Abstract
The combined effects of shade and drought on plant performance and the implications for species interactions are highly debated in plant ecology. Empirical evidence for positive and negative effects of shade on the performance of plants under dry conditions supports two contrasting theoretical models about the role of shade under dry conditions: the trade-off and the facilitation hypotheses. We performed a meta-analysis of field and greenhouse studies evaluating the effects of drought at two or more irradiance levels on nine response variables describing plant physiological condition, growth, and survival. We explored differences in plant response across plant functional types, ecosystem types and methodological approaches. The data were best fit using quadratic models indicating a humped-back shape response to drought along an irradiance gradient for survival, whole plant biomass, maximum photosynthetic capacity, stomatal conductance and maximal photochemical efficiency. Drought effects were ameliorated at intermediate irradiance, becoming more severe at higher or lower light levels. This general pattern was maintained when controlling for potential variations in the strength of the drought treatment among light levels. Our quantitative meta-analysis indicates that dense shade ameliorates drought especially among drought-intolerant and shade-tolerant species. Wet tropical species showed larger negative effects of drought with increasing irradiance than semiarid and cold temperate species. Non-linear responses to irradiance were stronger under field conditions than under controlled greenhouse conditions. Non-linear responses to drought along the irradiance gradient reconciliate opposing views in plant ecology, indicating that facilitation is more likely within certain range of environmental conditions, fading under deep shade, especially for drought-tolerant species.
Electronic supplementary material
The online version of this article (doi:10.1007/s00442-011-2196-5) contains supplementary material, which is available to authorized users.
Keywords: Competition, Facilitation, Interactive factors, Light, Trade-off, Water
Introduction
Plant species distribution and vegetation dynamics along environmental gradients are strongly driven by interspecific differences in resource uptake efficiency and tolerance to various stresses. Because levels of different resources often change simultaneously along spatial and temporal gradients, understanding the combined effects of multiple resource and stress levels on plant performance is of pivotal importance for the development of theoretical models in plant ecology as well as for predicting the effects of environmental change (Bazzaz 1979; Grime 1979; Huston 1994; Lavorel and Garnier 2002; Valladares and Niinemets 2008).
Light and water are key resources for plant growth and survival, and their interacting effects can shape the nature of plant–plant interactions in a wide range of terrestrial ecosystems. In nature, water and light levels are often negatively correlated. Dense canopies resulting from high plant production reduce irradiance in the understory of wet ecosystems, whereas low plant cover allows high light availability in dry systems (Givnish 1986; Huston 1994; Larcher 2003). Nevertheless, dry and shady conditions can occur simultaneously during dry periods in dense forests (Veenendaal et al. 1996) as well as under compact shrub architectures in deserts (Forseth et al. 2001; Tracol et al. 2011). How plants respond to the combination of light and water limitation has puzzled plant physiologists and ecologists for decades. Under limiting light and water availabilities, plant photosynthesis and carbon gain are lower, negatively affecting osmotic regulation and water use efficiency (Aranda et al. 2007). More generally, the trade-off hypothesis (Smith and Huston 1989) states that plants face a trade-off between drought and shade tolerances as a result of the contrasting structural and physiological adaptations required for tolerance to low levels of each of these resources separately. This hypothesis assumes that under dry conditions plants allocate relatively more biomass to roots than to aboveground structures. As a result, the ratio of respiring biomass to photosynthesizing biomass increases, increasing the light levels necessary to keep a positive carbon balance. High irradiance is expected to enhance photosynthetic rates during the active hours of the day and compensate in this way for the reductions in stomatal conductance under dry conditions. The trade-off hypothesis predicts therefore that plants become less tolerant to shade under dry conditions. Similarly, plants are expected to be less tolerant to drought in shady environments (Smith and Huston 1989). These predictions are expected to hold not only within a population but also across populations, and species, and have been used to explain temporal and spatial changes in species distributions along environmental gradients (Huston 1994).
However, in a wide variety of ecosystems where plants are exposed to water and thermal stress, shade usually enhances plant growth and survival (Holmgren et al. 1997; Callaway 2007; Gómez-Aparicio 2009; and citations therein). Shading reduces air and leaf temperatures preventing overheating (Turner et al. 1966; Franco and Nobel 1989) and reducing transpirational demands, particularly under dry conditions (Geiger 1965; Larcher 2003). In nature, plants may promote growth and survival of other plants under their crowns through different mechanisms leading to positive effects on plant water conditions. Plant canopies change environmental conditions in various ways, especially in dry ecosystems. Under a plant canopy, air and soil temperatures are cooler, reducing evapo-transpiration and often maintaining moisture in air and soils. Improved soil water infiltration and sometimes also plant hydraulic lift can further contribute to moister soils under shade. Indeed, amelioration of abiotic stress is thought to be one of the most significant mechanisms to explain facilitative interactions in a wide range of plant communities where plants grow and survive better under the shade of other plants (Holmgren et al. 1997; Callaway 2007; Gómez-Aparicio 2009).
In an influential paper, Bertness and Callaway (1994) suggested that facilitative interactions explained by amelioration of abiotic stress could in fact be expected to increase under more stressful conditions. In plant communities, this stress gradient hypothesis (SGH; Bertness and Callaway 1994) implies that the shade of neighbouring plants is expected to increasingly ameliorate drought stress as conditions become drier. Numerous papers have tested this prediction with contrasting outcomes maintaining an ongoing debate in plant ecology on the relative role of neighbouring individuals along stress and productivity gradients (Maestre et al. 2009). Particularly debatable is the role of shade at very dry conditions. Although the effect of nurse plants on microclimatic conditions is often positive for plants in the understory, competition for soil moisture may work in the opposite direction. As a result, the net effect of nurse plants on water conditions may be neutral or actually become negative under very dry conditions (Aguiar and Sala 1994; Kitzberger et al. 2000; Tielbörger and Kadmon 2000; Maestre and Cortina 2004; Valladares et al. 2008).
Experimental studies have provided evidence for both the ecophysiological trade-off hypothesis (Aranda et al. 2005, 2007; Valladares and Pearcy 2002; Valladares et al. 2005) and for the increased facilitation under increasing stress hypothesis (Holmgren 2000; Quero et al. 2006, 2008). Evidence in support for each of these apparently disparate theoretical models may be due to a number of different reasons. For instance, discrepancies can result from differences in the ranges of irradiance and water availability evaluated (Aranda et al. 2005), the type of response variables monitored (Quero et al. 2006, 2008), and the particular plant communities, species (Prider and Facelli 2004) or even phenotypes within species (Valladares et al. 2005) studied in each case. Clearly, a great difficulty in the search for general patterns has been the inherent complexity of a problem that scales physiological and biomass allocation mechanisms at the plant level to demographic and ecological consequences within populations and communities. The type of experiments and observations needed to approach those problems are clearly different in scale. Moreover, the type of evidence that can be obtained is different. Although extraordinarily difficult, greenhouse experiments can aim to maintain soil water conditions constant at increasing irradiance levels and compare the independent effects of water and light on physiological mechanisms and growth patterns (Sack and Grubb 2002). But under field conditions, this is practically impossible to achieve since as explained earlier water and light conditions are not independent in nature. Nevertheless, field experiments can assess the ecological consequences of different irradiance levels under a range of water conditions realizing that may be an uncontrollable interaction between them.
Understanding the conditions under which each of these theoretical models applies is not only relevant for the development of ecological theory but also has far reaching consequences for our predictions of the impact of increased drought on plant communities resulting from global climate change (Valladares and Niinemets 2008), and for our capacity for ecosystem restoration under unfavourable environmental conditions (Gómez-Aparicio et al. 2004, 2005; Gómez-Aparicio 2009). In this paper, we assess the relative role of shade on plant performance under dry conditions using a selection of published field and greenhouse studies where the interactive effects of light and water availability have been directly measured. This is a first large-scale numerical exploration of a pivotal problem that has been approached by numerous case studies. This meta-analysis aims to provide the empirical evidence supporting general emerging patterns and to highlight the current shortcomings in our understanding of key community ecology processes.
Materials and methods
Data collection
We searched observational and experimental studies that evaluated plant performance under two levels of water availability and two or more light levels in a full factorial design. Searches were conducted in ISI Web of Science (1945–2008) using the following keywords: (light or irradiance or shad*) and (water availability or water addition or drought or irrigation or rainfall or precipitation). In addition, we included those studies cited within the reference lists of the papers gathered. We decided not to include studies providing information on plant performance at only one light level because we wanted to focus our review on those studies explicitly designed to test water-light interactions. We considered studies conducted both under controlled conditions (e.g. greenhouse, growth chambers) and in the field. Because our objective was to explore drought effects along a gradient of light conditions (see “Data analysis” below), only studies that provided quantitative information on light levels were considered. The search from 2,274 papers yielded 65 suitable published studies that were grouped in 9 different datasets, depending on the plant performance estimator quantified: survival, whole-plant biomass, relative growth rate (RGR), root:shoot ratio, specific leaf area (SLA), maximum photosynthetic capacity (A max), stomatal conductance (gs), light compensation point (LCP), and maximum photochemical efficiency of photosystem II (F v/F m) (see Appendices S1 to S9 in Supporting Information). In cases where the light and water treatments were crossed with additional treatments that implied explicit manipulation of the environment (e.g. fertiliser addition), we included only the ambient treatment. For each case, we recorded (if available) any information provided by the authors indicative of the strength of the drought treatment at each light level (i.e. predawn leaf/shoot water potential, gravimetric/volumetric soil water content). When articles involved several species, each species was treated as a separate study. We decided to include several studies from the same paper because, although it tends to reduce the overall heterogeneity in effect sizes, excluding multiple results from a paper can underestimate effect sizes (Gurevitch and Hedges 1999; Karst et al. 2008). When data were only reported in graphical form, we used the data-grabbing software TechDig v.2.0 (Jones 1998) to obtain the quantitative information.
Data analysis
We used two different measures of effect size: the odds ratio metric for categorical data (survival), and the response ratio for continuous data (the eight remaining morphological and physiological variables). Survival data were summarised in a 2 × 2 contingency table, with columns representing treatments (high water vs. low water availability) and rows the possible outcomes (alive vs. dead). An odds ratio (OR) metric was obtained for each study, calculated as the ratio of the odds of survival under low water availability (experimental treatment) to the odds of survival under high water availability (control treatment). Because there were some studies where all individuals survived or died, and this could produce odds ratio values that require division by 0, the odds ratio were calculated adding 1 to the number of individuals in each category for every study (Hyatt et al. 2003; Maestre et al. 2005). Studies where survival was measured in units other than percentages (e.g. number of days until death; Sack 2004) were not included in the analysis due to the impossibility of calculating the OR. Values of the natural log of the odds ratio [ln(OR)] higher than 0 indicate a positive drought effect on performance, whereas values lower than 0 indicate a negative effect of drought. The effect size of drought for the eight continuous performance estimators was calculated using the natural log of the response ratio [ln(RR)] and its associated variance (v lnRR). The estimate of ln(RR) and v lnRR for each study is based on means, SDs, and replicate numbers for control and treatments (Hedges et al. 1999). As for odds ratios, positive ln(RR) values indicate positive drought effects and negative values negative drought effects on plant performance.
The effect of drought along the light gradient was first assessed for each of the nine entire datasets using weighted regressions (Rosenberg et al. 2000). For analyses purposes, light availability was expressed in a percentage scale. Although in absolute terms a given light percentage does not necessarily represent the same light quantity in systems differing in latitude or altitude, we consider that a relative scale is adequate to compare the response of species that naturally occur under different ranges of light availability (e.g. arid species vs. tropical species). Thus, independently of the absolute levels, 100% would represent the light that reaches an open microsite in each study case, and therefore the maximum light quantity that a species can receive at that site. Both linear and quadratic regressions were fit to account for non-linearity in the response, and the best model chosen using the Akaike Information Criterion corrected for small sample sizes (AICc). The absolute magnitude of the differences in AICc between alternate models provides an objective measure of the strength of empirical support for competing models (Burnham and Anderson 2002). The model with the strongest empirical support has the lowest AICc, and thus ΔAICc = 0. Models with ΔAICc between 0 and 2 are considered to have equivalent empirical support, models with ΔAICc = 2–10 are considered to have considerably less empirical support, and models with ΔAICc > 10 are considered to have essentially no empirical support (Burnham and Anderson 2002). To control for the fact that our meta-analytical approach does not explicitly take into account the potential consequences of a variation in the drought strength among light levels, we conducted additional analyses for the subset of studies that provided quantitative information on the water status of plants (predawn leaf/shoot water potential, Ψp) or soils (soil water content, SWC). In these analyses, the lnRR of Ψp (in the case of A max, gs and F v/F m) or SWC (for the rest of performance estimators) was included as a covariate.
For those performance estimators for which an effect of light was detected (i.e. the AICc of the linear and/or quadratic regressions was lower than the AICc of a null model that assumes no variation of drought effects with light), we explored the possibility of differences among groups of studies. Differential responses were considered to exist when a model that differentiated among groups (i.e. calculate different sets of parameters for each group) gave a more likely explanation of the data (i.e. lower AICc) than a model fit to all data combined. Groups of studies were created according to five different grouping variables: approach (i.e. controlled conditions vs. field study); ecosystem type or biome (i.e. semiarid, cold temperate, dry tropical, wet tropical); species leaf habit (i.e. winter deciduous vs. evergreen); species drought tolerance (i.e. intolerant vs. tolerant) and species shade tolerance (i.e. intolerant vs. tolerant). The semiarid category was used in broad sense to include different types of water-limited ecosystems (arid, semiarid, Mediterranean). For indoor controlled experiments, biome refers to the geographic area where the target species occurs in nature. Species were assigned to the different drought and shade tolerance groups based on the information provided by the authors in the reviewed papers and, when necessary, on additional published information about the ecological requirements of the species. Classifications in these two groups applied primarily to seedlings and saplings (following Niinemets and Valladares 2006). Only woody species were considered for the comparisons among leaf habits and drought/shade tolerances.
The possibility of publication bias (i.e. the greater possibility of publishing significant results) was tested by calculating the Rosenthal’s fail-safe number, that is, the number of studies with an effect size of zero that would be necessary to add to the meta-analysis to reduce the significance level of the observed mean to 0.05 (Rosenthal 1979). Funnel plots were also used as a graphical method to assess publication bias, and Spearman rank correlation tests were conducted to analyse the relationship between the standardised effect size and the standardised variance across studies (Borenstein et al. 2009). Significance of this test indicates that larger effect sizes are more likely to be published than smaller effects (Begg and Mazumdar 1994). Analyses were conducted using MetaWin v.2.0 (Rosenberg et al. 2000) and JMP v.7.0 (SAS Institute, Cary, NC).
Results
Overall patterns
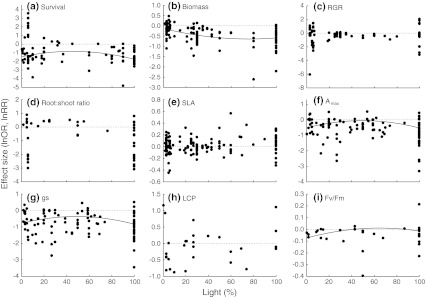
The number of experimental cases extracted for each of the nine response variables ranged between 29 and 136 (Table 1). We found evidence for variation in the effect of drought along the light gradient in 5 of the 9 variables (survival, whole plant biomass, A max, gs and F v/F m; Fig. 1; Appendices S1–S9). Moreover, in all these cases, the quadratic model was a better fit to the data (i.e. lower AICc) than the linear model. The same result was found for the subsets of studies for which a measure of the drought strength (i.e. Ψp or SWC) was included as a covariate in the analyses (Appendix S10). Therefore, we can assume that potential variations in the drought strength among light levels do not substantially affect the conclusions reached in this study. For 4 of these 5 variables (all but biomass), the negative effect of drought was maximum in the extremes of the light gradient, and minimum at intermediate light levels. For biomass, on the contrary, the negative effect of drought was ameliorated at the extremes of the light gradient, especially at the low-light extreme. The null (mean) model was the best fit for RGR, root:shoot ratio, SLA and LCP, indicating no variation in the effect of drought along the light gradient for these performance estimators (Fig. 1).
Table 1.
Model selection statistics (Akaike’s Information Criterion values; AICc) for three candidate models describing the variation of the drought effect along the light gradient
| Variable | n | AICc | R 2 | Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| Null | Linear | Polynomial | Intercept | Linear term | Quadratic term | |||
| Survival | 92 | 344.4 | 343.3 | 340.5 | 0.07 | −1.460 (−1.678, −1.242) | 0.028 (0.014, 0.042) | −0.00033 (−0.00051, −0.00019) |
| Biomass | 115 | 500.4 | 487.7 | 484.1 | 0.15 | −0.131 (−0.180, −0.082) | −0.013 (−0.015, −0.011) | 0.00016 (0.00013, 0.00019) |
| RGR | 91 | 296.3 | 297.9 | 299.9 | ||||
| Root:shoot ratio | 46 | 196.2 | 197.2 | 198.8 | ||||
| SLA | 136 | 447.3 | 448.7 | 450.1 | ||||
| Amax | 107 | 568.7 | 567.8 | 559.4 | 0.11 | −0.519 (−0.588, −0.450) | 0.018 (0.016, 0.020) | −0.00025 (−0.0027, −0.00023) |
| gs | 103 | 530.2 | 529.2 | 522.8 | 0.10 | −0.785 (−0.858, −0.712) | 0.017 (0.013, 0.021) | −0.00022 (−0.00024, −0.00020) |
| LCP | 29 | 114.4 | 116.7 | 117.3 | ||||
| Fv/Fm | 34 | 105.1 | 105.1 | 91.6 | 0.36 | −0.074 (−0.096, −0.052) | 0.003 (0.002, 0.004) | −0.00002 (−0.00002, −0.00002) |
The alternative models were fitted for each performance estimator using maximum likelihood methods. The best fitting model (i.e. lower AICc) is shown in italics. Goodness of fit (R 2) and parameter values (mean and 95% CI) are given for the best models. n number of experimental cases. Plant performance estimators were survival, whole-plant biomass, relative growth rate RGR, Root:shoot biomass ratio, specific leaf area SLA, maximum net photosynthetic rate A max, stomatal conductance gs, light compensation point LCP, predawn water potential Ψp and maximum photochemical efficiency of photosystem II F v /F m
Fig. 1.
Mean drought effect size (lnOR for survival, lnRR for continuous variables) along irradiance gradients for a survival, b biomass, c relative growth rate (RGR), d root:shoot ratio, e specific leaf area (SLA), f maximum net photosynthetic rate (A max), g stomatal conductance (gs), h light compensation point (LCP), and i maximum photochemical efficiency of photosystem II (Fv/Fm). Regression lines are shown for significant models only. Dotted lines indicate zero effect sizes
Among-group comparisons
Survival
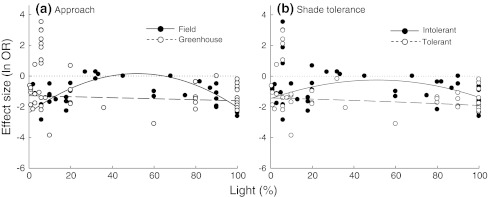
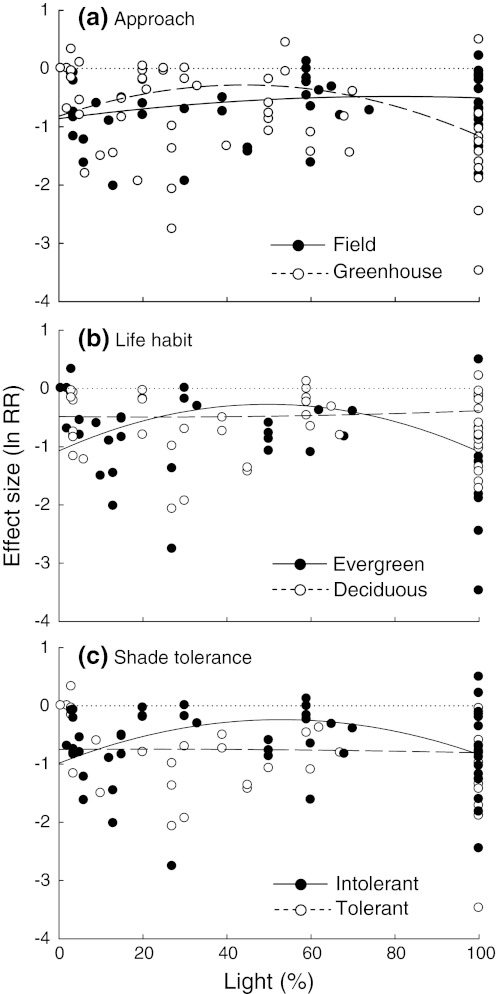
The fit of the statistical models to the empirical data improved substantially when studies were grouped according to two of the five grouping variables: approach and species shade tolerance (Appendix S11). The non-linear survival response to irradiance was strong under field conditions but very weak under controlled conditions. The effect of drought on the survival of shade-intolerant species showed a clear humped-back shape, varying from almost neutral effects at intermediate light levels to large negative effects at both extremes of the light gradient. In contrast, the survival of shade-tolerant species was strongly and negatively affected by drought throughout the whole light gradient (Fig. 2).
Fig. 2.
Comparison of drought effects on survival along the light gradient between groups of studies that differed in a their approach (field vs. greenhouse) and b species shade tolerance (intolerant vs. tolerant). Only those grouping variables for which differences among groups were detected are shown (see Appendix S11). Dotted lines indicate zero effect sizes
Biomass
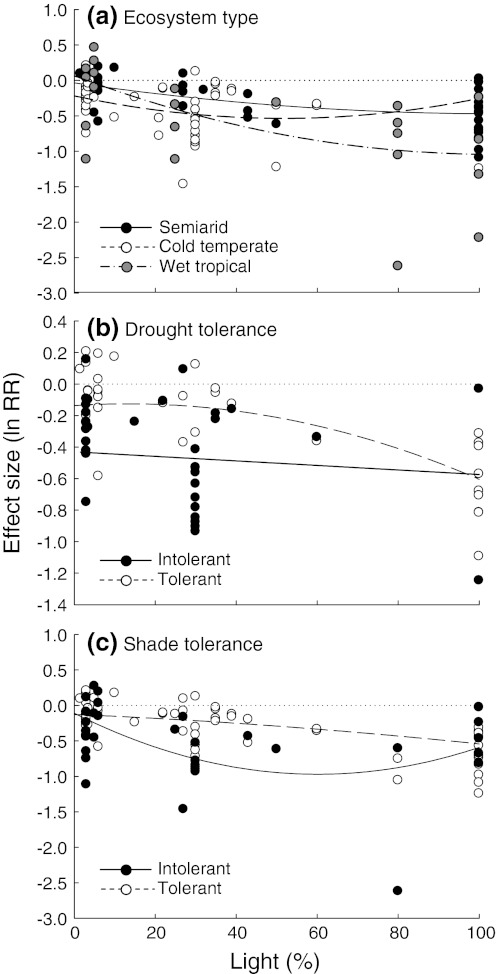
The response to drought along the light gradient varied among ecosystem types, species drought tolerance, and species shade tolerance, but not among leaf habits (Appendix S11). Differences among field and greenhouse studies were not explored due to the low sample size of field studies (only 2 of 115). Wet tropical species showed in general larger negative effects of drought, especially at high light (Fig. 3). Drought effects on biomass of drought-tolerant species varied from almost neutral to largely negative with increasing light availability, whereas drought-intolerant species showed large negative responses independently of the light level. Shade-intolerant species showed much stronger non-linear responses to the light gradient than shade-tolerant species.
Fig. 3.
Comparison of drought effects on whole-plant biomass along the light gradient between groups of studies that differed in a type of ecosystem (semiarid vs. cold temperate vs. wet tropical), b species drought tolerance (intolerant vs. tolerant), and c species shade tolerance (intolerant vs. tolerant). Only those grouping variables for which differences among groups were detected are shown (see Appendix S11). Dotted lines indicate zero effect sizes
Maximum net photosynthetic rate (Amax)
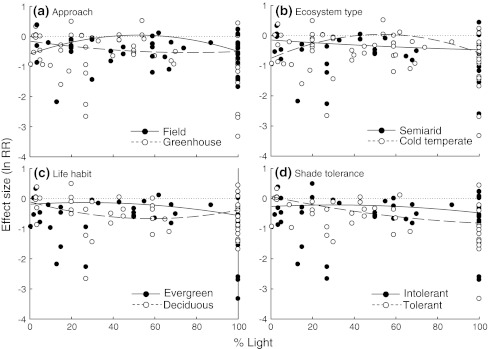
Grouping studies clearly improved the fit of the statistical models for all but one (species drought tolerance) of the five grouping variables tested (Appendix S11). We found a humped-back response of drought effects to light availability under controlled greenhouse conditions but not in the field, and in cold temperate but not in semiarid ecosystems (Fig. 4). Drought effects on A max of evergreen species varied from almost neutral to largely negative with increasing light availability, whereas winter deciduous species showed large negative responses independently of the light level. Regarding species shade tolerance, negative drought effects became stronger with light availability for shade-tolerant species, whereas the response of shade-intolerant species was of lower magnitude and more independent of the light level.
Fig. 4.
Comparison of drought effects on photosynthetic rate (A max) along the light gradient between groups of studies that differed in a their approach (field vs. greenhouse), b type of ecosystem (semiarid vs. cold temperate), c species leaf habit (evergreen vs. winter deciduous), and d species shade tolerance (intolerant vs. tolerant). Only those grouping variables for which differences among groups were detected are shown (see Appendix S11). Dotted lines indicate zero effect sizes
Stomatal conductance (gs)
The response to drought along the light gradient varied among type of approach, species leaf habit, and species shade tolerance, but not among biomes or species drought tolerance (Appendix S11). A stronger non-linear response was found in greenhouse than in field studies (Fig. 5). As for A max, drought effects showed a non-linear response to light in evergreen species, but were rather independent of the light level in deciduous species. The effect of drought on stomatal conductance of shade-intolerant species showed a clear humped-back shape, varying from almost neutral effects at intermediate light levels to large negative effects at both extremes of the light gradient. In contrast, conductance of shade-tolerant species was strongly negatively affected by drought throughout the whole light gradient (Fig. 5).
Fig. 5.
Comparison of drought effects on stomatal conductance (gs) along the light gradient between groups of studies that differed in a their approach (field vs. greenhouse), b species leaf habit (evergreen vs. deciduous), and c species shade tolerance (intolerant vs. tolerant). Only those grouping variables for which differences among groups were detected are shown (see Appendix S11). Dotted lines indicate zero effect sizes
Maximal photochemical efficiency (Fv/Fm)
Models differentiating among groups never showed a better fit to the data than models that grouped all species together. This failure to find among-group differences could be influenced by the low number of studies that measured this physiological variable, which limits the power of the analyses.
Assessment of publication bias
Fail-safe numbers tended to be large compared to the number of cases included in the meta-analyses. Rosenthal (1979) suggested that if the fail-safe number was larger than 5 times the sample size plus 10, it was safe to conclude that results were robust regarding publication bias. This was true for all variables except for root:shoot ratio, SLA and LCP (survival fail-safe number = 16,847.7; biomass = 37,148.7; RGR = 11,189.5; root:shoot ratio = 124.1; SLA = 0; A max = 35,303.3; gs = 82,433.1; LCP = 88.1; F v/F m = 317.8). Scatter plots of effect size against sample size of the nine datasets (not shown) exhibited a typical funnel shape, indicating that studies with small sample sizes (and generally low precision) showed a larger scatter around the true effect value than studies with larger sample sizes (and higher precision). Accordingly, the Spearman’s rank correlation tests between effect size and variance were not significant for most performance estimators, all but survival and root:shoot ratio (Rs = 0.35, P = 0.0005 for survival; Rs = −0.14, P = 0.22 for biomass; Rs = −0.01, P = 0.91 for RGR; Rs = −0.40, P = 0.004 for root:shoot ratio; Rs = 0.03, P = 0.68 for SLA; Rs = −0.06, P = 0.54 for A max; Rs = −0.14, P = 0.13 for gs; Rs = 0.04, P = 0.84 for LCP; Rs = −0.20, P = 0.26 for F v/F m). Overall, these results suggest that there was little publication bias in the studies included in the review and that the meta-analyses outputs were robust.
Discussion
Our results indicate that the interactive effects of light and water availability on plant performance tend to be non-linear with the negative effects of drought being lower at intermediate irradiance conditions and becoming more severe at higher or lower light levels. This general pattern was maintained when controlling for potential variations in the strength of the drought treatment among light levels. This humped-back shape response to drought along the irradiance gradient reconciliates contrasting views arising from ecophysiological (Holmgren 2000; Sack and Grubb 2002; Valladares and Pearcy 2002; Aranda et al. 2005; Quero et al. 2006) and plant–plant interactions studies (Smith and Huston 1989; Bertness and Callaway 1994; Holmgren et al. 1997; Maestre et al. 2009). Non-linear patterns of drought effects on plant performance along the irradiance gradient suggest that the positive effects of shade are more likely at intermediate light levels, and can be lost under deep shade. The stronger negative effects of drought at very low irradiance are congruent with the expectations of a trade-off between shade and drought tolerance at very low light levels. Our results also indicate that this loss of the positive influence of a canopy is more likely to occur in species that are relatively tolerant to high light and dry conditions, while shade-tolerant and drought-intolerant plants are very sensitive to dry conditions and are more commonly facilitated by a canopy over a larger gradient of shade levels. To fully appreciate how our results contribute to understand the interactive effects of water and light, we discuss in the following sections how sensitive plant traits, species and plant functional groups are to drought effects along different irradiance levels, and explain how these results contribute to reconcile contrasting views in ecological models.
Which plant traits are more sensitive to combined shade-drought?
Our quantitative synthesis shows that plant survival and several physiological variables related to stomatal and photosynthetic activity (A max, gs, F v/F m) are very sensitive to the interactive effects of light and water availability. For these estimators of plant performance, the negative effects of drought tend to be lower at intermediate irradiance conditions (Fig. 1).
At high irradiance levels, lower air humidity reduces stomatal conductance, which, in combination with high air temperatures, can strongly decrease photosynthetic rates and can eventually damage the photosynthetic apparatus, especially in dry soils (Valladares et al. 2005). Shade ameliorates these negative effects of drought but only to a certain extent. Controlled greenhouse experiments have demonstrated that combined limiting light and water conditions can directly reduce plant photosynthesis, reducing potential carbon gain, and negatively affecting osmotic regulation and water use efficiency (Aranda et al. 2007). In nature, water deficit could actually worsen under the very low light conditions found under dense canopies. Although plant canopies often generate cooler and moister atmospheres beneath them, which ameliorate plant water stress, their impact on soil water conditions is variable. Soil water availability in the understory results from mechanisms acting in opposite directions with a net result that is not easily predictable (Joffre and Rambal 1993; Larcher 2003). Lower evaporative demands (Breshears et al. 1998), improved soil water infiltration (Joffre and Rambal 1988), active hydraulic lift (Dawson 1993; Zou et al. 2005) and canopy dripping (Breshears et al. 1997) can all have positive effects on soil water availability in the understory. However, canopy interception can reduce the amount of water reaching the soil (Pressland 1973) and root competition for water can make understories drier than open patches (Abrams and Mostoller 1995; Valladares and Pearcy 2002). These negative effects on water availability can potentially override the positive ones and aggravate the effects of drought (Aguiar and Sala 1994; Kitzberger et al. 2000; Tielbörger and Kadmon 2000; Valladares et al. 2008). We found stronger non-linear effects of drought on plant survival in field than in greenhouse studies. Drought conditions may not only be more severe under field conditions but the duration of drought may be longer, which increases the probability of finding strong negative effects on survival at increasing irradiance.
We found the effects of drought on plant morphology and allometry (e.g. root:shoot ratio, SLA) to be less sensitive to changes in light conditions than the physiological and survival responses. Previous comparisons between physiological and structural responses to drought report comparable results (Quero et al. 2006, 2008). Although proportionally higher biomass allocation to roots and smaller and thicker leaves can potentially enhance acclimation to dry conditions, we found the effects of drought to be highly variable on both the root:shoot biomass ratio and the specific leaf area, and to be unaffected by changing light conditions. A plausible explanation may be found on how drought treatments were experimentally imposed. If drought treatments were applied by withholding water after a relatively long initial period of optimal water supply (as it was in several of the papers included in the review), plant morphology may become rather unresponsive for the remaining experimental time. In general, we found stronger coupling between physiological condition and survival than between those and plant morphology at the seedling stage.
How do species differ in their response to drought under different irradiance levels?
Species stress tolerance is an important aspect that may affect the way plants respond to drought at increasing irradiance levels. In general, species shade and drought tolerance are inversely correlated (Niinemets and Valladares 2006; but see Engelbrecht et al. 2007; Markesteijn et al. 2011). We found that the negative effects of drought on the performance of shade-tolerant species could be ameliorated by shade but tended to remain rather constant along the whole irradiance gradient. In contrast, drought effects on shade-intolerant species showed non-linear responses to the light gradient, as described previously. The difference between a linear and a humped-back shape response between plant functional groups indicates that very low light levels affect the physiological and morphological adaptations to cope with drought more strongly among species able to tolerate higher light and water stress. There are several underlying mechanisms potentially explaining this pattern. Drought-tolerant species typically allocate a large fraction of their biomass to roots, which cannot be accomplished under limiting light conditions, and the same applies to osmotic adjustment involving sugars to cope with dry soils which is hardly achieved with limited carbon gain under low light (Aranda et al. 2005, 2007; Robson et al. 2009).
We also found that wet tropical plant species exhibited the strongest negative drought effects with increasing light (Fig. 3). Because plant species from tropical rainforests grow mostly under conditions of high water availability and low irradiance, they tend to have large leaf area ratio and specific leaf area (Markesteijn and Poorter 2009), which likely increase their vulnerability to the increased temperature and water deficit found at higher irradiance. Indeed, drought sensitivity can strongly determine species distribution in tropical forests (Engelbrecht et al. 2007). These results suggest that facilitation may be an important yet overlooked mechanism in wet tropical ecosystems as recently suggested by theoretical (Holmgren and Scheffer 2010) and quantitative analysis of restoration experiences (Gómez-Aparicio 2009). In contrast, we found less variation of drought effects along the light gradient among species from arid and semiarid ecosystems.
In cold temperate systems, the response to the combined effects of drought and shade likely depends on a species leaf habit. We found that, in general, winter deciduous species showed large negative responses to drought independently of the light level whereas drought effects on evergreen species varied from almost neutral in shade to largely negative at high light conditions (Figs. 4, 5). A plausible explanation for this result would be that evergreen species have thicker leaves and often also deeper root systems that enhance tolerance to moderate drought, whereas deciduous species follow a strategy of higher assimilation rates during periods of favourable environmental conditions and a faster leaf turnover to avoid environmental stress (Mooney and Dunn 1970; Werner et al. 1999).
Reconciling physiological responses with ecological models
Our findings are highly relevant to understand the complex, interacting effects of water and light availabilities on plant performance and plant–plant interactions in nature and contribute to understand the discrepancies across conceptual models on the role of facilitative interactions in plant communities (Bertness and Callaway 1994; Maestre et al. 2009; Holmgren and Scheffer 2010). The stress gradient hypothesis (SGH) predicted the role of facilitative interactions to increase with the harshness of abiotic conditions (Bertness and Callaway 1994). We indeed found evidence of amelioration of drought stress under moderate shade but not under deep shade. Conceptual models had previously suggested switches between facilitation and competition along stress gradients. Holmgren et al. (1997) highlighted that net facilitation could only be expected when the positive effects outweigh the unavoidable negative effects of a nurse canopy shade on the plants growing underneath. Later on, Michalet et al. (2006) reasoned that, contrary to what is expected by the Stress Gradient Hypothesis model, facilitative interactions are probably insufficient to expand the niche of stress-intolerant species under the most stressful end of environmental conditions. Building on that, Maestre et al. (2009) predicted that the facilitation effects would not only depend on the life history of the interacting species (stress tolerance vs. competitive ability of benefactor and beneficiary) but also on the type of stress factor (resource vs. non-resource). They predicted (1) facilitation to increase with stressfulness of conditions when the abiotic stress gradient is not driven by resource limitation (e.g. temperature) or when the beneficiary (facilitated) species is stress-tolerant; and (2) a humped-back shape model with negative interactions overruling facilitation at both ends of the stress gradient especially when the stress gradient is driven by a resource like water. More recently, Holmgren and Scheffer (2010) concluded that, while facilitation will expand the range of conditions where an organism may occur, the largest absolute effects on growth will always occur under less stressful conditions. Our results indeed show a humped-back shape response of the positive effect of shade on drought amelioration at the plant level. Particularly for shade-intolerant species, the positive effects of shade peak at intermediate values, with detrimental effects at both ends of the light availability gradient. Stronger drought effects on plant performance under very low irradiance are in agreement with the trade-off hypothesis between shade and drought tolerance (Smith and Huston 1989).
Although the ecological literature on facilitative interactions is dominated by examples from ecosystems where abiotic stress is severe, we found that effects of shade on drought amelioration are particularly strong in wet tropical ecosystems. These results are in agreement with the models suggesting that facilitation may prevail in much milder environments than originally thought (Holmgren and Scheffer 2010). By reconciling opposing views and findings, our study provides a mechanistic insight on when and how positive plant–plant interactions become significant along gradients of abiotic stress.
Future prospects: how can we expand our understanding?
The current experimental evidence concentrates in relatively few species and growth forms and has been obtained primarily in drylands and cold temperate systems whereas few studies have been conducted in both dry and wet tropical ecosystems. Our knowledge is not only uneven across ecosystems but it is also biased by protocols and methodological practices. A major methodological difficulty is the control of constant soil water conditions along different irradiance levels which is particularly challenging under field conditions. This difficulty has stressed the differences in interpretation of the combined effects of shade and drought on plant responses among greenhouse versus field experimentalists. In general, greenhouse pot experiments under very controlled conditions report on physiological and morphological responses but often find no effects on survival. Field experiments, in contrast, often find demographic effects but lack a detailed description of the physiological or morphological responses associated with them. This is partly the result of the kind of questions addressed by plant ecologists and physiologists. Although both are interested in understanding the interactive effects of irradiance and water on plant performance, ecologists have emphasized population and community level questions regarding facilitation versus competition in plant–plant interactions (Smith and Huston 1989; Holmgren et al. 1997; Gómez-Aparicio et al. 2004, 2005; Gómez-Aparicio 2009) whereas physiologists have mostly concentrated in leaf and plant level mechanisms allowing individual plants to cope with the abiotic environment (Valladares and Pearcy 2002; Sack and Grubb 2002; Aranda et al. 2005; Quero et al. 2006).
Our analysis, therefore, should be considered as a first exploratory effort to synthesise our knowledge. In this meta-analysis, we included only the studies in which plant responses to interacting light and water levels had been evaluated in an effort to control for experimental biases. This strong condition unavoidably reduces the potential sample size because the study of interacting effects of multiple stresses has become more common only recently. The relatively small sample size, combined with the variability of responses found among ecosystem and plant functional types, certainly limited the explanatory power of our general models (Table 1).
The limitations we encountered highlight some promising paths for future work. We think that experimental work should assess the relationships between physiological, growth and demographic responses, across many species with contrasting functional types exposed to large gradients of shade and drought. We need ambitious experimental enterprises conducted under both field and greenhouse conditions, instead of small experiments focusing on particular species or plant responses. This would allow comparing how different plant functional types respond to the interactive effects of shade and drought within each type of ecosystem and controlling for the methodological approached used.
Better attention to understanding the effects of drought on dry and wet tropical ecosystems is particularly relevant to advance ecological knowledge but also to predict the potential consequences of climate change (Engelbrecht et al. 2007; Markesteijn et al. 2011). Tropical systems were very poorly represented in our study sample. Understanding the effects of drought on wet tropical understories is a priority for predicting the potential consequences of drier future climate expected for most of the world’s rainforests (Christensen et al. 2007), as well as for evaluating the potential role of facilitation in these ecosystems (Holmgren and Scheffer 2010).
The emerging humped-back shape response of drought effects along the light gradient merits attention, since it suggests that we may be able to reconcile the diversity of patterns found through numerous particular cases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Wageningen University for facilitating the stay of Lorena Gómez-Aparicio and José Luis Quero. This study was partly supported by the postdoctoral grant MCINN (Spain) to J.L.Q. (ref 2007-0572).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Glossary
Abbreviations
- Amax
Maximum photosynthetic capacity
- Fv/Fm
Maximum photochemical efficiency of photosystem II
- gs
Stomatal conductance
- LCP
Light compensation point
- RGR
Relative growth rate
- R:S
Root:shoot ratio
- SLA
Specific leaf area
- Ψp
Predawn leaf/shoot water potential
Contributor Information
Milena Holmgren, Phone: +31-317-485284, FAX: +31-317-484845, Email: milena.holmgren@wur.nl.
Lorena Gómez-Aparicio, Email: lorenag@irnase.csic.es.
José Luis Quero, Email: jose.quero@urjc.es.
Fernando Valladares, Email: valladares@ccma.csic.es.
References
- Abrams MD, Mostoller SA. Gas exchange, leaf structure and nitrogen in contrasting successional tree species growing in open and understory sites during a drought. Tree Physiol. 1995;15:361–370. doi: 10.1093/treephys/15.6.361. [DOI] [PubMed] [Google Scholar]
- Aguiar MR, Sala OE. Competition, facilitation, seed distribution and the origin of patches in a Patagonian steppe. Oikos. 1994;70:26–34. doi: 10.2307/3545695. [DOI] [Google Scholar]
- Aranda I, Castro L, Pardos M, Gil L, Pardos JA. Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak Quercus suber L seedlings. For Ecol Manag. 2005;210:117–129. doi: 10.1016/j.foreco.2005.02.012. [DOI] [Google Scholar]
- Aranda I, Pardos M, Puertolas J, Jimenez MD, Pardos JA. Water–use efficiency in cork oak (Quercus suber) is modified by the interaction of water and light availabilities. Tree Physiol. 2007;27:671–677. doi: 10.1093/treephys/27.5.671. [DOI] [PubMed] [Google Scholar]
- Bazzaz FA. The physiological ecology of plant succession. Annu Rev Ecol Syst. 1979;10:351–371. doi: 10.1146/annurev.es.10.110179.002031. [DOI] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- Bertness MD, Callaway RM. Positive interactions in communities. Trends Ecol Evol. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009. [Google Scholar]
- Breshears DD, Rich PM, Barnes FJ, Campbell K. Overstory-imposed heterogeneity in solar radiation and soil moisture in a semiarid woodland. Ecol Appl. 1997;7:1201–1215. doi: 10.1890/1051-0761(1997)007[1201:OIHISR]2.0.CO;2. [DOI] [Google Scholar]
- Breshears DD, Nyhan JW, Heil CE, Wilcox BP. Effects of woody plants on microclimate in a semiarid woodlands: soil temperature and evaporation in canopy and intercanopy patches. Int J Plant Sci. 1998;159:1010–1017. doi: 10.1086/314083. [DOI] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference. New York: Springer; 2002. [Google Scholar]
- Callaway RM. Positive interactions and interdependence in plant communities. Dordrecht: Springer; 2007. [Google Scholar]
- Christensen JH, Hewitson B, Busuioc A, Chen A, Gao X, Held I, Jones R, Kolli RK, Kwon WT, Laprise R, Magaña Rueda V, Mearns L, Menéndez CG, Räisänen J, Rinke A, Sarr A, Whetton P. Regional Climate Projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Climate Change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2007. pp. 847–940. [Google Scholar]
- Dawson TE. Hydraulic lift and water use by plants: implications for water balance, performance and plant–plant interactions. Oecologia. 1993;95:565–574. doi: 10.1007/BF00317442. [DOI] [PubMed] [Google Scholar]
- Engelbrecht BMJ, Comita LS, Condit R, Kursar TA, Tyree MT, Turner BL, Hubbell SP. Drought sensitivity shapes species distribution patterns in tropical forests. Nature. 2007;447:80–82. doi: 10.1038/nature05747. [DOI] [PubMed] [Google Scholar]
- Forseth IN, Wait DA, Casper BB. Shading by shrubs in a desert system reduces the physiological and demographic performance of an associated herbaceous perennial. J Ecol. 2001;89:670–680. doi: 10.1046/j.0022-0477.2001.00574.x. [DOI] [Google Scholar]
- Franco AC, Nobel PS. Effects of nurse plants on the microhabitat and growth of cacti. J Ecol. 1989;77:870–886. doi: 10.2307/2260991. [DOI] [Google Scholar]
- Geiger R. The climate near the ground. Cambridge: Harvard University Press; 1965. [Google Scholar]
- Givnish TJ. On the economy of plant form and function. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- Gómez-Aparicio L. The role of plant interactions in the restoration of degraded ecosystems: a meta-analysis across life-forms and ecosystems. J Ecol. 2009;97:1202–1214. doi: 10.1111/j.1365-2745.2009.01573.x. [DOI] [Google Scholar]
- Gómez-Aparicio L, Zamora R, Gómez JM, Hódar JA, Castro J, Baraza E. Applying plant facilitation to forest restoration: a meta-analysis of the use of shrubs as nurse plants. Ecol Appl. 2004;14:1128–1138. doi: 10.1890/03-5084. [DOI] [Google Scholar]
- Gómez-Aparicio L, Gómez JM, Zamora R, Boettinger JL. Canopy vs. soil effects of shrubs facilitating tree seedlings in Mediterranean montane ecosystems. J Veg Sci. 2005;16:191–198. doi: 10.1111/j.1654-1103.2005.tb02355.x. [DOI] [Google Scholar]
- Grime JP (1979) Plant strategies and vegetation processes. Wiley, New York
- Gurevitch J, Hedges LV. Statistical issues in ecological meta-analysis. Ecology. 1999;80:1142–1149. doi: 10.1890/0012-9658(1999)080[1142:SIIEMA]2.0.CO;2. [DOI] [Google Scholar]
- Hedges LV, Gurevitch J, Curtis P. The meta-analysis using response ratios in experimental ecology. Ecology. 1999;80:1150–1156. doi: 10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2. [DOI] [Google Scholar]
- Holmgren M. Combined effects of shade and drought on tulip poplar seedlings: trade-off in tolerance or facilitation? Oikos. 2000;90:67–78. doi: 10.1034/j.1600-0706.2000.900107.x. [DOI] [Google Scholar]
- Holmgren M, Scheffer M. Strong facilitation in mild environments: the stress gradient hypothesis revisited. J Ecol. 2010;98:1269–1275. doi: 10.1111/j.1365-2745.2010.01709.x. [DOI] [Google Scholar]
- Holmgren M, Scheffer M, Huston MA. The interplay of facilitation and competition in plant communities. Ecology. 1997;78:1966–1975. doi: 10.1890/0012-9658(1997)078[1966:TIOFAC]2.0.CO;2. [DOI] [Google Scholar]
- Huston MA. Biological diversity: the coexistence of species on changing landscapes. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Hyatt LA, Rosenberg MS, Howard TG, Bole G, Fang W, Anastasia J, Brown K, Grella R, Hinman K, Kurdziel J, Gurevitch J. The distance prediction of the Janzen–Connell hypothesis: a meta-analysis. Oikos. 2003;103:590–602. doi: 10.1034/j.1600-0706.2003.12235.x. [DOI] [Google Scholar]
- Joffre R, Rambal S. Soil water improvement by trees in the rangelands of southern Spain. Oecol Plant. 1988;9:405–422. [Google Scholar]
- Joffre R, Rambal S. How tree cover influences the water balance of Mediterranean rangelands. Ecology. 1993;74:570–582. doi: 10.2307/1939317. [DOI] [Google Scholar]
- Jones RB. TechDig, Version 2.0d. Illinois: Mundelein; 1998. [Google Scholar]
- Karst J, Marczak L, Jones MD, Turkington R. The mutualism–parasitism continuum in ectomycorrhizas: a quantitative assessment using meta-analysis. Ecology. 2008;89:1032–1042. doi: 10.1890/07-0823.1. [DOI] [PubMed] [Google Scholar]
- Kitzberger T, Steinaker DF, Veblen TT. Effects of climatic variability on facilitation of tree establishment in northern Patagonia. Ecology. 2000;81:1914–1924. doi: 10.1890/0012-9658(2000)081[1914:EOCVOF]2.0.CO;2. [DOI] [Google Scholar]
- Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functional groups. Springer, Berlin
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits revisiting the Holy Grail. Funct Ecol. 2002;16:545–556. doi: 10.1046/j.1365-2435.2002.00664.x. [DOI] [Google Scholar]
- Maestre FT, Cortina J. Do positive interactions increase with abiotic stress? A test from a semi-arid steppe. Proc R Soc Lond B. 2004;271:S331–S333. doi: 10.1098/rsbl.2004.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Valladares F, Reynolds JF. Is the change of plant–plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J Ecol. 2005;93:748–757. doi: 10.1111/j.1365-2745.2005.01017.x. [DOI] [Google Scholar]
- Maestre FT, Callaway RM, Valladares F, Lortie CJ. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J Ecol. 2009;97:199–205. doi: 10.1111/j.1365-2745.2008.01476.x. [DOI] [Google Scholar]
- Markesteijn L, Poorter L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J Ecol. 2009;97:311–325. doi: 10.1111/j.1365-2745.2008.01466.x. [DOI] [Google Scholar]
- Markesteijn L, Poorter L, Bongers F, Paz H, Sack L. Hydraulics and life history of tropical dry forest tree species: coordination of species’s drought and shade tolerance. New Phytol. 2011;191:480–495. doi: 10.1111/j.1469-8137.2011.03708.x. [DOI] [PubMed] [Google Scholar]
- Michalet R, Broker RW, Cavieres LA, Kikvidze Z, Lortie CJ, Pugnaire FI, Valiente-Banuet A, Callaway RM. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol Lett. 2006;9:767–773. doi: 10.1111/j.1461-0248.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- Mooney HA, Dunn EL. Photosynthetic systems of Mediterranean-climate shrubs and trees of California and Chile. Am Nat. 1970;104:447–453. doi: 10.1086/282679. [DOI] [Google Scholar]
- Niinemets U, Valladares F. Tolerance to shade, drought and waterlogging of temperate, northern hemisphere trees and shrubs. Ecol Monogr. 2006;76:521–547. doi: 10.1890/0012-9615(2006)076[0521:TTSDAW]2.0.CO;2. [DOI] [Google Scholar]
- Pressland AJ. Rainfall partitioning by arid woodland (Acacia anerua F. Muell.) in south–western Queensland. Aust J Bot. 1973;21:235–245. doi: 10.1071/BT9730235. [DOI] [Google Scholar]
- Prider JN, Facelli JM. Interactive effects of drought and shade on three arid zone chenopod shrubs with contrasting distributions in relation to tree canopies. Funct Ecol. 2004;18:67–76. doi: 10.1046/j.0269-8463.2004.00810.x. [DOI] [Google Scholar]
- Quero JL, Villar R, Marañón T, Zamora R. Interactions of drought and shade effects on four Mediterranean Quercus species: physiological and structural leaf responses. New Phytol. 2006;170:819–834. doi: 10.1111/j.1469-8137.2006.01713.x. [DOI] [PubMed] [Google Scholar]
- Quero JL, Villar R, Marañón T, Zamora R, Vega D, Sack L. Relating leaf photosynthetic rate to whole-plant growth: drought and shade effects on seedlings of four Quercus species. Funct Plant Biol. 2008;35:725–737. doi: 10.1071/FP08149. [DOI] [PubMed] [Google Scholar]
- Robson TM, Rodríguez-Calcerrada J, Sánchez-Gómez D, Aranda I. Summer drought impedes beech seedling performance more in a sub-Mediterranean forest understory than in small gaps. Tree Physiol. 2009;29:249–259. doi: 10.1093/treephys/tpn023. [DOI] [PubMed] [Google Scholar]
- Rosenberg MS, Adams DC, Gurevitch J. Metawin: statistical software for meta-analysis, Version 2. Sunderland: Sinauer; 2000. [Google Scholar]
- Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol Bull. 1979;86:638–641. doi: 10.1037/0033-2909.86.3.638. [DOI] [Google Scholar]
- Sack L. Responses of temperate woody seedlings to shade and drought: do trade-offs limit potential niche differentiation? Oikos. 2004;107:110–127. doi: 10.1111/j.0030-1299.2004.13184.x. [DOI] [Google Scholar]
- Sack L, Grubb PJ. The combined impacts of deep shade and drought on the growth and biomass allocation of shade-tolerant woody seedlings. Oecologia. 2002;131:175–185. doi: 10.1007/s00442-002-0873-0. [DOI] [PubMed] [Google Scholar]
- Smith T, Huston M. A theory of the spatial and temporal dynamics of plant communities. Vegetatio. 1989;83:49–69. doi: 10.1007/BF00031680. [DOI] [Google Scholar]
- Tielbörger K, Kadmon R. Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology. 2000;81:1544–1553. [Google Scholar]
- Tracol Y, Gutiérrez JR, Squeo FA. Plant area index and microclimate underneath shrub species from a Chilean semiarid community. J Arid Environ. 2011;75:1–6. doi: 10.1016/j.jaridenv.2010.08.002. [DOI] [Google Scholar]
- Turner RM, Alcorn SM, Olin G, Booth JA. Influence of shade, soil and water of saguaro seedling establishment. Bot Gaz. 1966;127:95–102. doi: 10.1086/336348. [DOI] [Google Scholar]
- Valladares F, Niinemets Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst. 2008;39:237–257. doi: 10.1146/annurev.ecolsys.39.110707.173506. [DOI] [Google Scholar]
- Valladares F, Pearcy RW. Drought can be more critical in the shade than in the sun: a field study of carbon gain and photoinhibition in a Californian shrub during a dry El Niño year. Plant Cell Environ. 2002;25:749–759. doi: 10.1046/j.1365-3040.2002.00856.x. [DOI] [Google Scholar]
- Valladares F, Dobarro I, Sánchez-Gómez D, Pearcy RW. Photoinhibition and drought in Mediterranean woody saplings: scaling effects and interactions in sun and shade phenotypes. J Exp Bot. 2005;56:483–494. doi: 10.1093/jxb/eri037. [DOI] [PubMed] [Google Scholar]
- Valladares F, Zaragoza-Castells J, Sánchez-Gómez D, Matesanz S, Alonso B, Portsmuth A, Delgado A, Atkin OK. Is shade beneficial for Mediterranean shrubs experiencing periods of extreme drought and late-winter frosts? Ann Bot Lond. 2008;102:923–933. doi: 10.1093/aob/mcn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenendaal EM, Swaine MD, Agyeman VK, Blay D, Abebrese IK, Mullins CE. Differences in plant and soil water relations in and around a forest gap in West Africa during the dry season may influence seedling establishment and survival. J Ecol. 1996;84:83–90. doi: 10.2307/2261702. [DOI] [Google Scholar]
- Werner C, Correia O, Beyschlags W. Two different strategies of Mediterranean macchia plants to avoid photoinhibitory damage by excessive radiation levels during summer drought. Acta Oecol. 1999;20:15–23. doi: 10.1016/S1146-609X(99)80011-3. [DOI] [Google Scholar]
- Zou CB, Barnes PW, Archer S, McMurtry CR. Soil moisture redistribution as a mechanism of facilitation in savanna tree–shrub clusters. Oecologia. 2005;145:32–40. doi: 10.1007/s00442-005-0110-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.