Summary
Initiation of the development of the anterior-posterior axis in the mouse embryo has been thought to take place only when the anterior visceral endoderm (AVE) emerges and starts its asymmetric migration. However, expression of Lefty1, a marker of the AVE, was recently found to initiate before embryo implantation. This finding has raised two important questions: are the cells that show such early, pre-implantation expression of this AVE marker the real precursors of the AVE and, if so, how does this contribute to the establishment of the AVE? Here, we address both of these questions. First, we show that the expression of another AVE marker, Cer1, also commences before implantation and its expression becomes consolidated in the subset of ICM cells that comprise the primitive endoderm. Second, to determine whether the cells showing this early Cer1 expression are true precursors of the AVE, we set up conditions to trace these cells in time-lapse studies from early peri-implantation stages until the AVE emerges and becomes asymmetrically displaced. We found that Cer1-expressing cells are asymmetrically located after implantation and, as the embryo grows, they become dispersed into two or three clusters. The expression of Cer1 in the proximal domain is progressively diminished, while it is reinforced in the distal-lateral domain. Our time-lapse studies demonstrate that this distal-lateral domain is incorporated into the AVE together with cells in which Cer1 expression begins only after implantation. Thus, the AVE is formed from both part of an ancestral population of Cerl-expressing cells and cells that acquire Cer1 expression later. Finally, we demonstrate that when the AVE shifts asymmetrically to establish the anterior pole, this occurs towards the region where the earlier postimplantation expression of Cer1 was strongest. Together, these results suggest that the orientation of the anterior-posterior axis is already anticipated before AVE migration.
Introduction
In the great majority of animal species, polarity of the embryo originates from the very beginning of embryonic development, due to the inheritance of maternal information that is either asymmetrically localised within the egg or becomes asymmetrically re-organised following fertilization (Gurdon, 1992; St Johnston and Nusslein-Volhard, 1992). Generation of this polarity often leads to the establishment of the major future body axis, the anterior-posterior axis (AP). However, the earliest stage at which the mammalian embryo develops polarity that will lead to the establishment of the AP axis is still unclear. Although a number of asymmetries have been documented both before and shortly after implantation, the link between these asymmetries and the development of the AP axis is not established (Zernicka-Goetz, 2005).
Until very recently, the establishment of the AP axis in the mouse embryo has been thought to be initiated only after embryo implantation. This is because at embryonic day 5.5 (E5.5), a distinct group of visceral endoderm cells, the AVE, forms at the distal tip of the egg cylinder. The subsequent unilateral movement of these cells towards the proximal region of the embryo establishes the future anterior pole (Srinivas et al., 2004; Thomas et al., 1998). This is in part due to the expression of a number of genes, such as Cer1 and Lefty1 within the AVE, that act as Nodal antagonists (Perea-Gomez et al., 2002; Yamamoto et al., 2004). Both the expression of genes specific to the AVE and its migration are regulated by signals derived from the extraembryonic ectoderm (Richardson et al., 2006; Rodriguez et al., 2005) that include BMP4 (Soares et al., 2005). Signalling from the epiblast, such as Nodal, is also essential for AVE formation as in the absence of Nodal, the AVE does not form ((Brennan et al., 2001), Robertson et al, 2003). Together these results demonstrate that asymmetries and signalling pathways established around E5.5 are essential events in the development of the AP axis.
Although the AVE appears only to be induced following implantation, the recent report that one of the AVE markers, Lefty1, is already expressed in a group of ICM cells in the implanting blastocyst has led to the suggestion that the establishment of the AP axis starts earlier than previously thought (Takaoka et al., 2006). Indeed, the expression of Hex, another gene expressed within the AVE, is also evident at the blastocyst stage (Thomas et al, 1998,). However, for this to be the case, cells that initiate such early expression of AVE markers have to be shown as the true progenitors of the AVE.
To address this question, we examined the spatial and temporal pattern of expression of another anterior marker, Cer1, and show that its expression is also initiated in a sub-group of ICM cells before embryo implantation. We also find that the initiation of Cer1 expression does not require embryo interaction with the uterus and therefore is independent from it. To determine whether the cells expressing this anterior marker truly contribute to the establishment of the AVE, we have established conditions allowing us to follow Cer1 expressing cells. This has enabled us to examine the dynamics of Cer1 expression from the earliest time point at which periimplantation mouse embryos can be cultured and observed by time-lapse microscopy until the AVE clearly emerges after E5.5. These studies demonstrate for the first time that some early Cer1 expressing cells do contribute to the AVE when it becomes established at E5.5. Thus, we conclude that the origin of the AVE is heterogeneous; it is formed by a subset of the ancestral population of Cer1 expressing cells and visceral endoderm cells which acquire expression of Cer1 only after implantation, particularly around E5.5.
Materials and Methods
Embryo collection
F1 (C57BL6 x CBA) and Cer1/GFP mice (Mesnard et al., 2004) were bred using a 7.00 to 19.00 light cycle. Embryos from F1xF1 and F1xCer1/GFP crosses were collected from natural matings. Periimplantation embryos were dissected in either M2 medium (for in situ hybridisation and immunostaining experiments) or in Dulbecco’s modified eagle medium without phenol red, (DMEM), containing sodium pyruvate and non-essential amino acids, supplemented with 10% Fetal Calf Serum (FCS) at 37°C (for 8.00 on the third and fourth day after plugging, respectively. All the other stages (E4.5-E6.25) were collected by using fine forceps to extract deciduae from the uterus and to remove the embryos from the uterine crypt. In some experiments (see below), the parietal endoderm was removed with fine syringe needles. E4.5, E4.75 E5.0 and E5.25 embryos were collected at 12.30, 19.00, 00.00 or 8.00 on the fourth or fifth day after plugging accordingly. All the embryos were analysed or fixed immediately after collection.
In situ hybridisation
Freshly collected embryos were fixed in 4% paraformaldehyde in PBS. In situ hybridisations were carried out as described (Wilkinson et al., 1990, Soares et al, 2005) except that the proteinase K treatment was omitted. ISH probes were labelled with digoxygenin-UTP.
Blastocyst dissociation and RT-PCR
E4.75 blastocysts were collected with fine forceps to extract deciduae from the uterus and to remove the embryos from the uterine crypt in M2 medium, washed in Ca2+ and Mg2+ free M2 and treated with 2.5% pancreatin, 0.5% trypsin/EDTA in Ca2+/Mg2+ free PBS at RT for 30-60 minutes. Blastocysts were rinsed in Ca2+/Mg2+ free M2 and transferred to a coverslip with Ca2+/Mg2+ free medium covered in mineral oil. A glass capillary pulled to a fine tip was used to mouth pipette embryos up and down into to single cells. UV light was used to separate GFP positive and negative cells which were washed in 0.25% polyvinylpyrrolidone (PVP) and then stored immediately at −80°C. RNA was extracted (Arctus Biosciences) according to manufacturers instructions and the following RT-PCR cycles were performed: Cer1, 58° annealing, 30 seconds; GFP, 58° annealing, 30 seconds; Lefty1, 55° annealing, 30 seconds; Hex, 58° annealing, 30 seconds; histone H2A, 59° annealing, 30 seconds. Respective forward and reverse primer sequences were as follows: Cer1: 5′-AGGAGGAAGCCAAGAGGTTC and 5′-CATTTGCCAAAGCAAAGGTT; GFP: 5′-CTGCTGCCCGACAACCA and 5′-CCATGTGATCGCGCTTCTC; Lefty1: 5′-CAATCCCTGTGTGTGCTCTTTG and 5′-AGTCACATTCCTCGAAGGTAAAAATT; Hex: 5′-GGTCAAGTGAGGTTCTCCAA and 5′-TCCTTTTTGTTGCTTTGAGG; H2A: 5′-GTCGTGGCAAGCAAGGAG and 5′-GATCTCGGCCGTTAGGTACTC.
Immunostaining
Embryos were fixed after removal of the parietal endoderm in 4% paraformaldehyde in PBS overnight at 4°C. It was essential to remove and/or puncture the parietal endoderm to prevent trapping of the secondary antibodies. Embryos were washed three times in PBS-T (0.1% Tween in PBS), dehydrated in 25%, 50%, 75% and 100% Methanol PBS-T and stored at -20°C overnight. After rehydration through methanol series, embryos were washed three times in PBS-T, blocked in 3% BSA, 10% DMSO in PBS-T and incubated with the primary antibodies overnight at 4°C (Cer1 antibody; R&D systems: MAB1986, dilution 1: 200; vHNF1/HNF1ß, provided by M.Pontoglio, (Gresh et al., 2004), dilution 1:200 and Lefty1 antibody, Abcam, dilution 1:200). Embryos were then washed, incubated overnight with the corresponding secondary antibodies, washed and stained with TOTO-3 (Molecular Probes). Confocal microscopy was performed using a 40X oil objective in a BioRad 1024 inverted Confocal Laser Microscope using the BioRad LaserSharp 2000 software. Whole embryos were analysed through Z-series sections (1.5 μm thickness) unless otherwise indicated, only single sections are shown in the figures as it is easier to appreciate the cellular details on these.
Imaging, culture and time-lapse analysis
Micrographs were taken using an inverted Nikon microscope and processed using IPLab software. For the culture of blastocysts, E3.5 blastocysts from F1xCer1-GFP crosses were flushed from the uterus in M2 medium. The zona pellucida was removed by brief exposure to Tyrod acid. Embryos were cultured in pre-equilibrated DMEM supplemented with 20% human cord serum (HCS) in a glass-bottom dish (MatTek). After 23 hours in the 37.5°C and in 5% CO2 incubator, embryos were observed on an inverted epifluorescent Zeiss Axiovert 200M microscope with a 20X objective, at 37.5°C and in 5% CO2. Multi-channels (green-fluorescence/transmission) multi-sections images were acquired every 30 minutes with a Hamamatsu ORCA ER CCD camera controlled by the AQM Advance 6 software (Kinetic imaging). 7 focal planes were acquired every 10.5 μm, with an exposure of 4 ms for transmitted light and 500 ms for green fluorescence, using a 50% transmission Chroma Neutral Density Filter. For time-lapse imaging of implanted blastocysts, E4.75 embryos were cultured in pre-equilibrated DMEM medium supplemented with 40% HCS and non-essential amino acids after removal of the mural trophectoderm. Time-lapse was recorded at 37.5°C in a 5% CO2 atmosphere using a 20X objective of an inverted Zeiss Axiovert microscope and the Kinetic Imaging software as above. Images were captured every 20 minutes in six different Z planes (covering 70-75 μm). Filming was started at 19:00 and stopped approximately after 12-14 hours of culture. Analysis of the movies was performed for individual embryos in all sections and Z-planes using the Volocity software (Improvision). The results we present here are derived from 5 movies and a total of 14 embryos analysed.
Since the rate of success of correct continuous development from E4.75 to E5.75 was low, we carried out three series of time-lapse observations: from E4.75 to E5.25, from E5.0 to E5.5 and from E5.25 to E5.75. The same parameters as above were followed in the time-lapse studies carried between E5.25 and E5.5, except that the embryos were collected at 6.30 on the 5th day after plugging and were imaged under a 10X objective for approximately 10 hours. Here, we show data from 11 embryos (3 movies). For embryos filmed from E5.0, we used the same parameters as for the embryos cultured from E5.25 except that the embryos were collected at 00.00 on the 5th day after plugging and allowed the embryos to develop for approximately 15 hours until the asymmetric shift of the AVE was observed. The data presented here is from 7 movies and a total of 9 embryos analysed. In all movies, the embryos that went out of field during the time-lapse were not taken into account for the analysis, nor were embryos included which appeared developmentally delayed.
To analyse the distribution of Cerl-GFP positive cells, regions of the embryo were defined as one of the following: Proximal – to include cells located at, above or below the extraembryonic/embryonic boundary; Lateral – cells located more distally in the embryonic half of the embryo; or Distal – the distal-most VE cell along the PD axis of the embryo, and the adjacent 2 cells; hence in our analysis, cells that are referred to as distal will comprise up to 3 cells. The boundaries were set by measuring embryos individually with respect to these boundaries.
Our culture conditions allowed correct embryonic growth during the 3 distinct time periods as each gave a reliable representation of in vivo growth and development between E4.75 and E5.25 (Supplementary figure 1). Moreover, formation of the AVE occurred normally (Supplementary Figure 2).
Results
Cer1 expression is initiated in the pre-implantation blastocyst and becomes asymmetric at early post-implantation stages
Cer1 expression is known to be already asymmetric by E5.5, before the AVE initiates its migration (Richardson et al., 2006; Yamamoto et al., 2004). To understand when and how this early asymmetry develops, we have examined Cer1 expression at successive stages, from the late blastocyst stage through early implantation, in three different ways. First, we used a transgenic line in which GFP is driven by the Cer1 promoter, that we have previously shown to faithfully reflect Cer1 expression (Mesnard et al., 2004). Secondly, we examined Cer1 expression by in situ hybridization to detect Cer1 mRNA. Thirdly, we used antibody staining to detect CER1 protein.
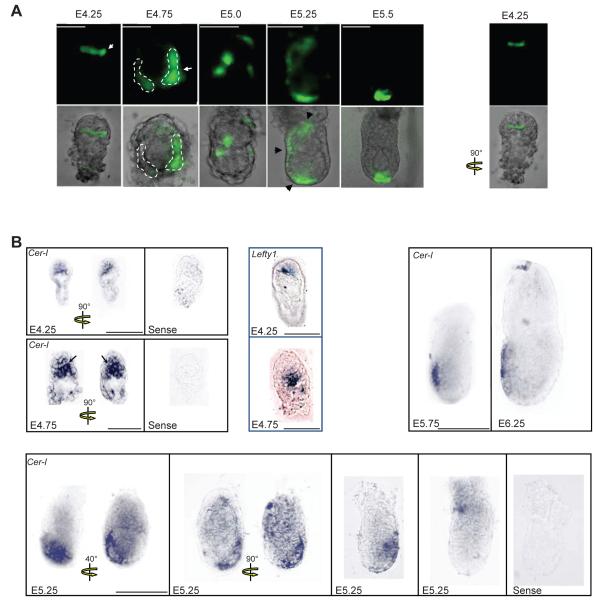
We found that the earliest time point in which we could detect Cer1 expression is in the expanded blastocysts, at E4.25, just before embryo implantation. In all embryos examined (n=14) expression of Cer1/GFP at this stage was evident in the primitive endoderm (PE). Interestingly, not all PE cells expressed Cer1/GFP at this stage and although there was no obvious spatial restriction of the Cer1/GFP to any specific region of the PE, the level of GFP fluorescence was clearly higher on one side of the bilaterally symmetrical embryo (Fig. 1A, E4.25, arrow, compare the two different optical views of the E4.25 embryo shown). At E4.75, in most embryos examined (64%, n=14), Cer1/GFP was evident on the two opposite sides of the PE, displaying a gradient of fluorescence intensity (Fig. 1A, E4.75, arrow). We confirmed this expression pattern of Cer1 by in situ hybridisation (n=9) (Fig. 1B, E4.75, black arrows). Since Lefty1, another marker of future AVE, has also been recently shown to be expressed at these early stages (Takaoka et al., 2006), we next examined whether we could detect a similar expression pattern for Lefty1 as that for Cer1. Although it is difficult to estimate the number of cells that express Cer1 or Lefty1 by in situ hybridisation it appeared that, at both E4.25 and E4.75, expression of Lefty1 was confined to a smaller region of the PE than that of Cer1 (Fig. 1B).
Figure 1. Spatial and temporal pattern of Cer1 expression in periimplantation mouse embryos.
A. Embryos derived from a transgenic line where GFP is driven by the Cer1 promoter were collected at the indicated stages and imaged under an inverted fluorescent microscope. Expression of Cer1 is detected in the primitive endoderm from E4.25. Note that between E4.25 and E4.75 one side of the bilaterally symmetrical embryo (white arrows) displays higher intensity of GFP fluorescence. Scale bar 50 μm.
In E5.0 and E5.25 embryos, Cer1/GFP expression was restricted to the visceral endoderm. Note in E5.25 embryos, GFP accumulation in a distal-lateral region, laterally around the boundary towards the extraembryonic region and in the proximal most region around the ectoplacental cone. Fluorescent (top) and merge (bottom) micrographies are shown. Shown are representative embryos of at least 10 embryos analysed per stage.
B. Endogenous expression of Cer1 recapitulates the pattern of Cer1/GFP expression. Embryos from wild-type crosses were collected at the indicated stages, fixed and processed for in situ hybridisation for Cer1. At E4.25, mRNA for Cer1 is found in the cells lining the blastocoeilic cavity. Later at E4.75, Cer1 is expressed in the primitive endoderm. Note that the staining tends to be stronger on one side of the primitive endoderm (black arrows). At E5.25 expression of Cer1 is variable within different regions of the visceral endoderm. Embryos incubated with the sense probe were stained for the same time as those with the antisense probe and both groups were treated in parallel under the same conditions. As control, we performed in parallel in situ hybridisation for embryos of later stages in all experiments, this shows that Cer1 expression is restricted to the AVE at E5.75 and E6.25. As indicated, embryos at E4.25 (n=5) and at E4.75 (n=6) were processed for in situ hybridization for Lefty1. Scale bar is 100 μm.
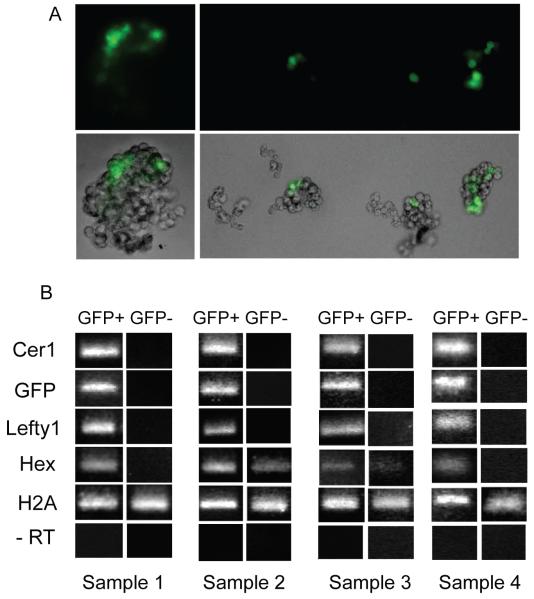
We further investigated whether the AVE markers Cer1, Lefty1 and Hex are co-expressed within the same population of cells. We recovered Cer1/GFP embryos at E4.75, a stage at which Lefty1 and Hex are already expressed (Takaoka et al., 2006; Chazaud and Rossant, 2006; Thomas et al, 1998) and dissociated them into single cells. Cells from individual embryos were classified as GFP-positive or GFP-negative cells upon brief exposure to UV fluorescence (Fig. 2B), pooled (~8 cells/sample) and processed for RT-PCR for Cer1, GFP, Lefty1 and Hex. RT-PCR analysis on both GFP positive and negative samples collected from the same embryo were run in parallel. Cer1 and GFP were both co-expressed, and were present only in cells which had been identified as GFP-positive under the fluorescence microscope, further confirming that the GFP transgene recapitulates endogenous Cer1 expression (Fig. 2). We detected Lefty1 transcripts in samples which were positive for both Cer1 and GFP, but samples which were negative for Cer1 and GFP were also negative for Lefty1 (Fig. 2B). This suggests that in late blastocysts, cells which express Cer1 also express Lefty1. In contrast, Hex transcripts were detected in cells which did not express Cer1 or GFP in half of the embryos (2/4), whereas in the other half, Hex transcripts were also present in samples which were Cer1 and GFP-positive (Fig. 2B). This analysis suggests that Cer1 and Lefty1 are co-expressed within some cells of the E4.75 stage blastocyst, but that Hex displays a broader expression pattern.
Figure 2. Lefty1 and Cer1 are co-expressed in the late blastocyst, whereas Hex has a broader expression pattern.
A. E4.75 Cer1/GFP transgenic embryos were dissociated into single cells and exposure to UV fluorescence was used to identify GFP positive (GFP+) and GFP negative cells (GFP-). Approximately 8 GFP+ and 8 GFP-cells were pooled and processed for RT-PCR per sample per embryo.
B. RT-PCR analysis of GFP+ ad GFP-cells from E4.75 dissociated blastocysts. Each sample represents cells dissociated from a single embryo. In all samples, Cer1 and GFP are co-expressed in the same cells, indicating that the transgene faithfully recapitulates endogenous Cer1 expression. Only the cells which express Cer1 also express Lefty1. In contrast, in half of the embryos, Hex is co-expressed in both Cer1 positive and negative cell samples, (Samples 2 and 3), whereas in the remaining, Hex is only expressed in cells positive for Cer1 (Samples 1 and 4).
Cells were processed for H2A and –RT for positive and negative controls, respectively.
At E5.0, we detected Cer1/GFP expression in opposite sides of the bilaterally symmetrical embryo in half of the egg cylinders examined (55%, n=11) (Supplementary Figure 2) and only on one side in the remaining half (Fig.1A). At E5.25, the position of Cer1/GFP expressing cells was variable. Cer1/GFP expression could be found in three different regions of the embryo: distal-lateral, lateral around the boundary towards the extraembryonic region and in the proximal most region around the ectoplacental cone (Fig. 1A, black arrowheads). A few embryos (16%) displayed only a distal patch within 1/3 of the distal most part of the egg cylinder. These three sites of expression could be found on either the same (47%, n=17) or opposite sides (53%) of the embryo, but when expression was observed on two opposite sides of the embryo, the fluorescence intensity was always stronger on one side than the other. The expression pattern of Cer1 at this stage was also confirmed by in situ hybridisation. After in situ hybridisation for Cer1, 5/17 embryos displayed expression in the distal-lateral and lateral-boundary regions, 4/17 in the distal-lateral and proximal regions and 7/17 displayed expression in all 3 regions (distal-lateral, lateral-boundary and proximal most). Only 1 embryo (1/17) displayed exclusively proximal expression with no expression in the lateral or distal VE. (Fig.1B).
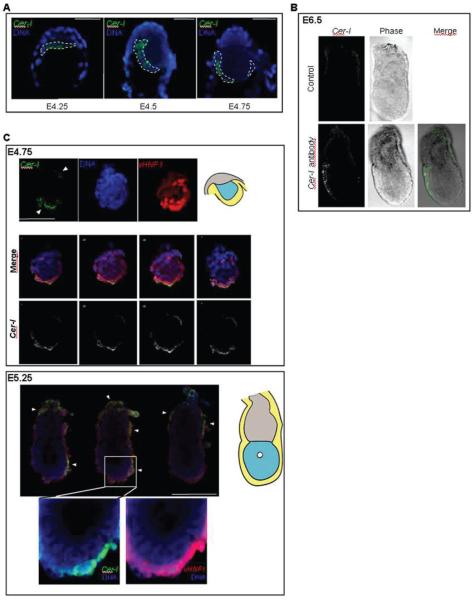
To gain insight into the distribution of CER1 protein at a single cell resolution level at these early stages, we carried out immunostaining from E4.25 until E5.25. This showed that the CER1 protein distributed along the PE at E4.25, with higher levels of fluorescence towards one side of the embryo. This tendency for the CER1 protein to accumulate with higher levels towards one side of the embryo was more evident at E4.5 and at E4.75 (Fig. 3A). Since a ‘tilt’ in the blastocyst at E4.25 has been suggested to be predictive of the orientation of the future AP axis (Smith, 1980), we analysed CER1 accumulation with respects to this tilt. The tilt forms in the region where the PE folds in the junction with the trophectoderm and we observed such folding in 7 out of 8 E4.25 embryos. Interestingly, in all of the cases CER1 accumulated towards the tilt (Fig. 3A). We confirmed the specificity of our CER1 antibody by carrying out immunostaining in E6.5 embryos and show that this antibody clearly marks the AVE as seen from the specific localisation of the VE cells that show fluorescence signal (Fig. 3, E6.5). To confirm that the cells expressing Cer1 at E4.75 and E5.25 corresponded to PE and VE respectively, we carried out immunostaining for CER1 together with vHNF1/HNF1ß, a PE marker (Barbacci et al., 1999; Coffinier et al., 1999). We found that the cells in which we detected CER1 were also labelled with vHNF1, confirming that CER1 positive cells were located within the PE and VE (Fig. 3C). Similar to the Cer1/GFP expression pattern, CER1 localisation in vHNF1 positive cells at E5.25 was present in three clearly localised patches: a distal-lateral patch, a lateral patch at the boundary level and in the proximal most region of the embryo (Fig. 3C, white arrowheads). These data indicate that the Cer1/GFP transgene mimics endogenous Cer1 expression at all stages analysed. We further confirmed this by performing double immunostaining using the CER1 antibody and a GFP antibody (Fig. 4). These experiments show that CER1 protein is progressively restricted to a subpopulation of the PE from E4.5.
Figure 3. Distribution of CER1 protein in periimplantation and early post-implantation mouse embryos.
A. Embryos were collected at E4.25, E4.5 or E4.75, fixed, and processed for immunostaining with the Cer1 antibody (green) and TOTO-3 (DNA, blue). Embryos were analysed under confocal microscopy. Cer1 localises to the cytoplasm of primitive endoderm cells and the protein is clearly enriched towards one side of the embryo (Left on the panel). Note that the polar trophectoderm and the parietal endoderm become very ‘sticky’ as seen with the accumulation of the TOTO-3 dye. Scale bar 50 μm.
B. The Cer1 antibody specifically stains the AVE of E6.5 embryos. Embryos at E6.5 were collected and processed as in A for immunostaining for Cer1. In the control panel, embryos were processed with the secondary antibody only. Shown are projections of z-series acquisitions. Embryos oriented with their anterior pole to the left.
C. Double immunostaining of E4.75 (top) and E5.25 (bottom) embryos with Cer1 and vHNF1 antibodies. Embryos were processed as in A. For both stages, a diagrammatic representation is shown at the right side of the merge panels. Primitive endoderm / visceral endoderm is shown in yellow, epiblast in blue and polar trophectoderm/extraembryonic ectoderm in grey.
For E4.75 embryos, stack projections of z-series images of single channel acquisitions for CER1 (green), DNA (blue) and vHNF1 (red) are shown in the top panel. Merge images of representative single optical sections of this same embryo and the corresponding greyscale image of the green channel (Cer1) are also shown. White arrowheads point to the regions of accumulation of CER1 in the primitive endoderm, where one side with bigger accumulation can be distinguished.
For E5.25 embryos, representative single optical merge images of the same embryo are shown at the top. Cer1 is shown in green, DNA in blue and vHNF1 in red. Sites of CER1 accumulation are indicated with white arrowheads. The region within the white square in the middle image is shown under a higher magnification in the bottom of the panel. CER1 is expressed in a subpopulation of visceral endoderm cells. Scale bar is 100 μm.
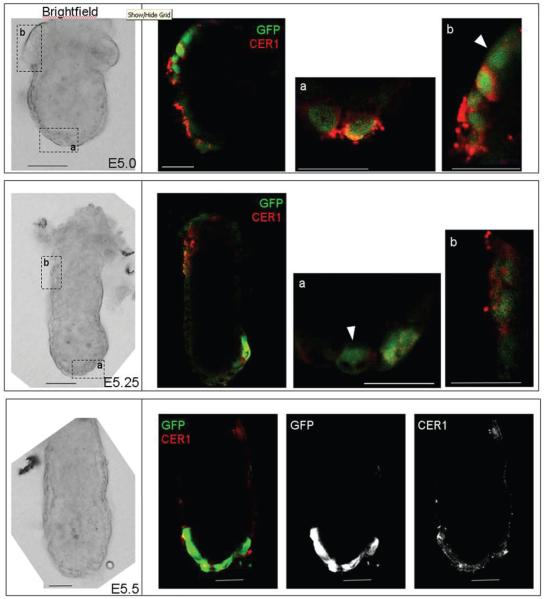
Figure 4. Double immunostaining for GFP and CER1 at early postimplantation stages.
Embryos were processed in parallel for immunostaining with an anti-GFP antibody (Green) and an anti-CER1 (red) antibody and analysed under an inverted confocal microscope. Individual optical sections for brightfield and fluorescence scanning are shown. Higher magnifications of the regions depicted by squares on the brightfield image are shown at the right (a and b). In the bottom panel greyscale images of the green (GFP) and red (CER1) channels are shown. Approximate stages are indicated, note the distinctive morphological features for these: at the earliest stage analysed the extraembryonic ectoderm has not yet expanded (top); in the E5.25 embryo the proamniotic cavity has not formed yet (middle), in contrast to the E5.5 embryo where AVE cells have started their migration (bottom). Note that the GFP localises to both nucleus and cytoplasmic whereas CER1 is exclusively cytoplasmic. All cells positive for GFP were also positive for CER1, however, some cells that were positive for GFP displayed very low levels of CER1 accumulation (arrowheads). We quantified the number of cells which exhibit these discrepancies and found that they were minimal (less than 1% of the cells showed either CER1 or GFP staining only throughout all the stages/embryos analysed) and therefore this would not have a major impact in our analyses. Scale bar is 30 μm. Note that the whole mount images are of the middle plane of the embryo, however, in the magnified images, the plane with the best resolution is presented.
Thus, three lines of evidence indicate that expression of Cer1 is initiated before implantation and, as embryo develops further, its expression is not uniform, with a group of cells containing higher levels of CER1 on one side of the embryo.
Expression of Cer1 is independent of interactions with the uterus
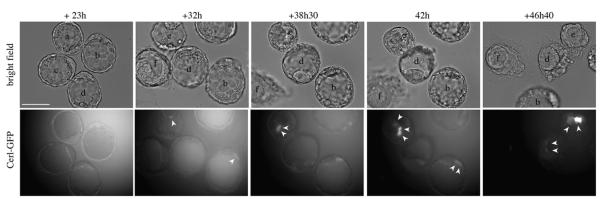
To determine whether the onset of expression of Cer1 in the blastocyst is independent of the interactions between the embryo and the uterus, we carried out time-lapse studies of early blastocysts collected at E3.5. Immediately on recovery, we did not observe any Cer1/GFP positive cells. These embryos were then cultured in vitro for approximately 23 hours and their development followed using time-lapse microscopy for a further 24 hours. All cultured blastocysts developed an expanded cavity and PE, which was seen as a distinct layer of cells lining the ICM (n=6, Fig.5). Cer1/GFP expression was detected after approximately 32 hours of culture. At this time, most embryos (5/6) had either 2 or 1 Cer1/GFP cells (that underwent division within an hour of acquiring Cer1/GFP expression). Interestingly, these cells were located towards one half of the PE. Of these 5 embryos, 4 remained with 2 Cer1/GFP positive cells at the end of the culture and in the fifth embryo, 5 cells acquired Cer1/GFP expression. The remaining embryo had 6 positive cells scattered on one half of the embryo at the beginning of the culture and one cell which acquired Cer1/GFP expression during culture. Similar results were observed when we recovered embryos at morula stage and cultured them in vitro as above (not shown).
Figure 5. Expression of Cer1 is independent of interactions with the uterus as it arises in the primitive endoderm after in vitro culture of blastocysts.
E3.5 blastocysts from F1XCer1/GFP crosses were flushed from the uterus and cultured under the microscope. Cer1/GFP expression is first detected after 32h of culture (white arrows). It is observed in the superficial ICM cells throughout the movie. The fluorescent images are only one of six z-planes, hence not all positive cells within an embryo are shown. Note also that the levels of expression, as seen from fluorescence intensity, can vary in different cells within the same and/or different embryos. Scale bar 50 μm.
Thus, similar to the findings of Lefty1 expression (Takaoka et al., 2006), the onset of expression of Cer1 is independent of embryo interactions with the uterus.
The population of Cerl positive cells at E5.25 derives largely from Cer1 expressing cells at E4.75
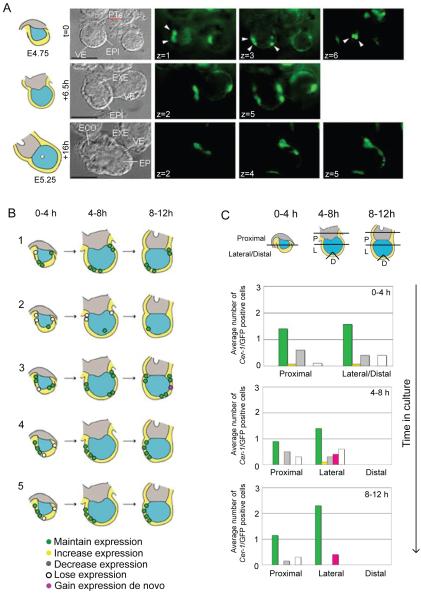
To determine whether Cer1 expressing cells at E4.75 do contribute to the Cer1 expressing domain at later stages, we started by following the behaviour of Cer1/GFP cells from E4.75 until E5.25 using time-lapse microscopy. Representative time points of the culture are shown in Figure 5A. For each embryo, we tracked the Cer1/GFP positive cells on 6 different optical planes at intervals of 20 minutes (Fig. 6A). The use of different optical planes was essential to identify all Cer1/GFP expressing cells. This also enabled us to exclude the possibility that expressing cells could be masked by/masking other positive cells. Therefore, in our images of single z-planes, not all of the positive cells are evident. This approach allowed us to follow individual Cer1/GFP cells from their original to their final location and also to monitor changes in their behaviour. The results of this analysis upon 5 of the 14 embryos examined that represent the different behaviours of Cer1-expressing cells are shown schematically in Figure 6B.
Figure 6. The population of Cer1 expressing cells at E5.25 is constituted mainly by cells that express Cer1 at E4.75.
A. Representative time lapse imaging of an E4.75 embryo cultured until approximately E5.25.
Embryos were recovered at E4.75 and cultured for 16 h under time-lapse microscopy. Images were collected at 6 different Z-planes along approximately 70 to 75 μm covering the diameter of the embryo at each time point and images were collected every 20 minutes. Images captured at time zero, after 6.5 and 16 (endpoint) h of culture are shown. A diagram showing the stage and orientation of the embryo is shown on the left side of the brightfield image. To illustrate how we identified the Cer1/GFP positive cells, different Z-planes of the green channel are shown (indicated by a z) on the right side of the corresponding brightfield image for each time point. This enabled us to ensure no cells were masked by/masking other positive cells and as such for one Z-plane, not all of the positive cells will be visible. For example, 7 Cer1/GFP positive cells can be identified through the Z-planes at E4.75 (depicted by white arrowheads). The different tissues of the embryo are indicated. PTe, polar trophectoderm; VE, visceral endoderm; EPI, epiblast; ECO, ectoplacental cone. Scale bar is 100 μm
B. Schematic examples of 5 of the embryos analysed with time-lapse microscopy. Three different time-points: start, middle and end, corresponding approximately to E4.75, E5.0 and E5.25, respectively are illustrated. The position and destiny of cells (circles) which express Cer1/GFP at E4.75 are indicated with a colour code: in green, the cells that maintain expression of Cer1/GFP (a cell was considered to maintain the expression when either it and/or its progeny were GFP positive at the endpoint); in white, a cell that loses Cer1/GFP expression; and in pink a cell that acquires de novo expression during culture. Note that all embryos have a distal or lateral-distal patch by E5.25.
C. Histograms illustrating both distribution and behaviour of Cer1/GFP expressing cells from E4.75. Positioning of cells was scored according to region within the embryo as shown in the diagram. Time in culture and behaviour of the expressing cells is indicated. Data is indicated as the average number of Cer1/GFP positive cells per embryo (n=14 embryos).
Our time-lapse recordings showed that the absolute number of Cer1/GFP positive cells is variable between individual embryos. But regardless of this variability, 75% of the total number of Cer1/GFP positive cells at E4.75 (n=49 cells in 14 embryos) maintained expression throughout culture until E5.25. Therefore, the original population of Cer1 expressing cells and their progeny contributes significantly to the Cer1 expressing cells at E5.25. In agreement, we observed that 25% of the Cer1/GFP positive cells at E5.25 are derived from cells acquiring Cer1/GFP expression between E4.75 and E5.25. We found that such de novo expression of Cer1/GFP could arise at any stage throughout the culture (Fig. 6B,C).
Our time-lapse studies revealed dynamic changes in the expression of Cer1/GFP at these early postimplantation stages. We observed both positive cells which lost Cer1/GFP expression, (80% of the embryos, 2 cells per embryo on average), and cells that began to express Cer1/GFP (73% of the embryos, between 1 and 3 cells per embryo during the period of culture). Moreover, in most of the embryos (93%) Cer1/GFP positive cells divided between E4.75 and E5.25 (2 cells per embryo on average) thus contributing to the increase in overall number of Cer1/GFP expressing cells.
We also examined whether we could detect any specific tendencies in the behaviour of Cer1/GFP positive cells and if so, whether they occurred after any specific time in culture or any particular region of the embryo. To evaluate this, we distinguished three time-periods of culture, 0-4, 4-8 and 8-12 hours, and analysed behaviour of Cer1/GFP cells according to their position in the embryo (Fig.6C). For the first 4 hours of culture, we analysed whether Cer1/GFP cells were located either at the Proximal or Lateral/Distal region of the embryo. During the remaining time in culture, the region of VE covering the embryonic/extraembryonic boundary became evident as the egg cylinder elongated. Thus, at these later stages we analysed the distribution of Cer1/GFP expressing cells in three regions: Proximal – cells located at, above, or below the boundary between embryonic and extraembryonic tissues; Lateral – cells within the embryonic region of the embryo; Distal – the distal-most cell according to the proximal-distal axis and its two adjacent cells. Therefore, in our analysis the distal region could include a maximum of 3 cells (see Fig.5C and Materials and Methods). To analyse the dynamics of Cer1 expression, we analysed several parameters: maintenance of expression, gain of expression, increase or decrease and/or loss of expression (Fig.6, 9A and Supplementary Information 4).
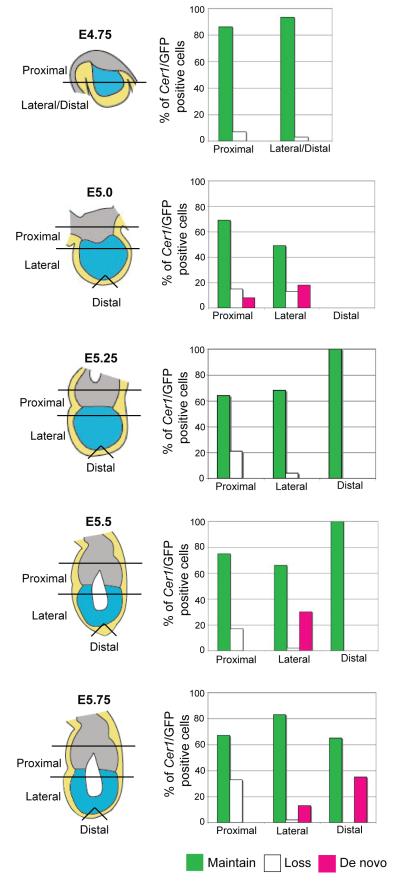
Figure 9. Summary of the contribution of ‘old’ and ‘new’ population of Cer1expressing cells to the AVE from E4.75.
Cer1/GFP positive cells that maintain (includes cells increasing, decreasing, dividing) Cer1/GFP expression are plotted versus the ones that lose Cer1/GFP expression. VE cells acquiring Cer1/GFP expression are also shown. It can be seen that during the formation of the AVE between E5.5 and E5.75, Cer1 expression is induced in the lateral and distal regions of the embryo but that also ‘precursors’ cells (in green) maintain Cer1 expression. Data is expressed as the percentage of the total number of Cer1/GFP positive cells in all embryos per region of the embryo and is derived from data presented in Figures 5,6,7.
During the first 4 hours of culture, both the proximal and lateral/distal regions of the embryo had an equivalent number of Cer1/GFP positive cells (2 cells per embryo on average, range of 0-4 cells per embryo; 30 positive cells/14 embryos). In both regions, most cells maintained their expression (86% proximally, 93% laterally/distally), with slight increases in the levels of expression (7% proximally, 1% laterally). Few cells lost Cer1/GFP expression during this time (less than 7% in either region). During this interval, we did not observe any de novo expression of Cer1/GFP.
In all of the embryos analysed within the next period of culture (4-8 hours), the total number of proximal Cer1/GFP positive cells was lower than during the first period (86% of original cells), but the number of lateral Cer1/GFP cells increased (30% more cells than in the first period). In contrast to the initial time period when no cells acquired Cer1/GFP expression de novo, VE cells in both proximal and lateral regions of the embryo started to express Cer1/GFP (8% and 20% of total Cer1/GFP labelled cells, respectively). There were no Cer1/GFP positive cells within the very distal tip of the embryo.
During the last 4 hours of culture (8-12 hours), the number of proximal Cer1/GFP positive cells decreased further (from 2 cells to 1 cell on average per embryo). In contrast, the number of lateral Cer1/GFP positive cells was now twice the number of cells found proximally. Again, there was no Cer1/GFP expression within the 3 distal-most cells at the tip of the embryo.
Thus, although expression of Cer1/GFP began in different regions of the embryo, most of the Cer1/GFP positive cells tended to accumulate laterally towards E5.25. This is due to cells within the proximal region (33% of cells in total) losing Cer1/GFP expression between E4.75 and E5.25. Moreover, when Cer1/GFP positive cells could be distinguished on opposite sides of the embryo at E4.75 (64% of the embryos), only the cells with the highest levels of fluorescence maintained the expression and constituted a lateral patch at the end of the culture (for example Fig. 6B, embryos , 3,4,5). This suggests that there is a tendency for only one side of the embryo to maintain Cer1/GFP expression after E4.75.
Thus, the population of Cer1 expressing cells in E5.25 embryos is mainly comprised of cells that already expressed Cer1 at E4.75 and/or their progeny. Moreover, the regulative mechanisms that induce/repress Cer1 expression also come into play at these stages.
Contribution of the ancient Cer1 population of cells to the development of the AVE
Having established the contribution of the original Cer1 expressing cells up to E5.25, we then questioned whether these early expressing cells contributed to the formation of the AVE. To this end, we conducted time-lapse analysis from both E5.0 and E5.25, until the AVE emerges at the distal tip of the embryo and becomes asymmetrically localised (Figure 7A). At this stage, cultured embryos showed an appropriate AVE formation as seen by the AVE markers LEFTY1 and CER1 at the end of the time in culture (Supplementary Figure 5).
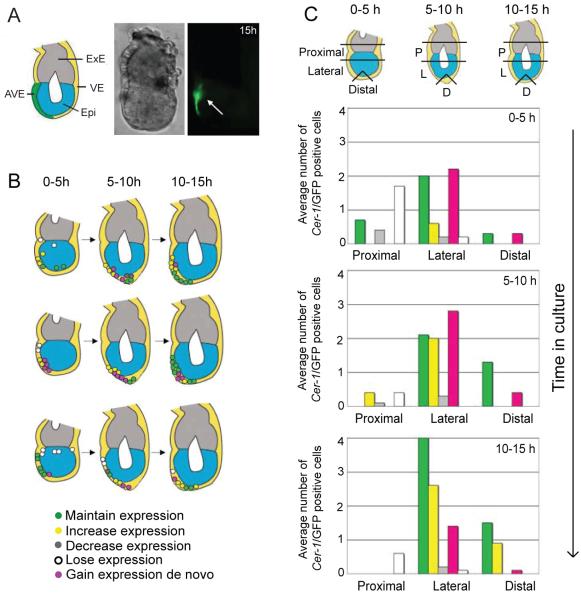
Figure 7. Time-lapse studies from E5.0 indicate that the AVE is composed of ‘precursor’ cells originally expressing Cer1/GFP and cells that are induced to express Cer1/GFP.
A. Representative embryo showing the stage at the end of culture from E5.0, where the clear asymmetric domain of the AVE is evident (arrowed). Brightfield and fluorescent images are shown.
B. Schematic images of Cer1/GFP expression in embryos cultured from E5.0. Dots represent localisation of and behaviour of expressing cells as indicated by the colour code below the diagrams. Three time frames during culture indicated.
C. Histograms illustrating both distribution and behaviour of Cer1/GFP expressing cells from E5.0. Positioning of cells was scored according to region within the embryo as shown in the diagram (Proximal: 2 cell lengths above and below the embryonic/extraembryonic boundary; Lateral: 1/3 total length of the egg cylinder below and distal to the proximal region, and Distal: the distal most cell according to the proximal-distal axis and 2 adjacent cells). Time in culture and behaviour of expressing cells is indicated. Data is indicated as the average number of Cer1/GFP positive cells per embryo for 9 embryos (for 0-5h and 5-10h) and 8 embryos for 10-15h.
Firstly, embryos were recovered at E5.0 and imaged every 20 minutes over 6 optical z-planes to detect Cer1/GFP expression. Changes in cell behaviour were scored as above (Fig. 7B-C, Fig.9B, Supplementary Information 6). Time in culture was recorded (0-5, 5-10, 10-15 hours) as was the position of expressing cells within the embryo: Proximal; Lateral; Distal. (Regions indicated schematically in Fig.7C). Of the 9 embryos analysed, 1 embryo went out of the field for the final time interval and therefore our analysis of this period includes 8 embryos. Schematic representation of the distribution and behaviour of Cer1/GFP positive cells of 3 of the embryos analysed are shown in Figure 7B.
During the first 5 hours of culture, proximal Cer1/GFP cells were evident in two thirds of the embryos examined (n=9, 4 cells on average per embryo, range from 1-7 cells per embryo). Despite variation in cell number, 73% of these positive cells underwent a progressive decrease and/or loss of expression (ranging from 100%-50% of cells in each embryo) with the remaining positive cells maintaining expression. Thus, at the end of this time period, 50% of embryos had cells in which expression was maintained (2-3 positive cells), whereas proximal expression was lost in the remaining embryos. During the next 5 hours, 55% of the embryos no longer had proximal Cer1/GFP expressing cells. Where proximal expression was evident (2 cells on average per embryo, range 2-3 cells per embryo), 90% of these cells were lost, with the remaining cells decreasing in levels of expression. At the end of culture, only one embryo retained a single proximal expressing cell from earlier stages. These observations support the progressive downregulation of proximal Cer1 expression from E5.0, evident from our previous time-lapse imaging of embryos at equivalent stages.
In around of 90% embryos examined, positive cells were observed laterally at the start of culture. The total number of cells was higher compared to those found proximally, (46 cells/8 embryos, range 3-8 cells per embryo, compared to 26 cells/6 embryos, range 1-7 cells per embryo). Furthermore, only 4% underwent a decrease in expression (compared to 73% of proximal cells), whilst 37% of lateral cells maintained expression. Where expression was downregulated, cells tended to be on one side of the embryo, resulting in an asymmetric domain of Cer1/GFP. Whereas no increases or de novo expression was evident proximally, a small number of lateral cells increased expression (9%), with a greater population of cells expressing Cer1/GFP de novo (50%). During the following 5 hours, all embryos had lateral Cer1/GFP expressing cells (7 cells on average per embryo, range from 2-12 cells per embryo), of which equal proportions maintained or increased expression (30% and 29% of positive cells, respectively). A further 36% of Cer1/GFP-expressing cells arose de novo, whilst a small proportion decreased levels of expression (5%). In contrast to the proximal region of the embryo, lateral Cer1/GFP positive cells were identified in all embryos examined during the final 5 hours of imaging (8 cells on average per embryo, range from 4-16 cells per embryo). Of these, a small fraction decreased and/or lost Cer1/GFP expression (9%, combined), whereas a third of expressing cells increased levels of expression and 44% maintained a constant level of Cer1/GFP expression. De novo Cer1/GFP expression was also evident, with 16% of cells acquiring expression during this time period. Therefore, cells located laterally largely maintained and increased levels of Cer1, as well as acquiring de novo Cer1/GFP expression, with only some degree of downregulation/loss of gene expression. In combination, this leads to an asymmetrical domain of Cer1/GFP positive expressing cells at a stage corresponding to E5.75.
Initially, no Cer1/GFP expressing cells were evident distally. However, during the subsequent 5 hours of imaging, distal Cer1/GFP positive cells were identified for the first time during culture in two thirds of the embryos (on average, 2.5 cells were expressing; that is, in 4/6 embryos, 3 cells were positive; in 1/6 embryos, 2 cells were positive and in 1/6 embryos, 1 cell was expressing). With the exception of one embryo with a single de novo expressing cell, the distal population comprised cells maintaining Cer1/GFP expression (2/6 embryos with distal positive cells) or a combination of a single de novo Cer1/GFP-expressing cell with 2 cells which had maintained Cer1/GFP expression (3/6 embryos with distal positive cells). During the final time period, all embryos had distal Cer1/GFP-expressing cells (3 cells on average, range from 2-3 cells per embryo) with either a constant or increasing level of expression.
To identify the origins of these Cer1 expressing cells, we compared the contribution of cells expressing Cer1/GFP at E5.0 (named here as ‘ancient population’), prior to expression in the distal tip, versus the contribution of cells acquiring Cer1 during culture. Only in one embryo we found that distally Cer1 expressing cells were derived exclusively from the ancient population. In the remaining 7 embryos, distal cells expressing Cer1 were a combination of both ancient Cer1 positive cells and cells initiating Cer1 expression after E5.0. Here, a greater proportion are derived from the ancient population (62% ancient cells compared to 38% newly expressing cells). Thus, Cer1 expressing cells within the distal region of the embryo were composed of a population of cells which had either maintained Cer1/GFP expression, or those in which Cer1 expression had been initiated after E5.0.
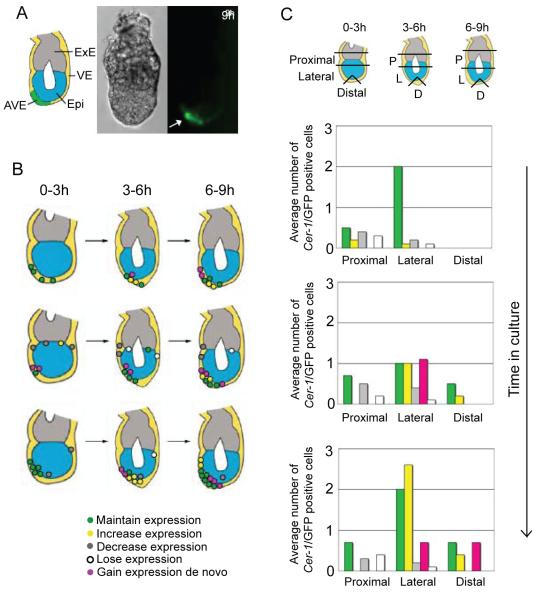
We then analysed the behaviour of Cer1 expressing cells in embryos dissected at E5.25. Embryos were cultured for approximately 10 hours when we could observe the asymmetrical positioning of the AVE (Fig. 8A, this stage is equivalent to the E5.5 LD stage described by Rivera-Perez et al., 2003). As before, embryos were imaged every 20 minutes over 6 optical z-planes to detect Cer1/GFP expression and cells were analysed according to time in culture (0-3, 3-6 and 6-9 hours) changes in behaviour (Fig. 8B, 9C) and position in the embryo: Proximal; Lateral or Distal (Fig. 8C). Out of 11 embryos analysed, 2 went out of the field for the final time interval and therefore the data for this period derives only from 9 embryos. Schematic representation of the distribution and behaviour of Cer1/GFP positive cells of 3 of the embryos analysed are shown in Figure 8B.
Figure 8. The AVE is composed of cells originally expressing Cer1 at early stages as well as of cells acquiring expression around E5.5.
A. Representative embryo showing the stage reached at the end of the culture from E5.25. Due to the high sensitivity acquisition conditions used, our time-lapse analysis was stopped at this point when the fluorescence intensity reached a level where we were no longer able to resolve single cells. Arrow points to the AVE. Brightfield and fluorescence images are shown.
B. Schematic images of Cer1/GFP expression in embryos cultured from E5.25. Dots represent localisation and behaviour of expressing cells as indicated by the colour code below the diagrams. Three time frames during culture indicated.
C. Histograms illustrating both distribution and behaviour of Cer1/GFP expressing cells from E5.25. Positioning of cells was scored according to region within the embryo as shown in the diagram (Proximal: 2 cell lengths above and below the embryonic/extraembryonic boundary; Lateral: 1/3 total length of the egg cylinder below the distal to the proximal region, and Distal: the distal most cell according to the proximal-distal axis and 2 adjacent cells). Time in culture and behaviour of the expressing cells is indicated. Data is indicated as the average number of Cer1/GFP positive cells per embryo for 11 embryos (for 0-3h and 3-6h) and 9 embryos for 6-9h.
In 45% (5/11) of the embryos analysed, Cer1/GFP positive cells were visible proximally during the first 3 hours of culture. These were either on one side of the embryo (3/5) or on the two opposite sides (2/5). As in the previous time-lapse studies, proximal cells tended to lose Cer1/GFP expression (50% of cells underwent either a decrease and/or loss of expression) whilst 21% of positive cells had constant levels of expression whilst no cells acquired Cer1/GFP expression proximally. The average cell number per embryo is plotted in Figure 8C.
During the following 3 hours of culture, the number of embryos with proximal Cer1/GFP positive cells remained constant (5/11). All embryos now had an asymmetrical distribution of Cer1/GFP expression (Fig. 8B), indicating that cells which lost expression were on the same side of the embryo. More than half of the Cer1/GFP positive cells underwent a decrease and/or loss of expression (59%), whilst a third maintained their levels of expression. No de novo expression was evident in this region (Fig.8C). Following a further 3 hours of culture, only 1/3 of embryos maintained Cer1/GFP expression. Of these cells, 50% underwent a decrease and/or loss of expression. This indicates a progressive downregulation and not uniform restriction of Cer1 expression around the embryonic/extraembryonic border of the embryo after E5.25.
Cer1/GFP expressing cells were identified in the lateral region at E5.25 in all embryos. Here, levels of Cer1/GFP expression remained largely constant: 79% of cells maintained expression, with some increases in levels of expression (6%). Only a small number showed decreased or loss of expression (10% and 5%, respectively). Cells acquiring Cer1/GFP expression de novo were not observed (Fig. 8C Thus, in contrast to the proximal positive cells of which more than half underwent changes in the levels of Cer1/GFP expression, lateral positive cells tended to maintain a constant level of Cer1 expression. During a further 3 hours of culture, all embryos continued to express Cer1/GFP laterally. Here, a third of positive cells resulted from de novo Cer1 expression (29%), with 56% of cells either increasing or maintaining levels of expression. Only low levels of loss of expression within the lateral region of the embryo were observed (2% of cells). This trend was maintained throughout the remaining 3 hours of culture: 80% of positive cells increased or maintained Cer1/GFP expression and 13% of cells acquired expression de novo, with few cells decreasing and/or losing expression (7%, combined). (Fig. 8C). Thus, there is a tendency for lateral positive cells to either increase or maintain levels of Cer1/GFP expression, with an increase in the number of cells acquiring Cer1 expression de novo.
We then examined the distal part of the egg cylinder, incorporating the distal-most cell across the proximal-distal axis and the two adjacent cells. Here, we found that only 36% (4/11) of E5.25 embryos had Cer1/GFP positive cells in this region at time zero (1 cell per embryo on average). However, the number of embryos with Cer1/GFP positive cells in this region increased with time. During the second 3 hours of culture, there was on average 1 Cer1/GFP positive cell in the distal region in all embryos examined (n=9 embryos). All these cells were originally located in the lateral region of the embryo and maintained their levels of Cer1/GFP expression. Interestingly, during the final 3 hours of culture, all embryos contained Cer1/GFP expressing cells distally, 2 cells on average per embryo.
These cells either maintained Cer1/GFP expression from the previous time point (1 cell on average per embryo) or acquired Cer1/GFP expression at this time (1 cell on average per embryo). Throughout culture, there was no decrease or loss of Cer1/GFP expression in the distal tip of the embryo (Fig. 8C). In contrast, some of these cells increased levels of Cer1/GFP expression at around E5.5. Thus, in accordance with our previous observations at the distal most region of the embryo, there is a progressive increase in the number Cer1/GFP expressing cells through both sustained maintenance of expression and de novo expression. Therefore, Cer1 expressing cells at the distal tip of the embryo were composed of cells which had either maintained Cer1/GFP expression, or those in which Cer1 expression arose after E5.0.
As the AVE undergoes its unilateral movement after E5.5, cells are thought to extend filopodia in response to guidance cues from the epiblast (Srinivas et al, 2004). In agreement with this observation, such extensions were evident as the AVE migrated asymmetrically after E5.5 (data not shown). We were unable to observe any signs of cell migration in these lateral precursor cells which become distally located at E5.5 in either of our time-lapse analyses in which AVE formation was evident. This indicates that it the displacement of these cells towards the distal tip of the embryo is likely to result from overall embryo growth and/or the pattern of cell division/ proliferation within the VE covering the embryonic part of the egg cylinder.
Our results from the time-lapse imaging of two time periods of periimplantation development show that: First, the expression of the AVE marker, Cer1, is evident prior to the formation of the AVE, with expression progressively downregulated across the proximal-distal axis of the embryo. Second, that a sub-group of these early Cer1 expressing cells maintain this expression, ultimately giving rise to the definitive AVE in co-operation with cells expressing Cer1 de novo.
Given that at E5.5 the AVE comprises cells located towards the distal tip of the embryo, we also analysed independently the origin of these distal/lateral Cer1 expressing cells. We refer to ‘AVE’ as the cells that express Cer1 in the lateral and distal regions of the embryo at the endpoint of our time-lapse study (around E5.75) (see Fig. 7A, on average 8 cells). We examined the contribution of Cer1 positive cells that were originally present at E5.25 versus the number of cells in the AVE that acquired Cer1 expression de novo after E5.25. We found in 27% (n=11) of the embryos analysed , the AVE population was composed exclusively of cells that expressed Cer1 at E5.25 and their progeny. Thus, in these embryos, the AVE was formed from “precursor” cells that had expressed Cer1 earlier (at E5.25). In the remaining 73% of embryos, 47% (SD 30%) of the AVE was formed by cells that expressed Cer1 at E5.25 or their progeny. The remaining Cer1 expressing cells (53%) originated from VE cells that began to express Cer1 after E5.25. By analysing these embryos together, it appears that, on average, 61% of the cells that form the AVE derive from precursor cells i.e. cells which were already Cer1 positive at E5.25. If we also take into account that at E5.25, 75% of the Cer1 positive cells can be traced back to E4.75 (section above), this suggests that on average, 45% of the AVE population derives from precursors already present at E4.75. Therefore, we conclude that the AVE is heterogeneous in its nature and comprises of both precursors that already express Cer1 in the implanting blastocyst and cells that acquire Cer1 expression at the egg cylinder stage between E4.75 and E5.5. Interestingly, in all of the embryos examined, “precursor” cells were located laterally within the embryo at E5.25 on the same side towards which the AVE migrates asymmetrically after E5.5. This suggests that the position of the lateral Cer1/GFP cells ‘dictates’ the side on which the AVE will shift anteriorily.
Discussion
We have found that the AVE is formed from both preimplantation precursor cells and by cells which show de novo gene expression after embryo implantation. Our results show that expression of Cer1, an AVE marker, is initiated at the blastocyst stage independently of the embryo’s interaction with the uterus. This Cer1 expression develops in the primitive endoderm and as the embryo implants and grows, Cer1-expressing cells show a tendency to gather only on one side of the egg cylinder. Our time-lapse studies demonstrate that a subgroup of these Cer1 expressing cells provide progenitors for the future AVE. However, our data also suggest that another yet-to-be discovered mechanism re-inforces AVE gene expression and induces Cer1 expression de-novo in adjacent cells at the distal tip of the egg cylinder around E5.5. Finally, we find that when the AVE shifts asymmetrically to establish the site of the anterior pole, this occurs towards the region in which strongest Cer1 expression was previously evident. Thus, these results strongly suggest that although the AVE is established only after embryo implantation, the orientation of the AP axis is already anticipated at periimplantation stages when the blastocyst transforms into the egg cylinder.
The expression of Cer1 restricted to some cells in the growing blastocyst, together with the recently reported early Lefty1 expression (Takaoka et al., 2006,), suggests that mouse embryo AP polarity originates earlier than previously expected. How does this polarity develop? Our results indicate that the onset of Cer1 expression occurs in the late blastocyst and can clearly be detected in a subset of adjacent cells in the primitive endoderm when this layer forms. This tendency of Cer1 expressing cells to gather together may indicate some spatial organization of the primitive endoderm at this early stage. Interestingly, as the blastocyst develops into the egg cylinder upon implantation, the expression of Cer1 becomes clearly asymmetric. In most embryos, Cer1 expression is only on one side of the bilaterally symmetrical egg cylinder, whereas in others it occurs on opposite sides. In embryos in which we could detect the characteristic folding of the visceral endoderm, Cer1 expression was on the same side as the folding. This suggests that the morphological asymmetry of the embryo which has been previously described (Smith, 1980) could correlate with molecular asymmetry in the expression of this anterior marker. This finding would provide some support to the observations of Smith (Smith, 1980) whereby some asymmetries within the implanting conceptus are predictive of the orientation and polarity of the AP axis. However, our observation of Cer1 expression in embryos recovered from the oviduct and cultured in vitro, suggests that initiation of Cer1 expression is independent of the embryo’s interaction with the uterus. This finding is in agreement with our previous studies indicating that the orientation of the AP axis follows the morphology of the embryo, rather than that of the uterus (Mesnard et al., 2004). The development of AP polarity independently of implantation has been also reported in other mammalian embryos, namely in the rabbit embryo (Viebahn et al., 1995). Together, these results provide strong support for the notion that the embryo is intrinsically able to initiate development of AP polarity in a manner that correlates with its asymmetric morphology. Whether this differential onset of anterior marker expression is a guide for or consequence of other asymmetries within the embryo remains unknown.
We found that some of the Cer1-expressing cells at these early periimplantation stages provide true precursors for the AVE which will only become apparent around E5.5. Our time-lapse studies demonstrate that as the periimplantation embryo grows, the one original coherent region of Cer1 expression becomes dispersed into 2 or 3 clusters of which one is located more proximally (at the level of the embryonic/extraembryonic boundary) and the other(s), more distally. This pattern of growth relates to the previously described morphogenesis of the egg cylinder in which VE growth is not coherent (Weber et al., 1999). Interestingly, the behaviour of these clusters of Cer1-expressing cells is different. As development progresses, expression of Cer1 in the proximal domain is progressively diminished: cells in this region either decrease their levels of expression or lose it entirely (Fig.6). In agreement with this trend, we have not observed any tendency for cells to initiate the Cer1 expression in this proximal region. In contrast, entirely the opposite happens to Cer1-expressing cells within the lateral region of the embryo. These cells not only maintain, but also often (54%), increase their Cer1/GFP expression levels. Thus, although expression of Cer1 is initially seen in different regions of the embryo at E4.75, most of the Cer1-expressing cells tend to accumulate in a lateral position towards E5.25. This lateral/distal population of Cer1 expressing cells comprises cells or daughters of cells which already expressed Cer1 at E4.75. In addition, we observed that in those embryos in which two opposite domains of Cer1 expression were present at E4.75, only the cells with the highest levels of Cer1 expression maintained their expression after E4.75. Moreover, in this lateral region some cells initiated Cer1 expression de novo, particularly around E5.5. Together, these results indicate that Cer1 expression becomes upregulated around E5.5. Thus, we conclude that overall there is a progressive down-regulation and unequal restriction of Cer1 expression around the embryonic/extraembryonic border of the embryo and upregulation of Cer1 expression in the lateral/distal region.
What then, is the origin of the AVE as a whole? Our studies indicate that the subset of the AVE expressing Cer1 comprises two different cell populations. On average ass many as 45% of AVE cells are members of lineages that have expressed Cer1 from the late blastocyst stage. The remainder are their neighbouring cells that have been induced to express Cer1 de novo from about E5.5 at the distal tip of the embryo. At the late blastocyst stage, we observed Cer1 expression in only a subset of PE cells that then become clustered together. Whether this expression results from signalling from neighbouring cells or arises stochastically remains unknown. However, we found that Cer1-expressing cluster of cells becomes dispersed upon growth of the PE into the VE and only the lateral-distal group of these cells will become incorporated into the future AVE. Moreover, the expression of Cer1 in these cells is reinforced. Cer1 expression in more proximally located groups declines with time, presumably as these cells have not received signals necessary to reinforce their anterior identity. The distal group of cells in which Cer1 persists is of particular interest. Do these cells represent a population that “seeds” the AVE proper? As they make a significant contribution to the founder population of the AVE, this is certainly a possibility. The upregulation of Cer1 expression in these cells occurs at around the same time that their non Cer1 expressing neighbouring cells are induced to express Cer1. Whether or not cells which start to express Cer1 at E5.5 had expressed and lost Cer1 at earlier stages, this indicates an important role of induction in AVE formation.
At these later stages of development, Nodal is required for AVE specification (Brennan et al., 2001). Removal of the extraembryonic ectoderm (ExE) has the opposite effect – most of the VE cells develop an anterior character (Richardson et al., 2006; Rodriguez et al., 2005). This could mean that the ExE provides an inhibitory signal upon the AVE inducing properties of Nodal. The recent finding that Nodal is also expressed in the PE (Mesnard et al., 2006; Takaoka et al., 2006) raises the question of whether Nodal may be involved in the specification of AVE precursors from this very early stage. As Cer1 (this study) and Lefty1 (Takaoka et al., 2006; Chazaud and Rossant, 2006) are not expressed uniformly in the PE, it will be important to determine whether Nodal (and also its co-receptor Cripto) has different levels of activity in different cells despite being expressed throughout the ICM.
It is noteworthy that there appears to be some variability in the expression of both Cerl (this study) and Lefty1 (Chazaud and Rossant, 2006) between embryos at the late blastocyst stage. While some authors describe asymmetric expression of Lefty1 (Takaoka et al., 2006), others state that Lefty1 shows varying degrees of expression: the expression is not uniform along the PE but in a gradient from the center to the periphery, Such variability in the expression of these early anterior markers could be due to the dynamic changes in their expression at these stages as indeed indicated by our time-lapse studies. Also other genes, such as Hex and Pem, have been recently reported to show varying levels of expression patterns within the PE at these early stages (Chazaud and Rossant, 2006). The mechanisms regulating these expression patterns remain unknown.
It also remains to be determined whether cells showing the early expression of Lefty1 in the PE reported by Takaoka et al also express Cer1. Our observation of Cerl expressing cells showing also expression of Lefty1 suggest that these two genes may be regulated by overlapping mechanisms. The dynamics and relative levels of expression of Lefty1 between cells were not examined in the study of Takaoka and colleagues and neither were the lineage relationships of such cells (Takaoka et al., 2006). It will therefore be important in the future to ascertain by lineage tracing studies whether early Lefty1-expressing cells are true progenitors of the AVE and if so whether, like Cer1-expressing cells, they contribute to just a proportion of the AVE. In this same vein, does Lefty1 expression show a similar pattern of upregulation as we here describe for Cer1? Furthermore, are cells which are induced to express Cer1 de novo positive for other AVE markers, such as Lefty1? The combination of transgenic lines tagged with different fluorescent proteins could be adopted to address this interesting possibility. It will also be essential to address how the different regulation of these transcription factors and signalling molecules act together to pattern the VE. We can speculate that it is the combinatorial effects and the local microenvironment provided by signalling stimuli together with the action of transcription factors such as Hex and Pem on their particular target genes that is important for the ultimate effects on the fate of the cells within the VE.
In all the embryos examined, “precursor” AVE cells were located laterally within the embryo. But perhaps most importantly, they were located on the same side of the embryo towards which the AVE becomes subsequently asymmetric. This suggests that the early position of the Cer1 expressing cells also predicts the side to which the AVE will shift. This supports the previous suggestion that the intrinsic information within the embryo “directs” the orientation of AVE migration and thus the orientation of the AP axis (Mesnard et al., 2004; Perea-Gomez et al., 2004).
Supplementary Material
Acknowledgements
We are grateful to Dr J.Belo for the Cer1/GFP trasngenic line, Prof. H. Hamada for the Lefty1 probe, Dr M.Pontoglio for the vHNF1 antibody and Dr S.Frankenberg for the Cer1 probe.and. We are grateful to BBSRC and Wellcome Trust grants to MZG which funded this work. MZG is Wellcome Senior Research Fellow. M.E.T-P is a recipient of an EMBO longterm fellowship (present address: Institut de Genetique et de Biologie Moleculaire et Cellulaire, 1 rue Laurent Fries, Strasbourg, France). L.R. held a BBSRC studentship and S.M.M was support by a Marie Curie Intra-European Fellowship within the Sixth European Framework Programme (present address: Department of Developmental Biology, Pasteur Institute, 25 rue du Dr. Roux, Paris, France).
References
- Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795–805. doi: 10.1242/dev.126.21.4795. [DOI] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–9. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Rossant J. Disruption of early proximodistal patterning and AVE formation in APC mutants. Development. 2006;133:3379–3387. doi: 10.1242/dev.02523. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Thepot D, Babinet C, Yaniv M, Barra J. Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999;126:4785–94. doi: 10.1242/dev.126.21.4785. [DOI] [PubMed] [Google Scholar]
- Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, et al. A transcriptional network in polycystic kidney disease. Embo J. 2004;23:1657–68. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB. The generation of diversity and pattern in animal development. Cell. 1992;68:185–99. doi: 10.1016/0092-8674(92)90465-o. [DOI] [PubMed] [Google Scholar]
- Mesnard D, Filipe M, Belo JA, Zernicka-Goetz M. The anterior-posterior axis emerges respecting the morphology of the mouse embryo that changes and aligns with the uterus before gastrulation. Curr Biol. 2004;14:184–96. doi: 10.1016/j.cub.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Mesnard D, Guzman-Ayala M, Constam DB. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development. 2006;133:2497–505. doi: 10.1242/dev.02413. [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Camus A, Moreau A, Grieve K, Moneron G, Dubois A, Cibert C, Collignon J. Initiation of gastrulation in the mouse embryo is preceded by an apparent shift in the orientation of the anterior-posterior axis. Curr Biol. 2004;14:197–207. doi: 10.1016/j.cub.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell. 2002;3:745–56. doi: 10.1016/s1534-5807(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Richardson L, Torres-Padilla ME, Zernicka-Goetz M. Regionalised signalling within the extraembryonic ectoderm regulates anterior visceral endoderm positioning in the mouse embryo. Mech Dev. 2006;123:288–296. doi: 10.1016/j.mod.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Mager J, Magnuson T. Dynamic morphogenetic events characterize the mouse visceral endoderm. Dev Biol. 2003;261:470–87. doi: 10.1016/s0012-1606(03)00302-6. [DOI] [PubMed] [Google Scholar]
- Robertson EJ, Norris NP, Brennan J, Bikoff EK. Control of early anterior-posterior patterning in the mouse embryo by TGF-ß signalling. Phil. Trans. R. Soc. Lond. B. 2003;358:1351–1358. doi: 10.1098/rstb.2003.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez TA, Srinivas S, Clements MP, Smith JC, Beddington RS. Induction and migration of the anterior visceral endoderm is regulated by the extraembryonic ectoderm. Development. 2005;132:2513–20. doi: 10.1242/dev.01847. [DOI] [PubMed] [Google Scholar]
- Smith LJ. Embryonic axis orientation in the mouse and its correlation with blastocyst relationships to the uterus. Part 1. Relationships between 82 hours and 4 1/4 days. J Embryol Exp Morphol. 1980;55:257–77. [PubMed] [Google Scholar]
- Soares ML, Haraguchi S, Torres-Padilla ME, Kalmar T, Carpenter L, Bell G, Morrison A, Ring CJ, Clarke NJ, Glover DM, et al. Functional studies of signalling pathways in periimplantation development of the mouse embryo by RNAi. BMC Dev Biol. 2005;5:28. doi: 10.1186/1471-213X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Rodriguez T, Clements M, Smith JC, Beddington RS. Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development. 2004;131:1157–64. doi: 10.1242/dev.01005. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Nusslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992;68:201–19. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Shiratori H, Meno C, Rossant J, Saijoh Y, Hamada H. The mouse embryo autonomously acquires anterior-posterior polarity at implantation. Dev Cell. 2006;10:451–9. doi: 10.1016/j.devcel.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Thomas PQ, Brown A, Beddington RS. Hex: a homeobox gene revealing periimplantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125:85–94. doi: 10.1242/dev.125.1.85. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Mayer B, Hrabe de Angelis M. Signs of the principle body axes prior to primitive streak formation in the rabbit embryo. Anat Embryol (Berl) 1995;192:159–69. doi: 10.1007/BF00186004. [DOI] [PubMed] [Google Scholar]
- Weber RJ, Pedersen RA, Wianny F, Evans MJ, Zernicka-Goetz M. Polarity of the mouse embryo is anticipated before implantation. Development. 1999;126:5591–8. doi: 10.1242/dev.126.24.5591. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Saijoh Y, Perea-Gomez A, Shawlot W, Behringer RR, Ang SL, Hamada H, Meno C. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–92. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M. Developmental cell biology: cleavage pattern and emerging asymmetry of the mouse embryo. Nat Rev Mol Cell Biol. 2005;6:919–28. doi: 10.1038/nrm1782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.