Abstract
Summary
Monthly minodronate at 30 or 50 mg had similar efficacy as 1 mg daily in terms of change in bone mineral density (BMD) and bone turnover markers with similar safety profiles. This new regimen provides patients with a new option for taking minodronate.
Introduction
Minodronate at a daily oral dose of 1 mg has been proven to have antivertebral fracture efficacy. In the present study, the efficacy and safety of oral minodronate at monthly doses of either 30 mg or 50 mg were compared with a daily dose of 1 mg.
Methods
A total of 692 patients with involutional osteoporosis were randomized to receive minodronate at either 30 or 50 mg monthly or a daily dose of 1 mg. The primary endpoint was the percent change from baseline in lumbar spine (LS) BMD at 12 months. Total hip BMD, bone turnover markers, serum calcium (Ca), and parathyroid hormone (PTH) levels were also evaluated.
Results
Minodronate at monthly doses of 30 or 50 mg were noninferior to the 1 mg daily dose in terms of change in LS-BMD. Changes in total hip BMD were also comparable. Although a transient decrease in serum Ca and increase in PTH levels were observed in all three groups at slightly different magnitudes and time courses, changes in bone turnover markers were comparable among the different dosage groups with a similar time course. Safety profiles were also comparable.
Conclusion
Minodronate at monthly doses of 30 or 50 mg has similar efficacy to the daily 1 mg dose in terms of BMD and bone turnover markers with similar tolerability.
Keywords: Bone mineral density, Bone turnover marker, Minodronate, Monthly bisphosphonate, Osteoporosis, PTH
Introduction
Minodronate is a nitrogen-containing bisphosphonate with a potent inhibitory effect on bone resorption. In a head-to-head comparison of the effects of minodronate with alendronate in postmenopausal osteoporosis patients, daily 1 mg minodronate resulted in similar increases in lumbar spine (LS) and total hip bone mineral density (BMD) after 12 months with similar safety profiles [1]. A randomized placebo-controlled trial conducted in Japan revealed that daily 1 mg minodronate reduced vertebral fractures by 59% in postmenopausal women with established osteoporosis [2]. Daily 1 mg minodronate has been approved to treat involutional osteoporosis in Japan.
Most oral bisphosphonates originally developed as a daily regimen have been shown to have equivalent efficacy with weekly and/or monthly regimens [3–7]. Since less frequent dosing, preferred by most patients, could result in better treatment compliance with better outcomes [8], we conducted a study to determine if minodronate could be administered as a monthly regimen. The present randomized, double-blind, active-controlled 1-year study was undertaken to determine whether or not once monthly oral minodronate at doses of 30 and 50 mg provides similar efficacy and safety as the 1-mg daily regimen in patients with involutional osteoporosis. The primary efficacy analysis was the test of the noninferiority of the mean percent change from baseline in lumbar spine bone mineral density (LS-BMD) in the monthly minodronate groups compared with the daily dose group after 1 year. The safety profiles of the monthly 30- and 50-mg regimens and the daily 1-mg regimen were also compared.
Materials and methods
Patient enrollment
We studied men and postmenopausal women with osteoporosis, aged 51 to 89 years, who had a BMD below 70% (T-score −2.6 at the LS) of the young adult mean (YAM) or a BMD below 80% (T-score −1.7 at the LS) of the YAM with at least one fragility fracture, as defined by the criteria of the Japanese Society for Bone and Mineral Research [9]. Vertebral fractures were assessed by X-ray films of the vertebrae and were diagnosed in accordance with the criteria of the Japanese Society for Bone and Mineral Research. Men with a total hip BMD below 70% (T-score −2.6 at the total hip) of the YAM were also eligible. Subjects were excluded if they had disorders such as primary hyperparathyroidism; Cushing's syndrome; premature menopause due to hypothalamic, pituitary or gonadal insufficiency, or other causes of secondary osteoporosis; or if there were any radiographic findings that might affect bone densitometry assessment. Subjects with peptic ulcer were excluded. Subjects were excluded if they had received bisphosphonate injections, strontium, or RANKL antibody at any time. Subjects were also excluded if they had taken oral bisphosphonates within the previous 1 year or for at least 30 days during the previous 2 years up until 1 year before the first dose of the study medication. Subjects were also excluded if they had taken glucocorticoids, calcitonin, vitamin K, active vitamin D compounds, or hormone replacement therapy within the previous 2 months; had serum calcium (Ca) levels above 10.6 mg/dL (2.6 mmol/L) or below 8.0 mg/dL (2.0 mmol/L); had serum creatinine levels above 1.5 mg/dL (133 μmol/L); or had clinically significant hepatic disorders.
This study was conducted in accordance with the principles that have their origin in the Declaration of Helsinki and was approved by the appropriate institutional review boards. All subjects gave written informed consent before undergoing any examination or study procedure, all of which were conducted in compliance with Good Clinical Practice. Eligibility of patients for enrollment was evaluated by H. Hagino—Rehabilitation Division, Tottori University Hospital, Yonago; M. Ito—Department of Radiology, Nagasaki University School of Medicine, Nagasaki; and T. Sone—Department of Nuclear Medicine, Kawasaki Medical School, Okayama.
Study design
This study was a randomized, double-blind, active-controlled, parallel-group, multicenter study conducted at 31 sites in Japan. Subjects who met all the entry criteria were enrolled and sequentially assigned an allocation number independent of study site. Subjects were randomized to take minodronate (Astellas Pharma Inc., Tokyo, Japan) at 1 mg daily, 30 mg monthly, or 50 mg monthly for 12 months. We selected 30-mg monthly dose because it is equivalent to 30-day daily dose of 1 mg. The dose of 50 mg dose was selected based on the pharmacokinetics study (data not shown) that demonstrated monthly bone exposure comparable to daily 1 mg would require 42- to 56-mg single monthly doses because of lower absorption with larger single doses.
Randomization was performed using a computerized system. Subjects were instructed to take their tablet on arising and 30 min before food with plain water. All subjects received daily calcium (610 mg) and vitamin D (400 IU) supplementation once a day after the evening meal. Compliance with the study treatment was assessed through medication diaries and by counting residual medication supplies.
Study outcomes
The primary endpoint of the study was the test of the noninferiority of the mean percent change from baseline in the lumbar spine (L2–L4) BMD at 12 months of treatment with the study medication. Secondary endpoints of the study included mean percent change from baseline in the total hip BMD, relative changes in bone turnover markers, and the occurrence of new morphometric vertebral and nonvertebral fractures.
Assessment of BMD
The lumbar spine (L2–L4) and the total hip were measured by dual-energy X-ray absorptiometry (DXA) at baseline and at 3, 6, 9, and 12 months to determine BMD. All 31 study centers involved in this trial were equipped with a Hologic QDR series for BMD measurements. A central facility (Department of Nuclear Medicine, Kawasaki Medical School, Okayama, Japan by T. Sone) performed quality assurance of the longitudinal adjustment. The DXA machines were adjusted for differences and each machine was calibrated with standardized phantoms.
Assessment of bone turnover
Serum and urine samples were collected at baseline and 1, 3, 6, 9, and 12 months for measurement of bone turnover markers, including urine type I collagen N-telopeptide (NTX; Osteomark, Inverness Medical Japan Co., Ltd., Tokyo, Japan), urine deoxypyridinoline (DPD; Osteolinks “DPD”; Quidel Corporation, San Diego, CA, USA) after acid hydrolysis, serum bone-specific alkaline phosphatase (BALP; AccessR OstaseR; Beckman Coulter, Inc., Brea, CA, USA), serum osteocalcin (BGP-IRMA; Mitsubishi Chemical Medience Corporation, Tokyo, Japan), serum Ca (Iatrofine Ca II; Mitsubishi Chemical Medience Corporation), and serum intact parathyroid hormone (PTH; ECLusys “PTH”; Roche Diagnostics K.K., Tokyo, Japan). Serum 25-hydroxyvitamin D (25(OH)D 125I RIA Kit; DiaSorin Inc., Saluggia, Italy) was also determined at baseline. When possible, the samples for each subject were collected around the same time of day to avoid the influence of daily fluctuations.
Assessment of vertebral fractures
Lateral radiographs of the thoracic and lumbar spine were taken at the screening visit to determine the presence of prevalent fractures. Subjects were enrolled based on a visual assessment of prevalent fractures in T4 to L4. All the radiologic specifications and the levels of vertebra at the thoracic and lumbar spine were standardized throughout the study sites. The assessment of prevalent fractures was made if the ratio of anterior or middle vertebral body height to the posterior vertebral body height was less than 0.8 [10]. Quantitative and semiquantitative techniques [11, 12] were used to identify incident vertebral fractures in order to determine efficacy. Lateral radiographs of the spine were performed at 12 months for the assessment of incident fractures. A new vertebral fracture was diagnosed if the anterior, posterior, or middle vertebral height had decreased by at least 15% and by 4 mm in a vertebra that was normal at baseline, or diagnosed semiquantitatively by grade progression [10]. Morphological diagnosis of fractures was made by quantitative and semiquantitative assessment of the images using the sequence of films at the central reading facilities of the University of Occupational and Environmental Health, Fukuoka, Japan by T. Nakamura.
Assessment of nonvertebral fractures
All nonvertebral fractures were identified symptomatically as clinical fractures, and only nontraumatic fractures assessed by investigators were reported. Suspected clinical fractures at six nonvertebral sites (humerus, radius/ulna, subclavia, pelvis, femur, and tibia/fibula) were adjudicated radiographically, and only radiographically confirmed fractures were listed.
Assessment of adverse events
All subjects were questioned about treatment-emergent adverse events (AEs) at each visit, and all adverse events reported were analyzed regardless of the investigators' assessments of causality. The Medical Dictionary for Regulatory Activities (Version 13.0J) was used to categorize reported adverse events.
Statistical analysis
The primary hypothesis of the study was that monthly minodronate (30, 50 mg) would be comparable to daily minodronate (1 mg) in terms of the mean percent change from baseline in LS-BMD after 12 months of treatment. The primary hypothesis was tested using an intention-to-treat (ITT) analysis. The ITT population comprised all randomized subjects. The primary analysis used a last observation carried forward approach for missing values. A Dunnett's test was used to determine the noninferiority of each of the monthly minodronate groups compared to the daily minodronate group. Noninferiority was to be declared if the lower bound of the two-sided 95% confidence interval (95% CI) of difference did not exceed the predefined noninferiority margin of −1.9%.
The group mean and standard deviation (SD) or standard error (SE) were calculated for the baseline characteristics, the percent changes from baseline in LS-BMD, total hip BMD, and bone turnover markers and were used to assess the significance of changes between each of the monthly minodronate groups and the daily minodronate group. A Dunnett's test was used to determine whether each of the monthly minodronate groups was significantly different from the daily minodronate group. A paired t test was used to determine whether each of the measured values was significantly different from the baseline. Statistical analyses were performed using SAS Drug Development (SAS Institute). The safety analysis included all subjects who received at least one dose of study medication in either treatment group.
Results
Patient disposition
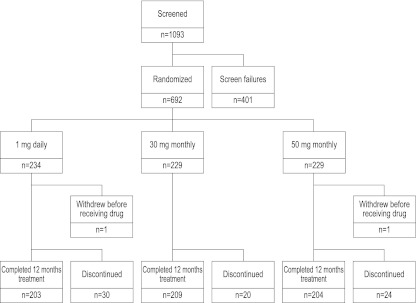
A total of 1,093 patients were screened; of these, 692 patients were randomized, and 690 patients received at least one dose of the study drug (Fig. 1). Baseline characteristics were similar in all three treatment groups (Table 1). A similar percentage of patients in each treatment group completed 12 months of the study (1 mg daily, 86.8%; 30 mg monthly, 91.3%; 50 mg monthly, 89.1%). The most common reason given for withdrawal was voluntary withdrawal: 19 (61.3%) in the 1 mg daily group; 10 (50.0%) in the 30 mg monthly group; and 10 (40.0%) in the 50 mg monthly group.
Fig. 1.
Enrollment and outcomes. A total of 1,093 patients were screened, of which 692 were randomized to take minodronate at 30 mg monthly (229 subjects), 50 mg monthly (229 subjects), or 1 mg daily (234 subjects)
Table 1.
Demographics and baseline characteristics of subjects
| 1 mg daily (n = 234) | 30 mg monthly (n = 229) | 50 mg monthly (n = 229) | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 2 (0.9) | 7 (3.1) | 5 (2.2) |
| Female | 232 (99.1) | 222 (96.9) | 224 (97.8) |
| Age (years) | 67.8 [6.870] | 68.6 [7.19] | 67.3 [6.53] |
| Body mass index (kg/m2) | 21.88 [3.101] | 21.87 [2.875] | 22.03 [3.248] |
| Menopause (years) | 50.0 [4.20] | 49.9 [3.81] | 49.5 [4.57] |
| Existing vertebral fractures, n (%) | 60 (25.6) | 61 (26.6) | 72 (31.4) |
| Lumbar BMD (g/cm2) | 0.6474 [0.06406] | 0.6527 [0.06023] | 0.6481 [0.06493] |
| Lumbar BMD (T-score) | −3.0551 [0.53830] | −3.0112 [0.50616] | −3.0494 [0.54561] |
| Total hip BMD (g/cm2) | 0.6684 [0.07949] | 0.6644 [0.08213] | 0.6685 [0.08765] |
| Total hip BMD (T-score) | −2.8791 [0.66802] | −2.9129 [0.69021] | −2.8784 [0.73656] |
| Serum 25(OH)D (ng/mL) | 27.0 [5.76] | 26.9 [5.94] | 25.8 [5.53] |
| Serum BALP (U/L) | 27.98 [9.165] | 27.07 [8.687] | 29.32 [14.321] |
| Serum osteocalcin (BGP, ng/mL) | 8.71 [2.756] | 8.61 [2.543] | 8.60 [2.205] |
| Serum intact PTH (pg/mL) | 42.2 [13.20] | 43.7 [14.45] | 44.1 [14.72] |
| Serum Ca (mg/dL) | 9.31 [0.343] | 9.29 [0.321] | 9.33 [0.335] |
| Urine DPD (nmol/mmol) | 6.47 [2.072] | 6.54 [2.145] | 6.38 [2.175] |
| Urine NTX (nmol BCE/mmol Cr) | 46.85 [21.527] | 45.67 [19.720] | 46.49 [20.692] |
Data are means [SD] for the indicated number of subjects in each group
LS and hip BMD
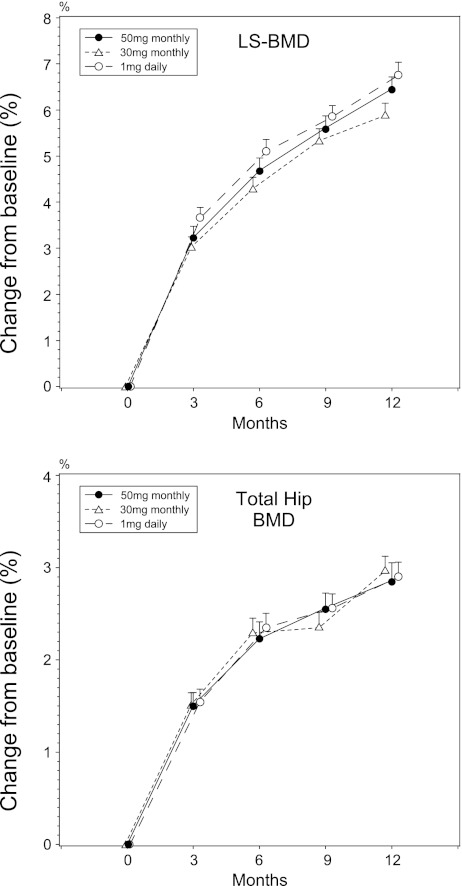
As shown in Fig. 2, both 30 and 50 mg monthly as well as 1 mg daily minodronate significantly increased LS-BMD from the baseline at all time points. Noninferiority of both monthly regimens to the daily regimen, with percent change in LS-BMD at 12 months as the end point, was determined. For 50 mg monthly minodronate, the estimated treatment difference (50 mg monthly–1 mg daily) was −0.294, with a 95% CI of −1.038 to 0.450, whereas for 30-mg monthly regimen, the difference was −0.873, with a 95% CI of −1.624 to −0.121. For both regimens, the lower bound of the 95% CI was more than the predefined noninferiority margin of −1.9%. At all the other time points, both monthly regimens were not inferior to the daily regimen by more than −1.9% (data not shown).
Fig. 2.
Changes in lumbar spine and total hip bone mineral density. Data are means ± SE
Total hip BMD also increased in all three regimens. The changes were not significantly different among treatment groups.
Bone turnover markers
Urinary NTX, DPD, serum BALP and BGP all significantly decreased from the baseline in all treatment groups (Fig. 3). There was no statistically significant difference in any of the markers at any time points among treatment groups.
Fig. 3.
Changes in bone turnover markers. Data are means ± SE
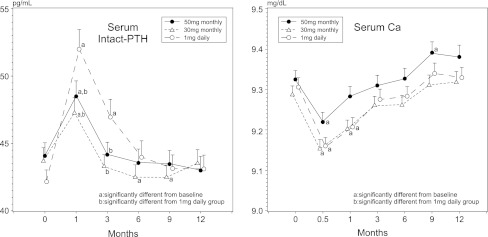
Serum Ca and PTH (Fig. 4)
Fig. 4.
Changes in serum calcium and parathyroid hormone levels. Data are means ± SE. a Significantly different from baseline, p < 0.05; b significantly different from 1 mg daily group, p < 0.05
A small but significant decrease in serum Ca level was observed in all treatment groups at 2 weeks. At 4 weeks, serum Ca levels were still significantly lower than the baseline value in the daily and 30 mg monthly groups but not in the 50 mg monthly group. Thereafter, the serum Ca level was not statistically different from the baseline in all the treatment groups. At 4 weeks, serum intact PTH significantly increased from the baseline in all the treatment groups, and the daily group showed higher PTH than both monthly groups. Increased PTH was maintained at 12 weeks in the daily and 50 mg monthly groups, but not in the 30 mg monthly group. Thereafter, PTH levels returned to baseline values and were not significantly different among groups.
Fracture
The incidences of vertebral and nonvertebral fracture were similar among treatment groups. Morphometric vertebral fracture occurred in six (2.6%) subjects in the daily minodronate group, five (2.2%) in the 30 mg monthly group, and two (0.9%) in the 50 mg monthly group. Nonvertebral fractures were reported in six subjects (2.6%) in the daily minodronate group (rib, femoral neck, ankle, and three radius fractures), five subjects (2.2%) in the 30 mg monthly group [radius and ulna (one), two feet (one), humerus (two), and foot (one)], and four subjects (1.7%) in the 50 mg monthly group [radius and wrist (one), rib (one), foot (one), and wrist (one)].
Safety
Overall, the drug-related AE profiles were similar in all treatment groups (Table 2). There were no deaths in any of the treatment groups.
Table 2.
Drug-related AEs [number of subjects (in percent) ≧ 1%]
| 1 mg daily (n = 234) | 30 mg monthly (n = 229) | 50 mg monthly (n = 229) | Total (n = 692) | |
|---|---|---|---|---|
| Drug-related AEs | 30 (12.8) | 32 (14.0) | 30 (13.1) | 92 (13.3) |
| Gastrointestinal disorders | 22 (9.4) | 16 (7.0) | 17 (7.4) | 55 (7.9) |
| Abdominal discomfort | 5 (2.1) | 4 (1.7) | 5 (2.2) | 14 (2.0) |
| Abdominal pain upper | 3 (1.3) | 3 (1.3) | 3 (1.3) | 9 (1.3) |
| Diarrhoea | 2 (0.9) | 4 (1.7) | 1 (0.4) | 7 (1.0) |
| Nausea | 3 (1.3) | 0 (0.0) | 2 (0.9) | 5 (0.7) |
| Investigations | 5 (2.1) | 11 (4.8) | 7 (3.1) | 23 (3.3) |
| Alanine aminotransferase increased | 0 (0.0) | 3 (1.3) | 0 (0.0) | 3 (0.4) |
| Gamma-glutamyltransferase increased | 0 (0.0) | 5 (2.2) | 0 (0.0) | 5 (0.7) |
| Blood alkaline phosphatase decreased | 5 (2.1) | 1 (0.4) | 3 (1.3) | 9 (1.3) |
Discussion
The present study demonstrated that monthly oral administration of minodronate at a dose of 30 and 50 mg resulted in similar increases in LS and hip BMD as daily administration at a dose of 1 mg. The changes in bone turnover markers were also similar between both monthly regimens and the daily regimen. Safety profiles for the monthly regimens were similar to that of the daily regimen. These results suggest that minodronate, for which a daily dose has been shown to have antivertebral fragility fracture (VFx) efficacy, can be administered monthly in the same manner as risedronate [7, 13] and ibandronate [14, 15].
In the present study, there was a transient decrease in the serum Ca level and a transient increase in the serum PTH level. The magnitudes and time courses of these changes were slightly different among different regimens. As shown in Fig. 3, although statistically nonsignificant, the magnitude of the inhibition of bone resorption markers was numerically different among groups especially at early time points. This may well be reflected to the differences in the changes of serum Ca and PTH. However, the responses in terms of BMD and bone turnover markers were not different among the three groups. Thus, the influence of subtle differences in Ca and PTH on bone was not clear. Similar transient changes in Ca and PTH were previously reported with oral alendronate [16, 17] and risedronate [18] without known effects on bone.
The major limitation of the present study was that it did not have the power to assess antifracture efficacy. However, BMD change has been accepted as a valid surrogate endpoint when evaluating a new dosage schedule for a bisphosphonate for which a fracture benefit has been established [3, 4, 7, 14, 19]. Thus far, no oral bisphosphonate has demonstrated antifracture efficacy with a weekly or monthly regimen in randomized controlled trials. The magnitude of BMD change by monthly minodronate in the present study was similar to that achieved by daily minodronate in the previous studies [1]. The changes in bone turnover markers were also comparable [1, 2]. These data suggest that the monthly and daily regimens of minodronate would be equally beneficial to bone. Another limitation in this study was that only a limited number of men were recruited. Thus, it was impossible to analyze whether or not minodronate would be equally effective to men as well. However, when the data from all three regimens were combined and analyzed using a per protocol set, the LS-BMD change from the baseline to the end of the study was 5.33% (95% CI 3.00–7.66) in men (n = 9), which was comparable to that in women (n = 605) [6.39% (6.09–6.70)]. The change in hip BMD was 1.10% (95% CI −0.34 to 2.53) in men (n = 8), which was smaller than that in women (n = 591) [2.94% (2.74–3.13)]. The changes in metabolic bone markers were numerically smaller in men compared with women: changes in serum BALP were −37.3% (−52.5 to −22.1) in men vs −54.1% (−55.3 to −52.9) in women; serum BGP were −43.8% (−50.7 to −36.9) in men vs −53.4% (−54.5 to 52.4) in women; urinary NTX were −49.3% (−65.0 to −33.5) in men vs −64.5% (−66.4 to −62.5) in women; and urinary DPD were −19.8% (−37.3 to −2.8) in men vs −26.9% (−28.7 to −25.0). Further studies would be needed to evaluate whether there would be sex difference in the responses to minodronate.
The present study demonstrated that oral minodronate administered monthly has comparable efficacy and safety to the daily regimen, which has been shown to have anti-VFx efficacy. This new monthly regimen will give patients with osteoporosis a new dosage option for minodronate, which may lead to better medication compliance for this bisphosphonate.
Acknowledgments
We thank Astellas Pharma Inc. for their scientific and technical support, Ono Pharmaceutical Co., Ltd. for providing supportive data and the following investigators and clinical sites in Japan which participated in this study: M. Harada, Naganuma Orthopedics & Rehabilitation Medical Institution; M. Jinnouchi, Nishi Waseda Orthopaedic Surgery; T. Nakamura, Medical Foundation Syukokai Abe Clinic; K. Akazawa, Akazawa Clinic; H. Hanashi, Medical Corporation Seikokai, New Medical Research System Clinic; D. Kubodera, Medical Corporation Eisinkai Kubodera Orthopaedic; H. Yamane, Toyooka-daiichi Hospital; M. Iwahashi, Medical Corporation Toyooka Orthopaedic Hospital; H. Kim, Yokohama Minoru Clinic, Shintoukai Medical Corporation; Y. Ohtake, The Kanazawa Hospital, Keisuikai Medical Corporation; T. Okawa, Okawa Orthopaedic Surgery Clinic; T. Sakata, Social Medical Corporation Reimei-kai Kitade Hospital; Y. Sakai, Medical Corporation Heiseikai Sunrise Sakai Hospital; R. Kikuno, Kikuno Hospital Medical Corporation Kikuno Association; J. Shiomi, Shiomi Orthopaedics; M. Kajitani, Koseinenkin Kochi Rehabilitation Hospital; S. Kawashita, Tonan Hospital; A. Myojin, Kohoku Hospital; T. Maeda, Maeda Hospital; M. Otani, Koryo Hospital; M. Morita, Susaki Kuroshio Hospital; M. Noguchi, Shinagawa East One Medical Clinic; M. Omata, Tiida Ohimachi Orthopedic Surgery Clinic; M. Nakayama, Tiida Yokohama Motomachi Clinic; K. Suzuki, Kenkokan Suzuki Clinic; H. Shimomura, Musashino Clinic; S. Wada, Wada Orthopedic Clinic; F. Omura, Koenji Orthopedic Surgery; K. Sakamoto, Nishikamata SeikeiGeka; Y. Nemoto, Iryohojin NemotoGeka; and T. Yokoyama, Kitashinagawa Third Hospital
Funding
This study was sponsored by Astellas Pharma Inc., and Ono Pharmaceutical Co., Ltd. The authors were supported in the editing and writing of this manuscript, and sponsored by Astellas Pharma Inc., and Ono Pharmaceutical Co., Ltd. The authors are fully responsible for the content and editorial decisions for this manuscript.
Conflicts of interest
Dr. R. Okazaki has received consulting/advisory fees and a research grant from pharmaceutical companies including Astellas, Ono, Eisai, Lilly, MSD and Teijin.
Dr. H. Hagino has received consulting/advisory fees and a research grant from pharmaceutical companies including Astellas, Ono, Ajinomoto, Asahi Kasei, Chugai, Eisai, Lilly, Mitsubishi Tanabe, MSD, Takeda, Taisho Toyama and Teijin.
Dr. M. Ito has received consulting/advisory fees and a research grant from pharmaceutical companies including Astellas, Ono, Asahi Kasei, Chugai, Daiichi Sankyo and JT.
Dr. T. Sone has received consulting/advisory fees and a research grant from pharmaceutical companies including Astellas, Ono, Asahi Kasei, Chugai, Daiichi Sankyo and Teijin.
Dr. T. Nakamura has received research grants and/or consulting fees from pharmaceutical companies including Astellas, Ono, Amgen, Asahi Kasei, Chugai, Daiichi Sankyo, Lilly, and Merck.
Dr. H. Mizunuma has received consulting/advisory fees and a research grant from pharmaceutical companies including Astellas, Ono and Chugai.
Dr. M. Fukunaga has received consulting/advisory fees from Astellas and Ono.
Dr. M. Shiraki has received consulting/advisory fees and a research grant from pharmaceutical companies including Astellas, Ono, Asahi Kasei, and Teijin.
Dr. Y. Nishizawa has received no consulting/advisory fees or research grants from any companies.
Dr. Y. Ohashi has received consulting/advisory fees and a research grant from pharmaceutical companies including Astellas, Ono, Chugai, Eisai, Daiichi Sankyo and MSD.
Dr. T. Matsumoto has received consulting/advisory fees and a research grant from pharmaceutical companies including Astellas, Ono, Asahi Kasei, Chugai, Daiichi Sankyo, JT, Lilly and Teijin.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Hagino H, Nishizawa Y, Sone T, Morii H, Taketani Y, Nakamura T, Itabashi A, Mizunuma H, Ohashi Y, Shiraki M, Minamide T, Matsumoto T. A double-blinded head-to-head trial of minodronate and alendronate in women with postmenopausal osteoporosis. Bone. 2009;44:1078–1084. doi: 10.1016/j.bone.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Morii H, Ohashi Y, Nakamura T. Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int. 2009;20:1429–1437. doi: 10.1007/s00198-008-0816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzoli R, Greenspan SL, Bone G, 3rd, Schnitzer TJ, Watts NB, Adami S, Foldes AJ, Roux C, Levine MA, Uebelhart B, Santora AC, 2nd, Kaur A, Peverly CA, Orloff JJ. Two-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis. J Bone Miner Res. 2002;17:1988–1996. doi: 10.1359/jbmr.2002.17.11.1988. [DOI] [PubMed] [Google Scholar]
- 4.Brown JP, Kendler DL, McClung MR, Emkey RD, Adachi JD, Bolognese MA, Li Z, Balske A, Lindsay R. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int. 2002;71:103–111. doi: 10.1007/s00223-002-2011-8. [DOI] [PubMed] [Google Scholar]
- 5.Uchida S, Taniguchi T, Shimizu T, Kakikawa T, Okuyama K, Okaniwa M, Arizono H, Nagata K, Santora AC, Shiraki M, Fukunaga M, Tomomitsu T, Ohashi Y, Nakamura T. Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab. 2005;23:382–388. doi: 10.1007/s00774-005-0616-5. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto H, Fukunaga M, Kushida K, Shiraki M, Itabashi A, Nawata H, Nakamura T, Ohta H, Takaoka K, Ohashi Y. Efficacy and tolerability of once-weekly administration of 17.5 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with 2.5-mg once-daily dosage regimen. J Bone Miner Metab. 2006;24:405–413. doi: 10.1007/s00774-006-0706-z. [DOI] [PubMed] [Google Scholar]
- 7.Delmas PD, McClung MR, Zanchetta JR, Racewicz A, Roux C, Benhamou CL, Man Z, Eusebio RA, Beary JF, Burgio DE, Matzkin E, Boonen S. Efficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosis. Bone. 2008;42:36–42. doi: 10.1016/j.bone.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Cotte FE, Fardellone P, Mercier F, Gaudin AF, Roux C. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010;21:145–155. doi: 10.1007/s00198-009-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–337. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 10.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. Jama. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 11.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 12.Wu CY, Li J, Jergas M, Genant HK. Comparison of semiquantitative and quantitative techniques for the assessment of prevalent and incident vertebral fractures. Osteoporos Int. 1995;5:354–370. doi: 10.1007/BF01622258. [DOI] [PubMed] [Google Scholar]
- 13.Ste-Marie LG, Brown JP, Beary JF, Matzkin E, Darbie LM, Burgio DE, Racewicz AJ. Comparison of the effects of once-monthly versus once-daily risedronate in postmenopausal osteoporosis: a phase II, 6-month, multicenter, randomized, double-blind, active-controlled, dose-ranging study. Clin Ther. 2009;31:272–285. doi: 10.1016/j.clinthera.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Miller PD, McClung MR, Macovei L, Stakkestad JA, Luckey M, Bonvoisin B, Reginster JY, Recker RR, Hughes C, Lewiecki EM, Felsenberg D, Delmas PD, Kendler DL, Bolognese MA, Mairon N, Cooper C. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322. doi: 10.1359/JBMR.050313. [DOI] [PubMed] [Google Scholar]
- 15.Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, Christiansen C, Rowell L, Mairon N, Bonvoisin B, Drezner MK, Emkey R, Felsenberg D, Cooper C, Delmas PD, Miller PD. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006;65:654–661. doi: 10.1136/ard.2005.044958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Kaneda K, Minaguchi H, Inoue T, Tomita A, Nagata Y, Nakashima M, Orimo H. A placebo-controlled, single-blind study to determine the appropriate alendronate dosage in postmenopausal Japanese patients with osteoporosis. The Alendronate Research Group. Endocr J. 1998;45:191–201. doi: 10.1507/endocrj.45.191. [DOI] [PubMed] [Google Scholar]
- 17.Tucci JR, Tonino RP, Emkey RD, Peverly CA, Kher U, Santora AC., 2nd Effect of 3 years of oral alendronate treatment in postmenopausal women with osteoporosis. Am J Med. 1996;101:488–501. doi: 10.1016/S0002-9343(96)00282-3. [DOI] [PubMed] [Google Scholar]
- 18.Zegels B, Eastell R, Russell RG, Ethgen D, Roumagnac I, Collette J, Reginster JY. Effect of high doses of oral risedronate (20 mg/day) on serum parathyroid hormone levels and urinary collagen cross-link excretion in postmenopausal women with spinal osteoporosis. Bone. 2001;28:108–112. doi: 10.1016/S8756-3282(00)00410-5. [DOI] [PubMed] [Google Scholar]
- 19.Cosman F, Borges JL, Curiel MD. Clinical evaluation of novel bisphosphonate dosing regimens in osteoporosis: the role of comparative studies and implications for future studies. Clin Ther. 2007;29:1116–1127. doi: 10.1016/j.clinthera.2007.06.009. [DOI] [PubMed] [Google Scholar]