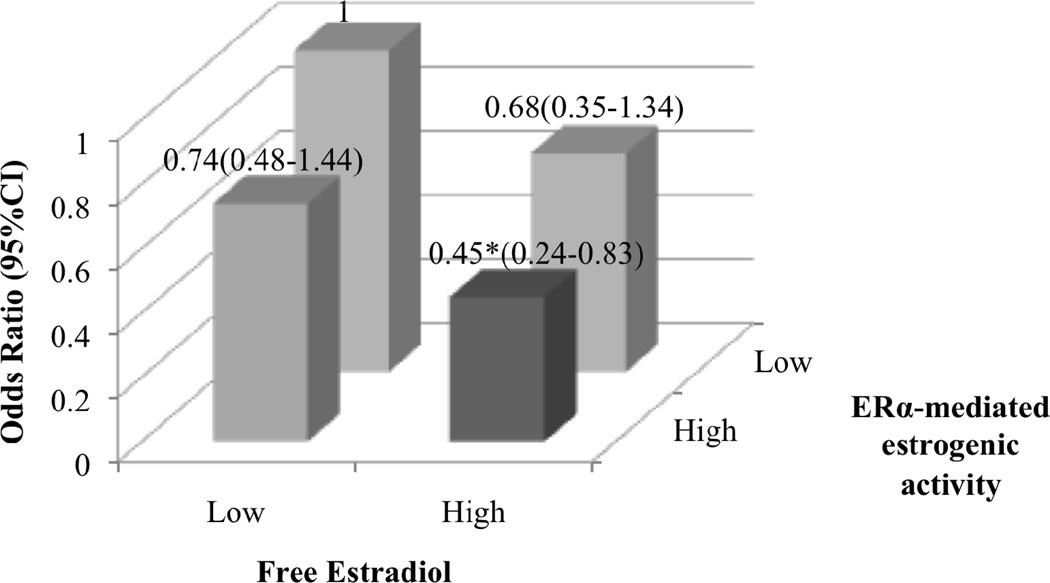
The positive correlations between ERα-mediated estrogen activity and serum free estradiol levels (Pearson’s correlation coefficient=0.253, P<0.0001), serum estrone levels (Pearson’s correlation coefficient=0.382, P<0.0001) and the sum of the two estrogens (Pearson’s correlation coefficient=0.384, P<0.0001) were generally weak. We examined the joint effects of free estradiol and ERα-mediated estrogenic activity on hip fracture risk (Figure 1). Since there are currently no biologically or clinically defined values of low and high levels for free estradiol or ERα-mediated estrogenic activity, for this analysis, subjects in the lowest 2 quintiles (Q1–Q2) were categorized as women with low level and those in the highest 3 quintiles (Q3–Q5) were categorized as women with high level (Figure 1). This approach was chosen because the difference in risk estimates was greatest between Q2 and Q3 for both biomarkers. Compared to women in low-level groups for both markers, women who were in high-level group for either free estradiol or ERα-mediated estrogenic activity had 26% to 32% reduction in hip fracture risk, although these risk estimates did not reach statistical significance due to small sample size (there were only 26 cases in the category with high level for free estradiol but low level for ERα-mediated estrogenic activity, and 28 cases in the category with low level for free estradiol but high level for ERα-mediated estrogenic activity). However, the risk reduction was greatest in women who were in high-level groups for both biomarkers. Compared with women in the lowest 2 quintiles of both free estradiol and ERα-mediated estrogenic activity, women in the highest 3 quintiles for both biomarkers had a statistically significant 55% reduction in hip fracture risk (OR=0.45, 95% CI=0.24–0.83) (Figure 1). The interaction term between these two biomarkers was not statistically significant (p=0.807), indicating that there was no multiplicative interaction between these 2 factors.
Figure 1. Associations between free estradiol and ERα-mediated estrogenic activity and hip fracture risk.
Low-Q1–Q2, High-Q3–Q5 of serum parameters levels. Women with both low Free Estradiol and ERα-mediated estrogenic activity are set as the reference group, Odds Ratio=1. Data adjusted for confounders as in Table 3. (*, P<0.05).
Discussion
We found that high levels of free estradiol and ERα-mediated estrogenic activity in sera were associated with reduced hip fracture risk in Chinese postmenopausal women. This reduction was still evident after adjustment for other known risk factors of hip fracture, including age and BMI. To our knowledge this is the first study in an Asian population, and supports the contention that ERα-mediated estrogenic activity in the sera of post-menopausal women may be sufficient to lower hip fracture risk.
Experimental studies have provided evidence for the bone-stabilizing effects mediated by ERα in maintaining bone mineral density [19, 20]. In this study, ERα mediated estrogenic activity was measured using a cell-based assay that does not rely on measurement of estradiol concentrations. Bioassays give an indication of the joint estrogenic activity of the total mixture, whereas chemical or immunoassay analyses only allow examination of known chemicals. In complex samples such as sera, it is reasonable to expect that many compounds are present that can contribute to overall ERα-mediated estrogenic activity. Dietary intake of phytoestrogens in common foods and nutraceuticals such as formononetin/biochanin (red clover), apigenin (celery), fisetin (apples), icariin/baicalein (folk medicines), galangin (ginger), quercetin (onions), kaempferol (tea), luteolin (oranges), or the intestinal metabolite equol may exert slight but cumulatively significant estrogenic effects [34]. Similarly a wide range of synthetic endocrine disrupting chemicals such as dioxins and polychlorinated biphenyls, bisphenol A, pesticides (endosulfan, toxaphene, and dieldrin) are estrogenic compounds that can exert biological effects at trace concentrations and have the potential to provoke additive estrogenic mixture effects at low doses, even at no observed effect levels [14, 15]. We used a cell line that constitutively express ERα and an ERα-driven luciferase reporter gene, the activity of which correlates well (R=0.83) with increases in summated estrogen levels in humans following ingestion of the estrogenic prodrug estradiol valerate [24].
Using this bioassay, we encountered the unexpected observation that the correlation between ERα-mediated estrogenic activity and free estradiol was relatively weak. Initially, this might seem surprising since estradiol, being the most potent natural estrogen, would be expected to contribute to the bulk of ERα-mediated estrogenic activity in sera. However, in post-menopausal women with low free estradiol levels, many other compounds discussed above can bind to ERα to increase its overall-mediated estrogenic activity and hence reducehip fracture risk. In addition, non-steroidal signaling pathways, including those mediated through growth factors and kinases, may impact on global ERα-mediated estrogenic activity. Concurring with results in this study, low correlation between estradiol and ERα-mediated estrogenic activity was also observed in another population-based study among post-menopausal women in Germany [35]. Our results showed that both free estradiol and ERα-mediated estrogenic activity appeared to influence hip fracture risk independently, and risk reduction was greatest in women who had high levels of both free estradiol and ERα-mediated estrogenic activity. The independent effects of these two biomarkers suggest that apart from the direct activation of ERα by free estradiol, both biomarkers may also act via independent mechanistic pathways in the prevention of osteoporotic hip fracture.
Conversely, free estradiol may also bind to other isoforms of estrogen receptors apart from ERα to mediate its protective effect on hip fracture risk. In addition to being a potent activator of ERα, estradiol is also a potent activator of ERβ, an ER isoform that can be detected in osteoblasts, osteoclasts and osteocytes of bone specimens from post-menopausal women, albeit at lower levels than ERα [18]. Despite the observation that mice with deficient ERβ-function (βERKO) have normal trabecular architecture, the possibility exists that a specific set of genes important for bone metabolism in women may be activated by estradiol binding to ERβ [17]. Besides transcriptional activation in the nucleus of bone cells, estradiol can also regulate bone metabolism through membrane bound receptors. Estradiol can activate a G-protein coupled receptor homolog (GPR30) or to membrane bound ER resulting in the activation of MAP kinase pathways [36] to regulate apoptosis in osteoclasts and osteoblasts [37]. In this context, a truncated variant of ERα (ERα36) lacking the transactivation domain can be activated by picomolar levels of estradiol to regulate bone remodeling. This variant ERα36 is reported to be strongly expressed in osteoblasts and osteoclasts of postmenopausal women with normal bone density and less so in those with osteoporosis. Using human bone cells in vitro, it was demonstrated that ERα36 was able to induce anti-apoptotic signaling on osteoblasts and the converse in osteoclasts through ERK1/2 and ROS pathways [38].
Notably, the mean BMI among the women in Western studies [4, 5] was higher than that among our leaner, Chinese women. Since high BMI is an established strong protectivefactor for hip fracture [39], it is possible that high BMI may compensate for the influence of low estradiol on bone loss in heavier women. Hence, the association between residual estradiol levels and hip fracture risk in menopausal women may be more evident in leaner Asians. In support of this hypothesis, women with no residual ovarian estradiol production due to bilateral oophorectomy after natural menopause had increased fracture risk, but this risk was reduced in those who were obese [40].
The strength of our study is the use of a comprehensive nationwide hospital database that captured practically all hip fracture cases, as Singapore is a small city state where all such cases are expected to be hospitalized. In addition, the use of questionnaire data and blood specimens collected before the occurrence of fracture reduces recall and reverse causality bias. Limitations of this study are that the results are based on a blood sample collected at single time point, and the lack of data regarding falls, family history of fragility fractures and weight loss. The natural fluctuation of estrogens that most likely occurred equally in both cases and controls could lead to the underestimation of the true associations between the biomarkers measured and the risk of hip fracture. In this cohort, among the women who gave blood for research, we limited the selection of cases to women who donated blood prior to the occurrence of hip fracture. Hence, only 140 out of a total of 871 fracture cases were included in this study. However, when we compared the women who gave blood prior to fracture (included in this study) with those who either did not give or gave blood after the fracture (not included in this study), these two groups were similar in other important factors such as age at fracture (mean age 73 years for both groups), BMI (mean of 23 kg/m2 in both groups) and diabetes status (20 percent were diabetic in both groups). Furthermore, willingness to donate blood for research should not influence the estrogen-hip fracture associations in this study. Hence, the results obtained from women who gave blood prior to fracture in this study would also be applicable to the other group.
Another limitation was the lack of information on use of drugs that can affect bone density and fracture risk such as glucocorticoids, proton pump inhibitors, selective estrogen receptor modulators and thyroid hormones. However, the use of such drugs is unlikely to be highly prevalent in population-based studies such as ours. We also do not have data on bone mineral density in our study population. However, while osteoporosis is often defined in terms of bone mineral density according to WHO guidelines, in the light of recent advances on the structural basis of skeletal fragility, it became clear that bone density represents only one of the contributors to bone strength. Even if bone mineral density is within acceptable range, disruption of bone microarchitecture or alteration in the amount and variety of proteins in bone can still increase the risk of fractures [41]. Hence, our study of hip fracture risk addresses this clinically most important and direct consequence of osteoporosis. Finally, the relatively small sample size does not give us sufficient power to detect significant difference in the levels of most of the blood parameters between cases and controls.
In conclusion, high levels of free estradiol and ERα-mediated estrogenic activity in sera were associated with decreased risk of osteoporotic hip fracture, and our data suggest that these two markers might act via independent mechanistic pathways in the prevention of osteoporosis. While recognizing that estrogen-independent processes such as oxidative stress have critical roles in trabecular bone strength [6], quantitative computed tomography shows that cortical bone, unlike trabecular bone, remains stable in women until after menopause [42, 43]. It is plausible the low, but supra-threshold, levels of estrogens in menopausal women reduces the risk of hip fractures by maintaining the strength of cortical bone, which comprises 80% portion of the skeleton [44]. The evaluation of therapeutic strategies including ultra-low-dose estrogen therapy such as a quarter of the regular dose [45] or selective ER agonists [46] may be warranted to reduce hip fracture for women with low serum estrogenicity.
Highlights.
> Lack of population studies on serum estrogens and hip fracture risk in Asian women. > We examine associations between estrogenic biomarkers hip fracture risk. > High levels of free estradiol were associated with reduced hip fracture risk. > ERα-mediated estrogen activity of serum was also associated with reduced hip fracture risk.
Supplementary Material
Acknowledgement
We thank Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study and Kazuko Arakawa and Renwei Wang for development of the cohort study database. We also thank the Ministry of Health in Singapore for assistance with the identification of hip fracture cases via database linkages. Finally, we acknowledge the founding, long-standing Principal Investigator of the Singapore Chinese Health Study – Mimi C. Yu.
Funding: The study was internally funded by Dept Obstetrics & Gynecology Pitch for funds, Biomedical Research Council, Singapore (R-174-000-116-305), National Medical Research Council, Singapore (R-174-000-137-275, NMRC/EDG/0011/2007), National Institutes of Health, USA (NCI RO1 CA55069, R35 CA53890, R01 CA80205, and R01 CA144034), and the Canadian Institutes of Health Research (MOP-15261).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors state that they have no conflicts of interests.
References
- 1.Haentjens P, Magaziner J, Colón-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, Boonen S. Meta-analysis: excess mortality after hip fracture among older women and men. Annals of Internal Medicine. 2010;152:380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riggs BL, Khosla S, Melton LJ., 3rd A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. Journal of Bone and Mineral Research. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. New England Journal of Medicine. 1998;339:733–738. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, LaCroix AZ, Wu L, Cauley JA, Jackson RD, Kooperberg C, Leboff MS, Robbins J, Lewis CE, Bauer DC, Cummings SR. Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. Journal of clinical endocrinology and metabolism. 2008;93:1796–1803. doi: 10.1210/jc.2007-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapurlat RD, Garnero P, Bréart G, Meunier PJ, Delmas PD. Serum estradiol and sex hormone-binding globulin and the risk of hip fracture in elderly women: the EPIDOS study. Journal of Bone and Mineral Research. 2000;15:1835–1841. doi: 10.1359/jbmr.2000.15.9.1835. [DOI] [PubMed] [Google Scholar]
- 6.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocrine Reviews. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. Journal of Clinical Investigation. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey AJ, Knott L. Molecular changes in bone collagen in osteoporosis and osteoarthritis in the elderly. Experimental Gerontology. 1999;34:337–351. doi: 10.1016/s0531-5565(99)00016-9. [DOI] [PubMed] [Google Scholar]
- 9.Seifert-Klauss V, Prior JC. Progesterone and bone: actions promoting bone health in women. J Osteoporos. 2010;2010:845180. doi: 10.4061/2010/845180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong SS, Liu XG, Chen Y, Guo Y, Wang L, Zhao J, Xiong DH, Xu XH, Recker RR, Deng HW. Association analyses of RANKL/RANK/OPG gene polymorphisms with femoral neck compression strength index variation in Caucasians. Calcified Tissue International. 2009;85:104–112. doi: 10.1007/s00223-009-9255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ursin G, Wilson M, Henderson BE, Kolonel LN, Monroe K, Lee HP, Seow A, Yu MC, Stanczyk FZ, Gentzschein E. Do urinary estrogen metabolites reflect the differences in breast cancer risk between Singapore Chinese and United States African-American and white women? Cancer Research. 2001;61:3326–3329. [PubMed] [Google Scholar]
- 12.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocrine Reviews. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 13.Vandewalle B, Lefebvre J. Opposite effects of estrogen and catecholestrogen on hormone-sensitive breast cancer cell growth and differentiation. Molecular and Cellular Endocrinology. 1989;61:239–246. doi: 10.1016/0303-7207(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 14.Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, Palimeri S, Panidis D, Diamanti-Kandarakis E. Endocrine Disruptors and Polycystic Ovary Syndrome (PCOS): Elevated Serum Levels of Bisphenol A in Women with PCOS. Journal of Clinical Endocrinology and Metabolism. 2011;96:E480–E484. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- 15.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Reviews. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. Journal of Steroid Biochemistry. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson S, Gustafsson JA. Estrogen receptors: therapies targeted to receptor subtypes. Clinical Pharmacology and Therapeutics. 2011;89:44–55. doi: 10.1038/clpt.2010.226. [DOI] [PubMed] [Google Scholar]
- 18.Batra GS, Hainey L, Freemont AJ, Andrew G, Saunders PT, Hoyland JA, Braidman IP. Evidence for cell-specific changes with age in expression of oestrogen receptor (ER) alpha and beta in bone fractures from men and women. Journal of Pathology. 2003;200:65–73. doi: 10.1002/path.1332. [DOI] [PubMed] [Google Scholar]
- 19.Hertrampf T, Schleipen B, Velders M, Laudenbach U, Fritzemeier KH, Diel P. Estrogen receptor subtype-specific effects on markers of bone homeostasis. Molecular and Cellular Endocrinology. 2008;291:104–108. doi: 10.1016/j.mce.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Seidlova-Wuttke D, Prelle K, Fritzemeier KH, Wuttke W. Effects of estrogen receptor alpha- and beta-selective substances in the metaphysis of the tibia and on serum parameters of bone and fat tissue metabolism of ovariectomized rats. Bone. 2008;43:849–855. doi: 10.1016/j.bone.2008.07.237. [DOI] [PubMed] [Google Scholar]
- 21.Soto AM, Maffini MV, Schaeberle CM, Sonnenschein C. Strengths and weaknesses of in vitro assays for estrogenic and androgenic activity. Best Practice and Research. Clinical Endocrinology and Metabolism. 2006;20:15–33. doi: 10.1016/j.beem.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Paris F, Servant N, Térouanne B, Balaguer P, Nicolas JC, Sultan C. A new recombinant cell bioassay for ultrasensitive determination of serum estrogenic bioactivity in children. Journal of Clinical Endocrinology and Metabolism. 2002;87:791–797. doi: 10.1210/jcem.87.2.8269. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Paris F, Sultan CS, Song RX, Demers LM, Sundaram B, Settlage J, Ohorodnik S, Santen RJ. Recombinant cell ultrasensitive bioassay for measurement of estrogens in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2005;90:1407–1413. doi: 10.1210/jc.2004-0766. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Lee L, Gong Y, Shen P, Wong SP, Wise SD, Yong EL. Bioassays for Estrogenic Activity: Development and Validation of Estrogen Receptor (ERalpha/ERbeta) and Breast Cancer Proliferation Bioassays to Measure Serum Estrogenic Activity in Clinical Studies. Assay and Drug Development Technologies. 2009;7:80–89. doi: 10.1089/adt.2008.154. [DOI] [PubMed] [Google Scholar]
- 25.Wong SP, Li J, Shen P, Gong Y, Yap SP, Yong EL. Ultrasensitive cell-based bioassay for the measurement of global estrogenic activity of flavonoid mixtures revealing additive, restrictive, and enhanced actions in binary and higher order combinations. Assay and Drug Development Technologies. 2007;5:355–362. doi: 10.1089/adt.2007.056. [DOI] [PubMed] [Google Scholar]
- 26.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutrition and Cancer. 2001;39:187–195. doi: 10.1207/S15327914nc392_5. [DOI] [PubMed] [Google Scholar]
- 27.Koh WP, Yuan JM, Sun CL, van den Berg D, Seow A, Lee HP, Yu MC. Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer Research. 2003;63:573–578. [PubMed] [Google Scholar]
- 28.Heng DM, Lee J, Chew SK, Tan BY, Hughes K, Chia KS. Incidence of ischaemic heart disease and stroke in Chinese, Malays and Indians in Singapore: Singapore Cardiovascular Cohort Study. Annals of the Academy of Medicine, Singapore. 2000;29:231–236. [PubMed] [Google Scholar]
- 29.Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clinical Chemistry. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 30.Niemi S, Mäentausta O, Bolton NJ, Hammond GL. Time-resolved immunofluorometric assay of sex-hormone binding globulin. Clinical Chemistry. 1988;34:63–66. [PubMed] [Google Scholar]
- 31.Langley MS, Hammond GL, Bardsley A, Sellwood RA, Anderson DC. Serum steroid binding proteins and the bioavailability of estradiol in relation to breast diseases. Journal of the National Cancer Institute. 1985;75:823–829. doi: 10.1093/jnci/75.5.823. [DOI] [PubMed] [Google Scholar]
- 32.Koh WP, Wu AH, Wang R, Ang LW, Heng D, Yuan JM, Yu MC. Gender-specific associations between soy and risk of hip fracture in the Singapore Chinese Health Study. American Journal of Epidemiology. 2009;170:901–909. doi: 10.1093/aje/kwp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh WP, Wang R, Ang LW, Heng D, Yuan JM, Yu MC. Diabetes and risk of hip fracture in the Singapore Chinese Health Study. Diabetes Care. 2010;33:1766–1770. doi: 10.2337/dc10-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Medica. 2008;74:1656–1665. doi: 10.1055/s-0028-1088304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widschwendter M, Lichtenberg-Frate H, Hasenbrink G, Schwarzer S, Dawnay A, Lam A, Menon U, Apostolidou S, Raum E, Stegmaier C, Jacobs IJ, Brenner H. Serum oestrogen receptor alpha and beta bioactivity are independently associated with breast cancer: a proof of principle study. Br J Cancer. 2009;101:160–165. doi: 10.1038/sj.bjc.6605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 37.Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651–1664. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie H, Sun M, Liao XB, Yuan LQ, Sheng ZF, Meng JC, Wang D, Yu ZY, Zhang LY, Zhou HD, Luo XH, Li H, Wu XP, Wei QY, Tang SY, Wang ZY, Liao EY. Estrogen receptor alpha36 mediates a bone-sparing effect of 17beta-estrodiol in postmenopausal women. Journal of Bone and Mineral Research. 2011;26:156–168. doi: 10.1002/jbmr.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporosis International. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 40.Melton LJ, 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. Journal of Bone and Mineral Research. 2003;18:900–905. doi: 10.1359/jbmr.2003.18.5.900. [DOI] [PubMed] [Google Scholar]
- 41.Nuti R, Brandi ML, Isaia G, Tarantino U, Silvestri S, Adami S. New perspectives on the definition and the management of severe osteoporosis: the patient with two or more fragility fractures. Journal of Endocrinological Investigation. 2009;32:783–788. doi: 10.1007/BF03346537. [DOI] [PubMed] [Google Scholar]
- 42.Riggs BL, Melton Iii LJ, 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. Journal of Bone and Mineral Research. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 43.Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. Journal of Bone and Mineral Research. 2008;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnick SL, Bonnick SL. Bone Densitometry in Clinical Practice. New York, NY: Humana Pr Inc; 2009. [Google Scholar]
- 45.Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042–1048. doi: 10.1001/jama.290.8.1042. [DOI] [PubMed] [Google Scholar]
- 46.Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, Lubrano C, Bonaiuto M, D'Anna R, Cannata ML, Corrado F, Adamo EB, Wilson S, Squadrito F. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Annals of Internal Medicine. 2007;146:839–847. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.